Abstract
C11H12O4, monoclinic, P21 (no. 4), a = 15.4586(2) Å, b = 7.80238(13) Å, c = 16.4003(2) Å, β = 91.0103(13)°, V = 1977.81(5) Å3, Z = 8, R gt (F) = 0.0413, wR ref (F2) = 0.1045, T = 180 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.16 × 0.13 × 0.12 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.90 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 73.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10,690, 6502, 0.023 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 6386 |
| N(param)refined: | 553 |
| Programs: | CrysAlisPRO [1], SHELX [2], Olex2 [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.11870 (10) | 0.7354 (3) | 0.29776 (10) | 0.0357 (4) |
| O2 | −0.08576 (10) | 0.4933 (3) | 0.48163 (11) | 0.0382 (4) |
| H2 | −0.120063 | 0.533356 | 0.448006 | 0.057* |
| O3 | 0.31167 (10) | 0.6880 (3) | 0.47316 (10) | 0.0334 (4) |
| O4 | 0.28372 (11) | 0.6905 (3) | 0.34198 (11) | 0.0458 (5) |
| C1 | 0.09698 (14) | 0.6621 (3) | 0.36961 (13) | 0.0265 (5) |
| C2 | 0.01304 (14) | 0.6156 (3) | 0.38727 (14) | 0.0272 (5) |
| H2A | −0.031420 | 0.635080 | 0.349462 | 0.033* |
| C3 | −0.00462 (14) | 0.5393 (3) | 0.46222 (14) | 0.0277 (5) |
| C4 | 0.06082 (14) | 0.5094 (3) | 0.51939 (14) | 0.0292 (5) |
| H4 | 0.048662 | 0.455217 | 0.568370 | 0.035* |
| C5 | 0.14425 (14) | 0.5609 (3) | 0.50296 (13) | 0.0261 (5) |
| C6 | 0.16449 (13) | 0.6356 (3) | 0.42781 (13) | 0.0256 (4) |
| C7 | 0.25485 (14) | 0.6750 (3) | 0.41041 (13) | 0.0300 (5) |
| C8 | 0.27887 (13) | 0.6868 (4) | 0.55708 (13) | 0.0292 (5) |
| H8 | 0.248437 | 0.794441 | 0.567728 | 0.035* |
| C9 | 0.21706 (14) | 0.5393 (3) | 0.56432 (14) | 0.0293 (5) |
| H9A | 0.194078 | 0.535786 | 0.618952 | 0.035* |
| H9B | 0.247085 | 0.432374 | 0.554473 | 0.035* |
| C10 | 0.35783 (15) | 0.6741 (4) | 0.61237 (15) | 0.0362 (6) |
| H10A | 0.385073 | 0.564769 | 0.604910 | 0.054* |
| H10B | 0.340812 | 0.685677 | 0.668114 | 0.054* |
| H10C | 0.397764 | 0.763727 | 0.599223 | 0.054* |
| C11 | 0.05132 (17) | 0.7581 (4) | 0.23809 (15) | 0.0415 (6) |
| H11A | 0.074389 | 0.812465 | 0.190660 | 0.062* |
| H11B | 0.006621 | 0.828727 | 0.260232 | 0.062* |
| H11C | 0.027648 | 0.648516 | 0.223245 | 0.062* |
| O5 | 0.40796 (10) | 0.8674 (3) | 0.27311 (11) | 0.0381 (4) |
| H5 | 0.374934 | 0.810969 | 0.301020 | 0.057* |
| O6 | 0.62498 (11) | 0.5890 (3) | 0.43905 (10) | 0.0351 (4) |
