Abstract
C24H27N5O9S, triclinic, P
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow needle |
| Size: | 0.42 × 0.25 × 0.09 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.18 mm−1 |
| Diffractometer, scan mode: | Oxford diffraction ruby, ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18,840, 4726, 0.037 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3319 |
| N(param)refined: | 386 |
| Programs: | CrysAlis [1], SHELX [2, 3], ORTEP-II [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1W | 0.7318 (4) | 0.1341 (2) | 0.38546 (13) | 0.0731 (6) |
| H1W | 0.793 (4) | 0.071 (4) | 0.3891 (18) | 0.080 (12)* |
| H2W | 0.687 (5) | 0.135 (4) | 0.343 (2) | 0.105 (13)* |
| C1 | 0.8596 (3) | 0.6705 (2) | 0.44288 (12) | 0.0406 (5) |
| H1A | 0.899496 | 0.631291 | 0.397487 | 0.049* |
| C2 | 0.8831 (3) | 0.8019 (2) | 0.45149 (13) | 0.0423 (5) |
| C3 | 0.8260 (3) | 0.8619 (2) | 0.52019 (14) | 0.0488 (6) |
| H3A | 0.843426 | 0.951428 | 0.525687 | 0.059* |
| C4 | 0.7454 (3) | 0.7899 (2) | 0.57858 (14) | 0.0471 (6) |
| H4A | 0.707587 | 0.830287 | 0.623806 | 0.056* |
| C5 | 0.5242 (3) | 0.3870 (2) | 0.68933 (13) | 0.0450 (6) |
| H5A | 0.492404 | 0.432940 | 0.733239 | 0.054* |
| C6 | 0.4900 (3) | 0.2546 (2) | 0.68768 (14) | 0.0460 (6) |
| C7 | 0.5369 (3) | 0.1868 (2) | 0.61980 (14) | 0.0479 (6) |
| H7A | 0.512541 | 0.097744 | 0.617645 | 0.057* |
| C8 | 0.6165 (3) | 0.2498 (2) | 0.55824 (13) | 0.0435 (6) |
| H8A | 0.645714 | 0.202972 | 0.514493 | 0.052* |
| C9 | 0.7419 (3) | 0.4554 (2) | 0.49519 (12) | 0.0369 (5) |
| N10 | 0.6365 (2) | 0.5839 (2) | 0.62940 (12) | 0.0427 (5) |
| H10A | 0.600 (3) | 0.621 (3) | 0.6684 (15) | 0.046 (7)* |
| C11 | 0.7753 (3) | 0.5937 (2) | 0.50233 (12) | 0.0368 (5) |
| C12 | 0.7191 (3) | 0.6539 (2) | 0.57073 (12) | 0.0384 (5) |
| C13 | 0.6568 (3) | 0.3857 (2) | 0.55845 (12) | 0.0376 (5) |
| C14 | 0.6055 (3) | 0.4530 (2) | 0.62626 (12) | 0.0390 (5) |
| N15 | 0.7899 (3) | 0.3967 (2) | 0.43089 (12) | 0.0461 (5) |
| H15A | 0.765 (3) | 0.314 (3) | 0.4262 (15) | 0.065 (9)* |
| H15B | 0.840 (3) | 0.441 (3) | 0.3920 (16) | 0.051 (8)* |
| N16 | 0.4102 (3) | 0.1874 (3) | 0.74878 (15) | 0.0650 (7) |
| H16A | 0.390 (4) | 0.228 (3) | 0.7893 (18) | 0.078* |
| H16B | 0.402 (4) | 0.102 (3) | 0.7495 (17) | 0.078* |
| O17 | 0.9597 (2) | 0.88627 (16) | 0.39651 (9) | 0.0519 (4) |
| C18 | 1.0196 (3) | 0.8305 (3) | 0.32493 (14) | 0.0523 (6) |
| H18A | 0.921682 | 0.792703 | 0.304783 | 0.063* |
| H18B | 1.111008 | 0.759103 | 0.332091 | 0.063* |
| C19 | 1.0909 (4) | 0.9401 (3) | 0.27360 (17) | 0.0769 (9) |
