Abstract
C19H25NO8, monoclinic, P21/n (no. 14), a = 10.149(2) Å, b = 12.338(3) Å, c = 15.898(3) Å, β = 97.58(3)°, V = 1973.2(7) Å3, Z = 4, R gt (F) = 0.0581, wR ref (F2) = 0.1842, T = 293(2) K.
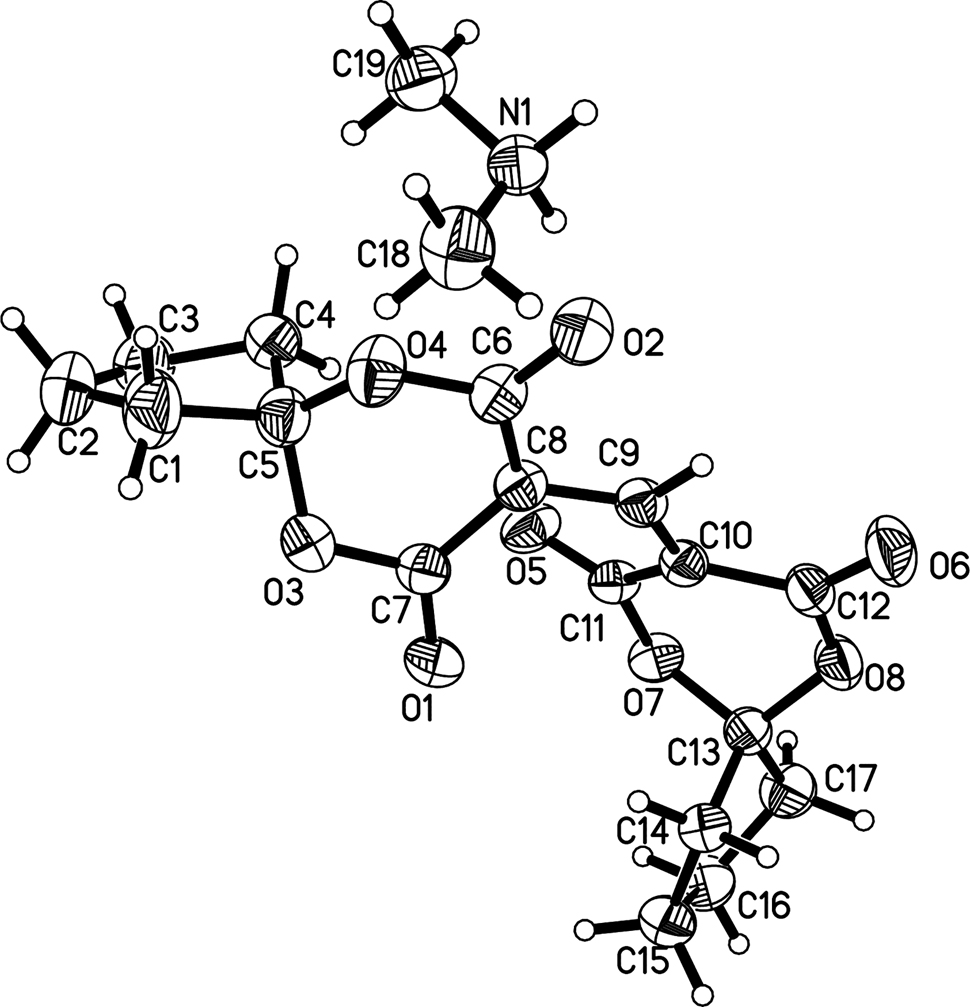
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.20 × 0.16 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Rigaku Spider Rapid IP, ω |
| θmax, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18636, 4510, 0.028 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3509 |
| N(param)refined: | 253 |
| Programs: | RAPID [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.0582 (2) | 0.7744 (3) | 0.16269 (19) | 0.1016 (8) |
| H1A | 0.016646 | 0.707336 | 0.141354 | 0.122* |
| H1B | 0.010620 | 0.802198 | 0.207187 | 0.122* |
| C2 | 0.0573 (3) | 0.8549 (2) | 0.0933 (2) | 0.1230 (11) |
| H2A | 0.028479 | 0.820712 | 0.039043 | 0.148* |
| H2B | −0.003830 | 0.913301 | 0.101207 | 0.148* |
| C3 | 0.1929 (3) | 0.8981 (2) | 0.09462 (15) | 0.1006 (8) |
| H3A | 0.191210 | 0.976620 | 0.091608 | 0.121* |
| H3B | 0.233136 | 0.870410 | 0.046872 | 0.121* |
| C4 | 0.2712 (2) | 0.86054 (15) | 0.17823 (12) | 0.0706 (5) |
| H4A | 0.364026 | 0.848653 | 0.172219 | 0.085* |
| H4B | 0.265458 | 0.913094 | 0.222977 | 0.085* |
| C5 | 0.20377 (18) | 0.75584 (16) | 0.19627 (12) | 0.0673 (4) |
| C6 | 0.33382 (17) | 0.68909 (14) | 0.32116 (10) | 0.0603 (4) |
| C7 | 0.37644 (18) | 0.62979 (13) | 0.17834 (10) | 0.0607 (4) |
| C8 | 0.42601 (16) | 0.65072 (12) | 0.26652 (9) | 0.0537 (4) |
| C9 | 0.54908 (16) | 0.61385 (12) | 0.30560 (9) | 0.0532 (4) |
| H9 | 0.555255 | 0.605403 | 0.364119 | 0.064* |
| C10 | 0.66246 (16) | 0.58808 (12) | 0.27147 (9) | 0.0521 (3) |
| C11 | 0.69319 (16) | 0.63315 (11) | 0.19257 (9) | 0.0523 (3) |
| C12 | 0.76714 (18) | 0.52760 (15) | 0.32288 (10) | 0.0634 (4) |
| C13 | 0.83700 (16) | 0.48106 (13) | 0.19135 (10) | 0.0567 (4) |
| C14 | 0.73605 (18) | 0.39344 (13) | 0.16208 (10) | 0.0608 (4) |
| H14A | 0.646568 | 0.422735 | 0.154736 | 0.073* |
| H14B | 0.740876 | 0.334662 | 0.202886 | 0.073* |
| C15 | 0.7741 (2) | 0.35405 (17) | 0.07757 (12) | 0.0766 (5) |
| H15A | 0.782773 | 0.275755 | 0.077907 | 0.092* |
| H15B | 0.706294 | 0.374459 | 0.031480 | 0.092* |
| C16 | 0.9053 (2) | 0.4065 (2) | 0.06607 (14) | 0.0832 (6) |
| H16A | 0.892135 | 0.464701 | 0.024814 | 0.100* |
