Abstract
C30H18F4IrN5·1.5[H2O], tetragonal, I41/a (no. 88), a = 37.5562(5) Å, b = 37.5562(5) Å, c = 9.2031(2) Å, V = 12980.7(4) Å3, Z = 16, R gt (F) = 0.0312, wRref(F2) = 0.1166, T = 300(2) K.
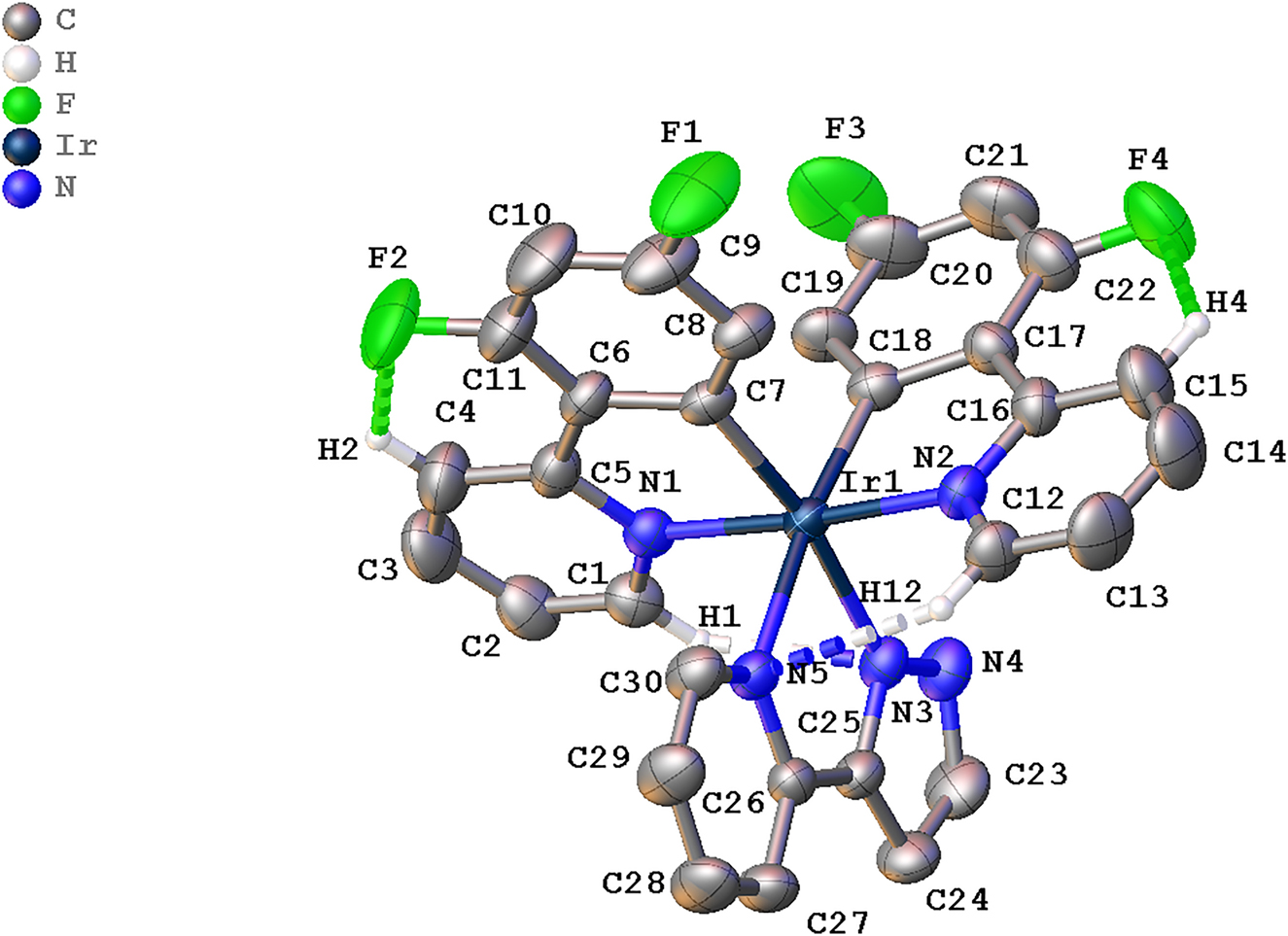
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.17 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 6.0 mm−1 |
| Diffractometer, scan mode: | Bruker APEXII, φ and ω |
| θmax, completeness: | 56.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 13,239, 9148, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7183 |
| N(param)refined: | 686 |
| Programs: | Bruker [1], SHELX [2–4], OLEX2 [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Ir1 | 0.64748 (2) | 0.29196 (2) | 0.03393 (2) | 0.03014 (9) |
| C1 | 0.65569 (13) | 0.22476 (13) | 0.2093 (5) | 0.0424 (9) |
| H1 | 0.631831 | 0.228325 | 0.232043 | 0.051* |
| F1 | 0.75746 (11) | 0.35698 (13) | −0.2427 (5) | 0.0954 (15) |
| F2 | 0.77911 (9) | 0.24709 (12) | −0.0291 (5) | 0.0877 (13) |
| C2 | 0.67257 (16) | 0.19575 (14) | 0.2657 (6) | 0.0532 (12) |
| H2 | 0.660575 | 0.180003 | 0.326451 | 0.064* |
| F3 | 0.68777 (15) | 0.33183 (13) | 0.5719 (4) | 0.1024 (16) |
| C3 | 0.70777 (18) | 0.19030 (16) | 0.2307 (7) | 0.0659 (16) |
| H3 | 0.719884 | 0.170681 | 0.267520 | 0.079* |
| F4 | 0.63174 (13) | 0.41718 (10) | 0.2821 (5) | 0.0847 (12) |
| C4 | 0.72486 (15) | 0.21384 (16) | 0.1415 (7) | 0.0607 (14) |
| H4 | 0.748551 | 0.209951 | 0.116722 | 0.073* |
| N1 | 0.67198 (9) | 0.24840 (9) | 0.1225 (4) | 0.0348 (7) |
| N2 | 0.62643 (9) | 0.33781 (10) | −0.0490 (4) | 0.0363 (7) |
| N3 | 0.59654 (9) | 0.27379 (10) | 0.0923 (4) | 0.0357 (7) |