| H19A | 1.135541 | 0.904707 | 0.225752 | 0.115* |
| H19B | 1.185597 | 0.977456 | 0.295143 | 0.115* |
| H19C | 0.997388 | 1.008646 | 0.266940 | 0.115* |
| C20 | 0.4250 (3) | 0.8183 (2) | 0.87314 (13) | 0.0489 (6) |
| C21 | 0.3201 (3) | 0.9241 (3) | 0.90505 (15) | 0.0550 (7) |
| H21A | 0.264189 | 0.988947 | 0.875000 | 0.066* |
| C22 | 0.2998 (3) | 0.9320 (3) | 0.98266 (16) | 0.0617 (8) |
| C23 | 0.3796 (4) | 0.8388 (3) | 1.02930 (16) | 0.0678 (8) |
| H23A | 0.363562 | 0.845236 | 1.081267 | 0.081* |
| C24 | 0.4833 (4) | 0.7364 (3) | 0.99612 (15) | 0.0630 (7) |
| C25 | 0.5067 (4) | 0.7241 (3) | 0.91954 (14) | 0.0567 (7) |
| H25A | 0.577624 | 0.652328 | 0.898883 | 0.068* |
| C26 | 0.4524 (4) | 0.8048 (3) | 0.78883 (14) | 0.0559 (7) |
| O27 | 0.3892 (3) | 0.8980 (2) | 0.74987 (11) | 0.0800 (6) |
| O28 | 0.5351 (3) | 0.7005 (2) | 0.76663 (10) | 0.0846 (7) |
| N29 | 0.1918 (4) | 1.0471 (3) | 1.0164 (2) | 0.0857 (9) |
| O30 | 0.1335 (4) | 1.1341 (3) | 0.97468 (18) | 0.1085 (9) |
| O31 | 0.1675 (4) | 1.0493 (3) | 1.08421 (16) | 0.1292 (11) |
| N32 | 0.5765 (5) | 0.6351 (3) | 1.04375 (16) | 0.0856 (8) |
| O33 | 0.6839 (4) | 0.5532 (3) | 1.01343 (16) | 0.1097 (9) |
| O34 | 0.5397 (5) | 0.6401 (3) | 1.11118 (15) | 0.1347 (11) |
| S35a | 0.91775 (19) | 0.48519 (17) | 0.21541 (8) | 0.0899 (7) |
| S35Ab | 1.0676 (3) | 0.4653 (2) | 0.21896 (14) | 0.0777 (10)* |
| O36 | 0.9633 (4) | 0.5044 (3) | 0.29074 (13) | 0.1162 (9) |
| C37 | 1.0633 (11) | 0.5732 (7) | 0.1553 (3) | 0.215 (4) |
| H37A | 1.038831 | 0.667222 | 0.162712 | 0.323* |
| H37B | 1.049005 | 0.554808 | 0.104265 | 0.323* |
| H37C | 1.183293 | 0.546662 | 0.165257 | 0.323* |
| C38 | 0.9980 (8) | 0.3198 (5) | 0.1953 (4) | 0.182 (3) |
| H38A | 1.006934 | 0.308948 | 0.142027 | 0.274* |
| H38B | 0.917494 | 0.258810 | 0.220362 | 0.274* |
| H38C | 1.113454 | 0.301585 | 0.212543 | 0.274* |
-
aOccupancy: 0.635 (3).bOccupancy: 0.365 (3).
Source of material
All compounds were purchased from Sigma-Aldrich and used without purification. 6,9-Diamino-2-ethoxyacridine-dl-lactate monohydrate (0.03 g, 0.083 mmol) and 3,5-dinitrobenzoic acid (0.035 g, 0.165 mmol) were dissolved in 12 mL of an ethanol/water mixture (5:1 v/v) and heated in order to dissolve the sample. The solution was allowed to evaporate for a few days. Next recrystallization was performed. The sample was dissolved in 10 mL of methanol and added a few drops of CH2Cl2 and 3 mL dimethylsulfoxide (DMSO). The solution was allowed to evaporate for a few days to give yellow crystals (m.p. = 260.2 °C).