| H16B | 0.965695 | 0.353697 | 0.046959 | 0.100* |
| C17 | 0.96002 (19) | 0.45015 (19) | 0.15271 (14) | 0.0777 (5) |
| H17A | 1.010821 | 0.395182 | 0.186477 | 0.093* |
| H17B | 1.016304 | 0.512755 | 0.147783 | 0.093* |
| C18 | 0.0416 (2) | 0.6622 (2) | 0.46716 (17) | 0.0914 (6) |
| H18A | 0.068424 | 0.592019 | 0.489224 | 0.137* |
| H18B | −0.036059 | 0.685346 | 0.490754 | 0.137* |
| H18C | 0.021790 | 0.658278 | 0.406501 | 0.137* |
| C19 | 0.1189 (2) | 0.85049 (17) | 0.45933 (14) | 0.0816 (6) |
| H19A | 0.193778 | 0.896636 | 0.476634 | 0.122* |
| H19B | 0.100555 | 0.850048 | 0.398523 | 0.122* |
| H19C | 0.042706 | 0.877115 | 0.482775 | 0.122* |
| N1 | 0.14923 (14) | 0.73976 (12) | 0.49000 (8) | 0.0606 (4) |
| H1C | 0.221315 | 0.716586 | 0.469052 | 0.073* |
| H1D | 0.168302 | 0.741391 | 0.546262 | 0.073* |
| O1 | 0.42965 (15) | 0.57573 (12) | 0.12902 (8) | 0.0790 (4) |
| O2 | 0.35362 (14) | 0.68833 (12) | 0.39906 (7) | 0.0756 (4) |
| O3 | 0.25108 (13) | 0.66764 (11) | 0.15063 (8) | 0.0734 (4) |
| O4 | 0.21593 (13) | 0.72885 (12) | 0.28451 (9) | 0.0760 (4) |
| O5 | 0.64653 (14) | 0.71522 (9) | 0.15935 (8) | 0.0704 (4) |
| O6 | 0.77449 (17) | 0.51038 (15) | 0.39792 (8) | 0.0931 (5) |
| O7 | 0.79328 (11) | 0.58501 (9) | 0.15707 (7) | 0.0595 (3) |
| O8 | 0.86736 (12) | 0.49047 (11) | 0.28114 (7) | 0.0714 (4) |
Source of material
IR spectra were performed on FT IR-650 instrument. The starting material 6,10-dioxaspiro[4.5]decane-7,9-dione (1.84, 0.01 mol), dissolved in absolute ethanol (25 mL) was added to a round-bottom flask and magnetically stirred at 25 °C. Then, 1,1-dimethoxy-N,N-dimethylmethanamine (1.19 g, 0.01 mol) was added dropwise to the mixture for half an hour. The reaction mixture was kept stirring for 2.5 h, then 1,1-dimethoxy-N,N-dimethylmethan amine (0.119 g, 0.001 mol) was added again. The above mixture was set aside for another 2 h. Then the solution is cooled and evaporated at room temperature. The precipitate was collected by filtration, washed three times and dried. Yield 27.6%. M.p.: 137.8–138.1 °C. The colorless block-shaped crystals of C19H25NO8 were obtained by evaporation of a solution (Vpetroleumether:Vacetone = 2:1). Peaks at 1717, 1672, 1253, 1147 cm−1 are attributed to the stretching vibrations of C=O and C–O bands of 1,3-dioxane ring.