| N4 | 0.57685 (11) | 0.27745 (12) | 0.2147 (4) | 0.0445 (9) |
| N5 | 0.63172 (9) | 0.25913 (9) | −0.1479 (4) | 0.0347 (7) |
| C5 | 0.70696 (11) | 0.24395 (12) | 0.0866 (5) | 0.0409 (9) |
| C7 | 0.69758 (11) | 0.30045 (12) | −0.0351 (4) | 0.0359 (8) |
| C8 | 0.71044 (12) | 0.32917 (14) | −0.1166 (5) | 0.0461 (10) |
| H8 | 0.695571 | 0.348211 | −0.138808 | 0.055* |
| C9 | 0.74513 (14) | 0.32907 (18) | −0.1637 (7) | 0.0622 (15) |
| C10 | 0.76857 (14) | 0.30188 (19) | −0.1371 (7) | 0.0682 (17) |
| H10 | 0.791865 | 0.302443 | −0.171265 | 0.082* |
| C11 | 0.75579 (13) | 0.27377 (17) | −0.0572 (7) | 0.0568 (13) |
| C6 | 0.72136 (11) | 0.27235 (13) | −0.0028 (5) | 0.0410 (9) |
| C18 | 0.65564 (11) | 0.32442 (12) | 0.2028 (4) | 0.0367 (8) |
| C12 | 0.61342 (13) | 0.34102 (14) | −0.1837 (5) | 0.0470 (10) |
| H12 | 0.614090 | 0.321299 | −0.244806 | 0.056* |
| C19 | 0.67119 (14) | 0.31548 (14) | 0.3362 (5) | 0.0480 (10) |
| H19 | 0.680073 | 0.292693 | 0.352109 | 0.058* |
| C13 | 0.59899 (18) | 0.37248 (18) | −0.2362 (7) | 0.0672 (16) |
| H13 | 0.589790 | 0.373741 | −0.329876 | 0.081* |
| C20 | 0.67312 (19) | 0.34091 (18) | 0.4433 (6) | 0.0649 (16) |
| C14 | 0.5986 (2) | 0.40149 (17) | −0.1474 (8) | 0.0736 (18) |
| H14 | 0.589105 | 0.422896 | −0.180209 | 0.088* |
| C21 | 0.66007 (19) | 0.37524 (18) | 0.4280 (7) | 0.0677 (16) |
| H21 | 0.661624 | 0.391802 | 0.502903 | 0.081* |
| C15 | 0.61235 (18) | 0.39910 (15) | −0.0087 (7) | 0.0635 (15) |
| H15 | 0.612183 | 0.418883 | 0.052084 | 0.076* |
| C22 | 0.64488 (16) | 0.38352 (15) | 0.2981 (6) | 0.0563 (13) |
| C16 | 0.62659 (13) | 0.36662 (12) | 0.0405 (5) | 0.0431 (10) |