Experimental details
The solvent molecule is disordered. The site-occupancy factors are 0.635(3) and 0.365(3). All H atoms bound to N/O-atoms were located on a difference Fourier map and refined freely. All H atoms bound to aromatic C atoms were placed geometrically (C–H = 0.93 Å) and refined using a riding model with Uiso(H) = 1.2Ueq(C). All H atoms from the methyl group were positioned geometrically (C–H = 0.98 Å) and refined using a riding model, with Uiso(H) = 1.5Ueq(C).
Comment
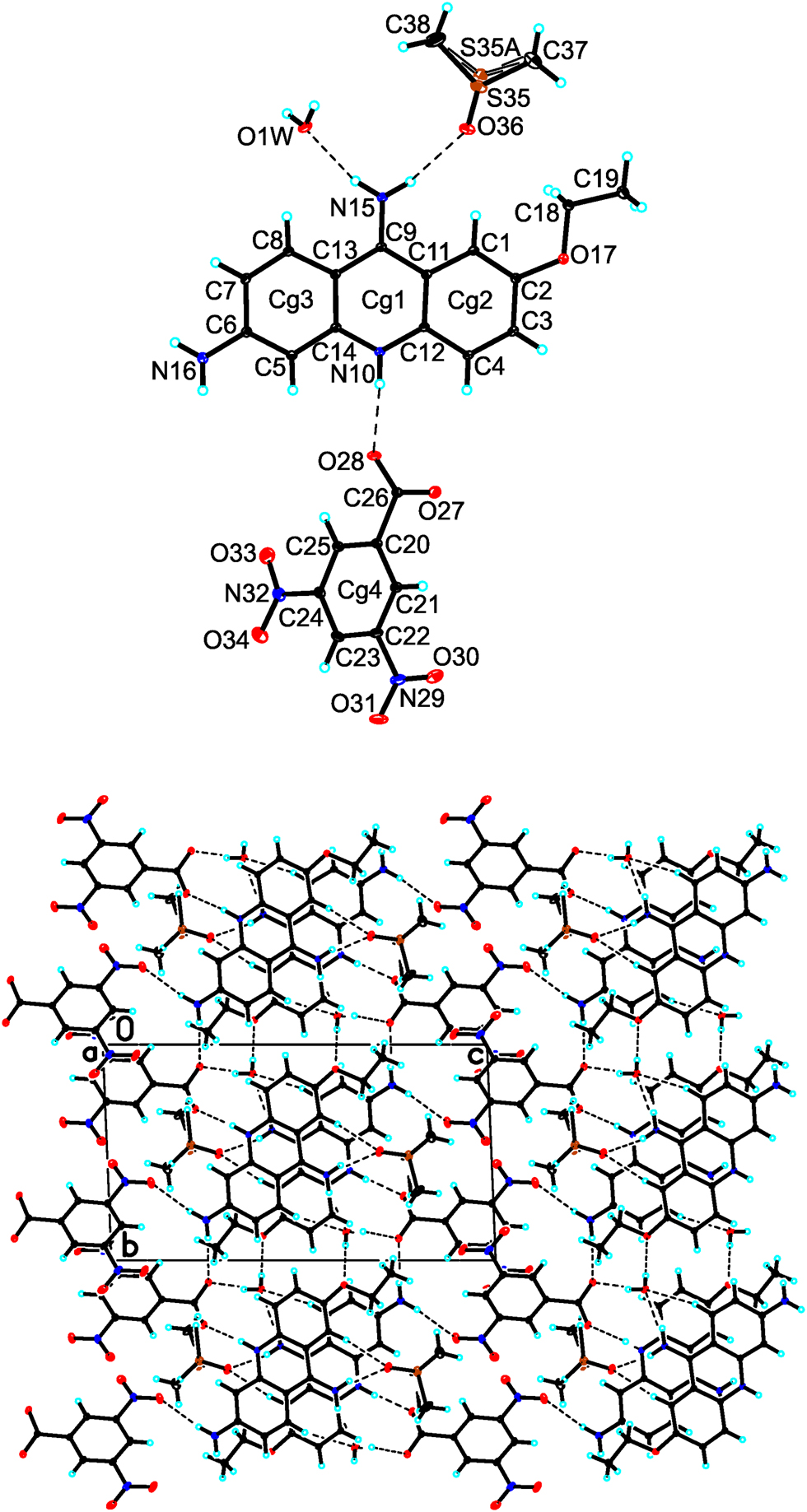
An acridine derivative – 6,9-diamino-2-ethoxyacridine – has antiviral and antibacterial properties, and it is also helpful in curing various infections [5], [6], [7]. As a continuation of my recent study on the acridine derivatives [8], [9], [10], in this paper, the crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate dimethylsulfoxide monosolvate monohydrate is described.