| O7 | 0.78551 (10) | 0.6210 (3) | 0.38987 (10) | 0.0351 (4) |
| O8 | 0.80964 (10) | 0.7069 (3) | 0.26558 (10) | 0.0313 (4) |
| C44 | 0.75444 (13) | 0.6762 (3) | 0.32636 (13) | 0.0268 (5) |
| C12 | 0.55230 (14) | 0.8666 (3) | 0.23338 (14) | 0.0288 (5) |
| H12 | 0.536747 | 0.931754 | 0.187971 | 0.035* |
| C13 | 0.48998 (14) | 0.8180 (3) | 0.28878 (14) | 0.0282 (5) |
| C14 | 0.51317 (14) | 0.7245 (3) | 0.35811 (13) | 0.0281 (5) |
| H14 | 0.471355 | 0.693561 | 0.395447 | 0.034* |
| C15 | 0.59892 (14) | 0.6775 (3) | 0.37133 (13) | 0.0262 (4) |
| C16 | 0.66248 (13) | 0.7198 (3) | 0.31381 (13) | 0.0250 (5) |
| C17 | 0.63707 (13) | 0.8181 (3) | 0.24576 (13) | 0.0256 (4) |
| C18 | 0.70565 (14) | 0.8732 (3) | 0.18732 (14) | 0.0294 (5) |
| H18A | 0.679855 | 0.890185 | 0.133576 | 0.035* |
| H18B | 0.730440 | 0.981355 | 0.205244 | 0.035* |
| C19 | 0.77542 (14) | 0.7406 (3) | 0.18286 (13) | 0.0287 (5) |
| H19 | 0.751372 | 0.634627 | 0.159763 | 0.034* |
| C20 | 0.85162 (15) | 0.7972 (4) | 0.13335 (15) | 0.0376 (6) |
| H20A | 0.871677 | 0.906554 | 0.152661 | 0.056* |
| H20B | 0.834327 | 0.806418 | 0.077016 | 0.056* |
| H20C | 0.897339 | 0.714481 | 0.138869 | 0.056* |
| C21 | 0.56364 (17) | 0.5605 (4) | 0.50141 (15) | 0.0397 (6) |
| H21A | 0.516201 | 0.494228 | 0.479830 | 0.060* |
| H21B | 0.542557 | 0.668659 | 0.520686 | 0.060* |
| H21C | 0.590832 | 0.499474 | 0.545753 | 0.060* |
| O9 | 1.02791 (10) | 0.3470 (3) | 0.05186 (10) | 0.0385 (4) |
| O10 | 0.80644 (11) | 0.2873 (3) | 0.25194 (10) | 0.0379 (4) |
| H10 | 0.775040 | 0.304914 | 0.212003 | 0.057* |
| O11 | 1.21349 (10) | 0.2112 (3) | 0.22487 (10) | 0.0390 (4) |
| O12 | 1.17659 (11) | 0.1847 (3) | 0.09616 (11) | 0.0473 (5) |
| C31 | 1.15305 (15) | 0.2186 (4) | 0.16495 (14) | 0.0342 (5) |
| C22 | 0.88945 (15) | 0.2825 (3) | 0.22878 (14) | 0.0306 (5) |
| C23 | 0.91322 (14) | 0.3167 (4) | 0.14829 (14) | 0.0308 (5) |
| H23 | 0.871473 | 0.345647 | 0.109156 | 0.037* |
| C24 | 0.99985 (15) | 0.3067 (4) | 0.12767 (13) | 0.0300 (5) |
| C25 | 1.06338 (14) | 0.2574 (3) | 0.18601 (14) | 0.0290 (5) |
| C26 | 1.03678 (15) | 0.2300 (3) | 0.26683 (14) | 0.0303 (5) |
| C27 | 0.95162 (15) | 0.2438 (4) | 0.28778 (14) | 0.0332 (5) |
| H27 | 0.935583 | 0.227112 | 0.341588 | 0.040* |
| C28 | 1.10551 (15) | 0.1868 (4) | 0.32939 (14) | 0.0362 (6) |
| H28A | 1.112133 | 0.063335 | 0.332757 | 0.043* |
| H28B | 1.088174 | 0.228217 | 0.382413 | 0.043* |