| C17 | 0.64213 (12) | 0.35950 (12) | 0.1826 (5) | 0.0411 (9) |
| C23 | 0.54855 (14) | 0.25609 (16) | 0.1942 (6) | 0.0542 (12) |
| H23 | 0.530536 | 0.253044 | 0.262395 | 0.065* |
| C24 | 0.54912 (13) | 0.23919 (14) | 0.0613 (6) | 0.0492 (11) |
| H24 | 0.532243 | 0.223814 | 0.022174 | 0.059* |
| C25 | 0.58086 (11) | 0.25059 (11) | −0.0001 (5) | 0.0366 (8) |
| C26 | 0.59935 (10) | 0.24331 (11) | −0.1348 (5) | 0.0360 (8) |
| C27 | 0.58567 (13) | 0.22247 (14) | −0.2467 (6) | 0.0484 (11) |
| H27 | 0.563581 | 0.211562 | −0.236529 | 0.058* |
| C28 | 0.60511 (15) | 0.21806 (16) | −0.3729 (6) | 0.0595 (14) |
| H28 | 0.596573 | 0.203823 | −0.447892 | 0.071* |
| C29 | 0.63769 (14) | 0.23538 (16) | −0.3855 (6) | 0.0562 (13) |
| H29 | 0.650870 | 0.233614 | −0.470719 | 0.067* |
| C30 | 0.65008 (12) | 0.25491 (14) | −0.2719 (5) | 0.0454 (10) |
| H30 | 0.672215 | 0.265821 | −0.280451 | 0.055* |
Source of material
The chemicals iridium(III) trichloride hydrate (99.9%, Sigma-Aldrich), 2-(2,4-difluorophenylpyridine) (97%, Sigma-Aldrich), 3-(dimethylamino)-1-(2-pyridyl)-2-propen-1-one (95%, Sigma-Aldrich) and hydrazine hydrate (50–60%, Sigma-Aldrich) were used without further purification.
The ancillary ligand pyridylpyrazole (PyPz) was obtained by mixing 3-(dimethylamino)-1-(2-pyridyl)-2-propen-1-one (30 mmol, 5.50 g) and hydrazine hydrate (5 ml) in ethanol (7 ml). The solution was heated to 60 °C and stirred for 30 min. Next, distilled water (37.5 ml) was added into the solution and left at temperatures 4–6 °C overnight. A white precipitate was formed. The solid was filtered with Büchner filter and washed with distilled water to give a white powder (2.85 g, 63%) [6].