The title compound crystallizes in the triclinic P
Funding source: University of Gdańsk
Award Identifier / Grant number: 539-T080-B027-22
Award Identifier / Grant number: DS 531-T080-D738-22
Acknowledgements
I am grateful to Professor Artur Sikorski for critically reading and feedback on an early version of the manuscript.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Research of Young Scientists grant (BMN) no. 539-T080-B027-22 (University of Gdańsk) and DS 531-T080-D738-22 (University of Gdańsk).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlis CCD and CrysAlis RED, Version 1.171.36.24; Yarnton: UK, 2012.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122.https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Johnson, C. K. ORTEP II, Report ORNL-5138; Oak Ridge National Laboratory: Oak Ridge, TN, 1976.Search in Google Scholar
5. Oie, S., Kamiya, A. Bacterial contamination of commercially available ethacridine lactate (acrinol) products. J. Hosp. Infect. 1996, 34, 51–58. https://doi.org/10.1016/s0195-6701(96)90125-5.Search in Google Scholar PubMed
6. Petrikaite, V., Tarasevišius, E., Pavilonis, A. New ethacridine derivatives as the potential antifungal and antibacterial preparations. Medicina 2007, 43, 657. https://doi.org/10.3390/medicina43080084.Search in Google Scholar
7. Koelzer, S. C., Held, H., Toennes, S. W., Verhoff, M. A., Wunder, C. Self- induced illegal abortion with Rivanol: a medicolegal–toxicological case report. Forensic Sci. Int. 2016, 268, e18–e22. https://doi.org/10.1016/j.forsciint.2016.08.028.Search in Google Scholar PubMed
8. Mirocki, A., Sikorski, A. Influence of halogen substituent on the self-assembly and crystal packing of multicomponent crystals formed from ethacridine and meta-halobenzoic acids. Crystals 2020, 10, 79. https://doi.org/10.3390/cryst10020079.Search in Google Scholar
9. Mirocki, A., Sikorski, A. The influence of solvent on the crystal packing of ethacridinium phthalate solvates. Materials 2020, 13, 5073. https://doi.org/10.3390/ma13225073.Search in Google Scholar PubMed PubMed Central
10. Mirocki, A., Sikorski, A. Structural characterization of multicomponent crystals formed from diclofenac and acridines. Materials 2022, 15, 1518. https://doi.org/10.3390/ma15041518.Search in Google Scholar PubMed PubMed Central
11. Kowalska, K., Trzybiński, D., Sikorski, A. Influence of the halogen substituent on the formation of halogen and hydrogen bonding in co-crystals formed from acridine and benzoic acids. CrystEngComm 2015, 17, 7199–7212. https://doi.org/10.1039/c5ce01321f.Search in Google Scholar
12. Sikorski, A., Trzybiński, D. Networks of intermolecular interactions involving nitro groups in the crystals of three polymorphs of 9-aminoacridinium 2,4-dinitrobenzoate·2,4-dinitrobenzoic acid. J. Mol. Struct. 2013, 1049, 90–98. https://doi.org/10.1016/j.molstruc.2013.06.031.Search in Google Scholar
13. Sikorski, A., Trzybiński, D. Synthesis and structural characterization of a cocrystal salt containing acriflavine and 3,5-dinitrobenzoic acid. Tetrahedron Lett. 2014, 55, 2253–2255. https://doi.org/10.1016/j.tetlet.2014.02.074.Search in Google Scholar
© 2022 Artur Mirocki, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