| C29 | 1.19049 (15) | 0.2668 (4) | 0.30723 (14) | 0.0362 (6) |
| H29 | 1.184786 | 0.391920 | 0.308074 | 0.043* |
| C30 | 1.26421 (18) | 0.2139 (6) | 0.36337 (17) | 0.0525 (8) |
| H30A | 1.274193 | 0.093058 | 0.357983 | 0.079* |
| H30B | 1.249735 | 0.239711 | 0.418716 | 0.079* |
| H30C | 1.315567 | 0.275398 | 0.349102 | 0.079* |
| C41 | 0.96584 (17) | 0.4097 (5) | −0.00603 (15) | 0.0453 (7) |
| H41A | 0.994415 | 0.440147 | −0.055468 | 0.068* |
| H41B | 0.937651 | 0.508835 | 0.015750 | 0.068* |
| H41C | 0.923620 | 0.322261 | −0.017400 | 0.068* |
| O13 | 0.71649 (11) | 0.2265 (3) | 0.02857 (12) | 0.0397 (4) |
| O14 | 0.68208 (11) | 0.3443 (3) | 0.14428 (11) | 0.0406 (5) |
| O15 | 0.52403 (11) | 0.3026 (3) | 0.19775 (10) | 0.0398 (4) |
| O16 | 0.30999 (10) | 0.1394 (3) | −0.00258 (10) | 0.0387 (4) |
| H16 | 0.277822 | 0.156006 | 0.035851 | 0.058* |
| C33 | 0.56455 (15) | 0.2413 (3) | 0.06262 (14) | 0.0295 (5) |
| C34 | 0.65577 (15) | 0.2743 (3) | 0.08202 (15) | 0.0327 (5) |
| C32 | 0.49920 (15) | 0.2543 (3) | 0.12123 (14) | 0.0305 (5) |
| C42 | 0.41374 (15) | 0.2181 (3) | 0.10082 (14) | 0.0313 (5) |
| H42 | 0.371252 | 0.223186 | 0.140192 | 0.038* |
| C43 | 0.39216 (14) | 0.1738 (3) | 0.02059 (14) | 0.0297 (5) |
| C35 | 0.45472 (15) | 0.1655 (3) | −0.03923 (14) | 0.0309 (5) |
| H35 | 0.439303 | 0.137992 | −0.092711 | 0.037* |
| C36 | 0.54001 (14) | 0.1986 (3) | −0.01821 (14) | 0.0289 (5) |
| C37 | 0.60949 (15) | 0.1943 (4) | −0.08069 (15) | 0.0346 (5) |
| H37A | 0.618999 | 0.308958 | −0.101531 | 0.042* |
| H37B | 0.591335 | 0.121907 | −0.125943 | 0.042* |
| C38 | 0.69245 (16) | 0.1259 (4) | −0.04365 (17) | 0.0395 (6) |
| H38 | 0.683867 | 0.006247 | −0.027594 | 0.047* |
| C39 | 0.76802 (18) | 0.1364 (6) | −0.1005 (2) | 0.0573 (9) |
| H39A | 0.776540 | 0.253348 | −0.116717 | 0.086* |
| H39B | 0.756306 | 0.067496 | −0.147965 | 0.086* |
| H39C | 0.819267 | 0.094975 | −0.073127 | 0.086* |
| C40 | 0.45859 (17) | 0.3108 (4) | 0.25805 (15) | 0.0418 (6) |
| H40A | 0.414461 | 0.390105 | 0.240841 | 0.063* |
| H40B | 0.483794 | 0.348421 | 0.308884 | 0.063* |
| H40C | 0.433549 | 0.199221 | 0.264962 | 0.063* |
Source of material
All solvents were of analytical grade. The title compound was isolated and purified from the secondary metabolites of Talaromyces sp. Colorless crystals were obtained by slow-evaporative solution of the methanol at room temperature.