The precursor complex [Ir(dfppy)2Cl]2 (Di-μ-chloro-bis{bis[2-(2,4-difluorophenyl)pyridinato-κ2C, N)iridium(III)) was synthesized using Nonoyama’s reaction [7], where iridium(III) trichloride hydrate (1 mmol, 0.30 g) and 2-(2,4-difluorophenylpyridine) (2.5 mmol, 0.48 g) were poured into a round bottom flask containing 2-ethoxyethanol (30 ml) and distilled water (10 ml). The solution was brought to refluxing temperature and was heated for 24 h under inert conditions. A yellow precipitate ([Ir(dfppy)2Cl]2) was formed and collected using Büchner filtration and used for the next step.
2-(1H-pyrazol-3-yl-κN)pyridine-κ2N-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III), [Ir(dfppy)2PyPz] was obtained by adding PyPz into[Ir(dfppy)2Cl]2 in 30 ml dichloromethane under inert condition. The mixture was stirred and refluxed for 7 h. Crystals of the title compound were obtained through slow evaporation in a mixture of dichloromethane and acetone solvents.
Experimental details
All hydrogen atoms were positioned geometrically and allowed to ride on their respective parent atoms with C–H distances = 0.93 Å, and with Uiso(H) = 1.2Ueq for aryl H atoms. The diffuse electron density of part of the water molecule was removed with the solvent-mask procedure implemented in OLEX2 [5]. There were 240 electrons found in a volume of 3620 Å3 in two voids per unit cell. This is consistent with the presence of 1.5[H2O] per formula unit which accounts for 252 electrons per unit cell.
Comment
Iridium(III) complexes are well known that some of them are light-emitting and were used for various applications such as organic light-emitting diodes (OLEDs) [8], light-emitting electrochemical cells (LECs) [9] and sensors [10]. Previous work has demonstrated that choosing the right ancillary or cyclometalating ligand is very crucial in determining the desired photophysical properties [11] and these can be done by modifying the ligand structures [12]. Over the years, extensive studies have been made on iridium(III) complexes with various conjugated aromatic compounds especially phenylpyridyl derivatives for tuning the emission color of the complexes [13]. In the present work, the synthesis and crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κ2N-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N) iridium(III), [Ir(dfppy)2PyPz] is described.
The title compound shows an octahedral geometry where the iridium metal is coordinated with two difluorophenylpyridine (dfppy) groups and a PyPz ring via C, N and N, N atoms, respectively (Figure 1). The octahedral geometry of this complex is similar to the geometry of archetypal iridium(III) complexes [14]. The complex crystallizes in the tetragonal I41/a space group whereby a similar complex was reported to crystallize in the monoclinic P21/c space group [15]. The coordination of the iridium metal center with nitrogen atoms of PyPz moiety gives slightly longer Ir–N distances, ca 2.1 Å than that of dfppy moiety [Ir1–N1 = 2.046(4) Å; Ir1–N2 = 2.043(4) Å]. This observation is also associated with a smaller bite angle for the former with Ir(III) center [N3–Ir1–N5 = 76.31(14)°] compared to the latter chelating ligands [N2–Ir1–C18 = 80.54(16)°; N1–Ir1–C7 = 80.32(16)°] [16], [17], [18]. The PyPz ring is essentially planar, with a distortion of 4.24° between the two mean planes. The dfppy and PyPz moieties are twisted with a dihedral angles of 85.6–92.5°. The observed bond distances for Ir–C and Ir–N of dfppy are similar to the corresponding bond lengths of related structures [19, 21].
The packing of the title compound is dominated by weak intramolecular interactions and exhibits a column along c-axis. Two C–H⃛F interactions (C4–H4⃛F2 and C15–H15⃛F4) between fluorine atom of the phenyl ring and hydrogen atom of the pyridine ring from the same moiety, dfppy are observed (Figure). Besides, another two C–H⃛N interactions involving the dfppy moieties and pypz (C1–H1⃛N4 and C12–H12⃛N5) help to stabilize the structure. The hydrogen bonds involving the electronegative F atoms engendering two intramolecular S(6) motifs whereas the C–H⃛N interactions gave the S(6) and S(5) motifs. The presence of the electronegative F atom has influenced the X-ray structure. This hypothesis was further corroborated when an analogous crystal (2-(1H-pyrazol-1-yl-κN)pyridine-κ2N-bis(2-(2,4-difluorophenyl)πyridinato-κ2C,N) iridium(III) hexafluorophosphate), [Ir(dfppy)2(PzPy)]PF6 exhibited similar intramolecular interactions between fluorine of difluorophenyl and hydrogen atom from the adjacent pyridine rings [20]. In addition, no observable π⃛π interaction is exhibited by the complex, albeit with the presence of six heterocyclic rings. A previously study also reported no discernable π⃛π interactions for Ir(III) complex consisting of seven heterocyclic rings [18].