Experimental details
All hydrogen atoms were placed in calculated positions and refined using a riding model with the relative isotropic parameters. Uiso values of hydrogen atoms were set to 1.2Ueq of the parent atoms.
Comment
The endophytic fungus Talaromyces sp. was isolated from mangrove tree Sonneratia apetala in Dongzhai harbor, Hainan Province, and identified by 18S DNA gene sequence [4]. The specimen was stored at Guangzhou Medical University, Guangzhou, P. R. China. This fungus strain was fermented statically by using rice medium (85 g rice, 160 mL coarse sea salt solution with a mass concentration of 2 g/L) in a 1 L Erlenmeyerflask at 28 °C for fourty days. The culture was repetitively extracted with MeOH solvent and then the extract was further fractionated by silica gel chromatography using a stepwise isocratic solvent system according to the increasing polarity starting from 100% petroleum to 100% methanol using normal-phase vacuum liquid chromatography (VLC) to obtain nine fractions (Fr. A–I). The title compound was isolated and purified from Fraction F (20% petroleum/80% ethyl acetate) through silica gel column chromatography [5]. Its structure was elucidated by comprehensive analysis of spectroscopic data and confirmed by X-ray crystallography.
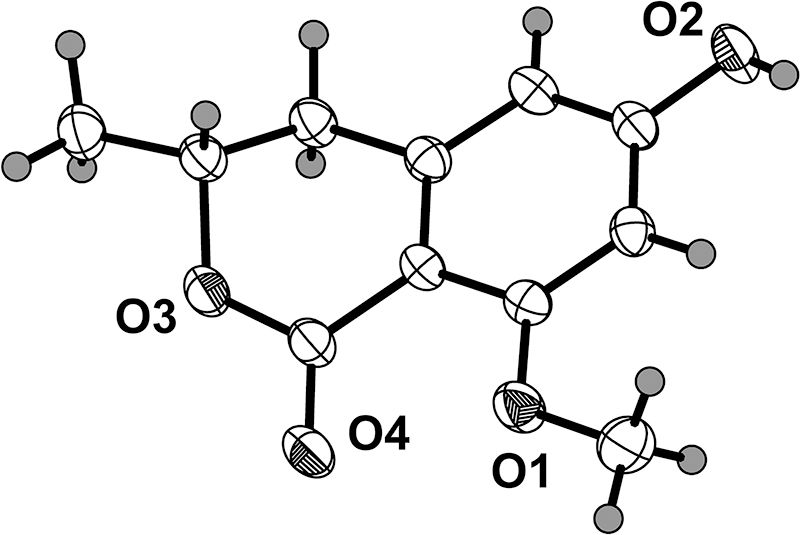
The asymmetric unit of the title compound contains four crystallographically independent molecules (only one of the molecules is shown in the figure). And the crystal structure of two similar compounds, (R)-8-hydroxy-6-methoxy-3-methylisochroman-1-one) [6] and (R)-6,8-dihydroxy-3-methylisochroman-1-one [7], have been previously reported. The absolute configuration of the chiral center at C-2 both was determined to be R.
The title compound has been also reported from Penicillium sp. and Talaromyces thailandiasis., containing its 1H NMR, 13C NMR data [6], [7], [8], [9]. It exhibited a moderate cytotoxic activity against the mouse lymphoma cells (L5178Y) with IC50 value of 13.70 µM and weak anti–HIV activities with the IC50 values of 69.3 µM [6, 9]. What’s more, the X-ray crystallography data of the title compound was reported here for the first time. The geometric parameters are similar to those reported for the (−)-(3R)-6-methoxy-mellein [10].