Funding source: Ministry of Higher Education http://dx.doi.org/10.13039/501100003093
Funding source: Universiti Kebangsaan Malaysia http://dx.doi.org/10.13039/501100004515
Award Identifier / Grant number: FRGS/1/2018/STG01/UKM/01/3
Acknowledgments
We would like to thank the Department of Chemical Sciences, Faculty of Science and Technology (UKM) for providing the experimental facilities and Center for Research and Instrumentation Management (UKM) for the X-ray analysis.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was financially supported by the Ministry of Higher Education (MOHE) (http://dx.doi.org/10.13039/501100003093) and Universiti Kebangsaan Malaysia (UKM) (http://dx.doi.org/10.13039/501100004515) for FRGS/1/2018/STG01/UKM/01/3 research grant and the studentship for AAL.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX3, SAINT and SADABS. Bruker AXS Inc., Madison, WI, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
5. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
6. Mark-Lee, W. F., Chong, Y. Y., Law, K. P., Ahmad, I. B., Kassim, M. B. Synthesis, structure and density functional theory (DFT) study of a Rhenium(I) pyridylpyrazol complex as a potential photocatalyst for CO2 reduction. Sains Malays. 2018, 47, 1491–1499; https://doi.org/10.17576/jsm-2018-4707-17.Search in Google Scholar
7. Nonoyama, M. Benzo[h]quinolin-10-yl-N iridium(III) complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768; https://doi.org/10.1246/bcsj.47.767.Search in Google Scholar
8. Ma, H., Liu, D., Li, J., Mei, Y., Li, D., Ding, Y., Wei, W. Sky-blue iridium complexes with pyrimidine ligands for highly efficient phosphorescent organic light-emitting diodes. New J. Chem. 2020, 44, 8743–8750; https://doi.org/10.1039/d0nj01262a.Search in Google Scholar
9. Baranoff, E., Bolink, H. J., Constable, E. C., Delgado, M., Häussinger, D., Housecroft, C. E., Nazeeruddin, M. K., Neuburger, M., OrtíE, Schneider, G. E., Tordera, D., Walliser, R. M., Zampese, J. A. Tuning the photophysical properties of cationic iridium(III) complexes containing cyclometallated 1-(2,4-difluorophenyl)-1H-pyrazole through functionalized 2,2′-bipyridine ligands: blue but not blue enough. Dalton Trans. 2013, 42, 1073–1087; https://doi.org/10.1039/c2dt32160b.Search in Google Scholar PubMed
10. Zhang, K. Y. Luminescent rhenium(I) and iridium(III) complexes for intracellular labeling, sensing, and photodynamic therapy applications. In Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells; Lo, K. K.-W., Ed.; Elsevier Inc., 2017; pp. 91–117.10.1016/B978-0-12-803814-7.00003-4Search in Google Scholar
11. Huang, Y.-C., Li, Z.-B., Guo, H.-Q., Mu, D., Li, H.-Y., Lu, A.-D., Li, T.-Y. Synthesis, structures, photophysical properties, and theoretical study of four cationic iridium(III) complexes with electron-withdrawing groups on the neutral ligands. Inorg. Chim. Acta. 2019, 496, 119060; https://doi.org/10.1016/j.ica.2019.119060.Search in Google Scholar
12. Martínez-Alonso, M., Cerdá, J., Momblona, C., Pertegás, A., Junquera-Hernández, J. M., Heras, A., Rodríguez, A. M., Espino, G., Bolink, H., Ortí E. Highly stable and efficient light-emitting electrochemical cells based on cationic iridium complexes bearing arylazole ancillary ligands. Inorg. Chem. 2017, 56, 10298–10310; https://doi.org/10.1021/acs.inorgchem.7b01167.Search in Google Scholar PubMed
13. Giridhar, T., Lee, J. H., Cho, W., Yoo, H., Moon, C. K., Kim, J. J., Jin, S. H. Highly efficient bluish green phosphorescent organic light-emitting diodes based on heteroleptic iridium(III) complexes with phenylpyridine main skeleton. Org. Electron. 2014, 15, 1687–1694; https://doi.org/10.1016/j.orgel.2014.04.028.Search in Google Scholar
14. Sunesh, C. D., Choe, Y. Synthesis and characterization of cationic iridium complexes for the fabrication of green and yellow light-emitting devices. Mater. Chem. Phys. 2015, 156, 206–213; https://doi.org/10.1016/j.matchemphys.2015.03.001.Search in Google Scholar
15. Su, N., Shen, C. Z., Zheng, Y. X. Efficient electroluminescence of sky-blue iridium(III) complexes for organic light-emitting diodes. Dyes Pigments 2018, 159, 100–106; https://doi.org/10.1016/j.dyepig.2018.06.011.Search in Google Scholar
16. Orselli, E., Albuquerque, R. Q., Fransen, P. M., Fröhlich, R., Janssen, H. M., De Cola, L. 1,2,3-Triazolyl-pyridine derivatives as chelating ligands for blue iridium(III) complexes. Photophysics and electroluminescent devices. J. Mater. Chem. 2008, 18, 4579–4590; https://doi.org/10.1039/b805324c.Search in Google Scholar
17. Mark-Lee, W. F., Chong, Y. Y., Kassim, M. B. Supramolecular structures of rhenium(I) complexes mediated by ligand planarity via the interplay of substituents. Acta Crystallogr. 2018, C74, 997–1006; https://doi.org/10.1107/s2053229618010586.Search in Google Scholar PubMed
18. Chong, Y. Y., Mark-Lee, W. F., Ahmad, I., Kassim, M. B. Crystal structure of 1-[3-(trifluoromethyl)cinnamoyl]-3-(pyridin-2-yl-κN) pyrazole-κ2N-bis(2-phenylpyridinato-k2C,N) iridium(III) hexafluorophosphate complex, [C40H28F3IrN5O]PF6. Z. Kristallogr. NCS 2020, 235, 825–829; https://doi.org/10.1515/ncrs-2020-0029.Search in Google Scholar
19. Yang, C. H., Mauro, M., Polo, F., Watanabe, S., Muenster, I., Fröhlich, R., De Cola, L. Deep-blue-emitting heteroleptic iridium(III) complexes suited for highly efficient phosphorescent OLEDs. Chem. Mater. 2012, 24, 3684–3695; https://doi.org/10.1021/cm3010453.Search in Google Scholar
20. He, L., Duan, L., Qiao, J., Wang, R., Wei, P., Wang, L., Qiu, Y. Blue-emitting cationic iridium complexes with 2-(1H-pyrazol-1-yl)pyridine as the ancillary ligand for efficient light-emitting electrochemical cells. Adv. Funct. Mater. 2008, 18, 2123–2131; https://doi.org/10.1002/adfm.200701505.Search in Google Scholar
21. Sykes, D., Parker, S. C., Sazanovich, I. V., Stephenson, A., Weinstein, J. A., Ward, M. D. d→f energy transfer in Ir(III)/Eu(III) dyads: use of a naphthyl spacer as a spatial and energetic “stepping stone”. Inorg. Chem. 2013, 52, 10500–10511; https://doi.org/10.1021/ic401410g.Search in Google Scholar PubMed PubMed Central
© 2022 Aqilah Abdul Latiff et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