Funding source: Guangzhou Education Bureau Yangcheng Scholars Project
Award Identifier / Grant number: 202032774
Funding source: National Students’ Training Programs for innovation and Entrepreneurship
Award Identifier / Grant number: 202110570035, 202010570028
Funding source: Special Funds for Undergraduates’ Scientific and Technological Innovation Training Programs in Guangdong
Award Identifier / Grant number: pdjh2021b0417, pdjh2020b0487
Funding source: Undergraduate Training Programs for Innovation and Entrepreneurship in GZHMU
Award Identifier / Grant number: 2021A074, 2020A068, 2019A076
Funding source: High-level University Construction Fund of Guangdong Province
Award Identifier / Grant number: 06-410-2107242, 06-410-2107249, 06-410-2107273
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Guangzhou Education Bureau Yangcheng Scholars Project (202032774), National Students’ Training Programs for innovation and Entrepreneurship (202110570035 and 202010570028), Special Funds for Undergraduates’ Scientific and Technological Innovation Training Programs in Guangdong (pdjh2021b0417 and pdjh2020b0487), Undergraduate Training Programs for Innovation and Entrepreneurship in GZHMU (2021A074, 2020A068, and 2019A076) and High-level University Construction Fund of Guangdong Province (06-410-2107242, 06-410-2107249, and 06-410-2107273).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Agilent Technologies. CrysAlisPRO Software System, Version 1.171.37.35; Agilent Technologies UK Ltd: Oxford, UK, 2011.Search in Google Scholar
2. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Houbraken, J., López–Quintero, C. A., Frisvad, J. C., Boekhout, T., Theelen, B., Franco–Molano, A. E., Samson, R. A. Penicillium araracuarense sp. nov., Penicillium elleniae sp. nov., Penicillium penarojense sp. nov., Penicillium vanderhammenii sp. nov. and Penicillium wotroi sp. nov., isolated from leaf litter. Int. J. Syst. Evol. Microbiol. 2011, 61, 1462–1475. https://doi.org/10.1099/ijs.0.025098-0.Search in Google Scholar PubMed
5. Zou, T. G., Xue, K. C., Zhu, J. L., Lin, L. Y., Huang, H. B., Tao, Y. W. Study on the endophytic fungus Penicillium sp. KF22 polyketide secondary metabolites of autumn eggplant in Dongzhaigang, Hainan. Chin. Pharmaceut. J. 2021, 56, 1557–1562.Search in Google Scholar
6. Fahmi, E. M., Ghabbour, H. A., Nathalie, L., Fabien, F. V., Mohamed, M. New bioactive chlorinated cyclopentene derivatives from the marine-derived fungus Phoma sp. Med. Chem. Res. 2018, 27, 1885–1892.10.1007/s00044-018-2201-1Search in Google Scholar
7. Thakur, J. P., Haider, R., Singh, D. K., Kumar, B. S., Vasudev, P. G., Luqman, S., Kalra, A., Saikia, D., Negi, A. S. Bioactive isochromenone isolated from Aspergillus fumigatus, endophytic fungus from Bacopa monnieri. Microbiol. Res. 2015, 6, 5800. https://doi.org/10.4081/mr.2015.5800.Search in Google Scholar
8. Orfali, R. S., Aly, A. H., Ebrahim, W., Proksch, P. Isochroman and isocoumarin derivatives from hypersaline lake sediment-derived fungus Penicillium sp. Phytochem. Lett. 2015, 13, 234–238. https://doi.org/10.1016/j.phytol.2015.06.003.Search in Google Scholar
9. Dethoup, T., Manoch, L., Kijjoa, A., Pinto, M., Gales, L., Damas, A. M., Silva, A. M. S., Eaton, G., Herz, W. Merodrimanes and other constituents from Talaromyces thailandiasis. J. Nat. Prod. 2007, 70, 1200–1202. https://doi.org/10.1021/np0680578.Search in Google Scholar PubMed
10. Wen, Q. I., Wang, L. L., Wen, H., Wang, W. N., Zhang, L., Yuan, D. Isolation and identification of chemical constituents in roots and rhizomes of Asarum heterotropoides Fr. Schmidt var. mandshuricum (Maxim.) Kitag. J. Shenyang Pharm. Univ. 2014, 31, 681–686. 691.Search in Google Scholar
© 2022 Hong Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