Experimental details
The structure was solved by SHELXT-2015 [2] and refined with SHELXL-2015 [3]. The value of Uiso(H) is 1.2 times Ueq of all C(H) groups, all C(H,H) groups, all N(H,H) groups and 1.5 times of all C(H,H,H) groups. The secondary C(H, H) and methylic H are both refined with riding coordinates.
Comment
New spiro compounds and their derivatives have been extensively studied in the biological field such as cytotoxicity [5], antimycobacterial [6], anti-inflammatory [7], cytotoxic activity [8, 9], antifungal activity [10], antitumor activity [11], antioxidants and anti-cancer agents [12]. Furthermore, application of spiro compounds in other aspects has also drawn great attention such as green corrosion inhibitors for mild steel [13], organic semiconductor [14] and emitting materials [15]. Based on above reasons, a lot of spiro compounds were prepared by our group for 10 years [16], [17], [18], [19]. As part of our ongoing research, a new salt was received.
The title compound is a salt containing one C17H17O8 anion and one C2H8N cation. The two 6,10-dioxaspiro groups are linked to the central C(9) atom, which forms a conjugated system (C(8)=C(9)–C(10)). From the bond length data, the bond lengths of C8–C9 (1.396(2) Å), C9–C10 (1.373(2) Å) are same as that of our earlier report C(10)–C(11) (1.396(2) Å), C(8)–C(10) (1.373(2) Å) [4]. The bond angle of C(8)–C(9)–C(10) 130.33(14)° is consistent with that of C(8)–C(10)–C(12) 131.80(16)° [4]. The two cyclopentane rings of the C17H17O8 anion both show half chair conformations. However, two 1,3-dioxane-4,6-dione rings both show envelope conformations.
There exists one kind of N(1)–H(1C)⋯O(2) intramolecular hydrogen bonds and one kind of N(1)–H(1D)⋯O(5) intermolecular hydrogen bonds in the title compound. The distance of N(1)⋯O(2) (2.754(19) Å) is in agreement to what was previously reported (2.750(2)°) [4]. The distance of N(1)⋯O(5) is 2.753(19) Å), symmetry code: −1/2 + x, 3/2 − y, 1/2 + z.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22108208
Funding source: Foundation of State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering
Award Identifier / Grant number: 2021-K02
Funding source: National Innovation and Entrepreneurship Training Program for College Students
Award Identifier / Grant number: 202111067020
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This project was supported by the National Natural Science Foundation of China (Grant No. 22108208), the Foundation of State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering (Grant No. 2021–K02) and the National Innovation and Entrepreneurship Training Program for College Students (Grant No. 202111067020).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku/MSC. RAPID–AUTO; Rigaku/MSC Inc.: The Woodlands, Texas, USA, 2004.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Zeng, W., Jiang, J., Jiang, G., Li, Y. Synthesis, characterization, and fluorescence properties of two new heterocyclic compounds containing 1,5-dioxaspiro group. Crystals 2018, 8, 269; https://doi.org/10.3390/cryst8070269.Search in Google Scholar
5. Akaev, A.-A., Villemson, E.-V., Vorobyeva, N.-S., Majouga, A.-G., Budynina, E.-M., Melnikov, M.-Y. 3-(2–Azidoethyl)oxindoles: advanced building blocks for one-pot assembly of spiro [pyrrolidine-3, 3′-oxindoles]. J. Org. Chem. 2017, 82, 5689–5701; https://doi.org/10.1021/acs.joc.7b00529.Search in Google Scholar PubMed
6. Bharkavi, C., Kumar, S.-V., Ali, M.-A., Osman, H., Muthusubramanian, S., Perumal, S. One-pot microwave assisted stereoselective synthesis of novel dihydro-2′H- spiro[indene-2,1′-pyrrolo-[3,4-c]pyrrole]- tetraones and evaluation of their antimycobacterial activity and inhibition of AChE. Bioorg. Med. Chem. Lett. 2017, 27, 3071–3075; https://doi.org/10.1016/j.bmcl.2017.05.050.Search in Google Scholar PubMed
7. Kumar, R.-S., Antonisamy, P., Almansour, A.-I., Arumugam, N., Periyasami, G., Altaf, M., Kim, H.-R., Kwon, K.-B. Functionalized spirooxindole-indoli zinehybrids: stereoselective green synthesis and evaluation of anti-inflammatoryeffect involving TNF-a and nitrite inhibition. Eur. J. Med. Chem. 2018, 152, 417–423; https://doi.org/10.1016/j.ejmech.2018.04.060.Search in Google Scholar PubMed
8. Nagaraju, B., Kovvuri, J., Babu, K.-S., Adiyala, P.-R., Nayak, V.-L., Alarifi, A., Kamal, A. A facile one pot C–C and C–N bond formation for the synthesis of spiro- benzodiazepines and their cytotoxicity. Tetrahedron 2017, 73, 6969–6976; https://doi.org/10.1016/j.tet.2017.10.060.Search in Google Scholar
9. Konyar, D., Andac, C.-A., Buyukbingol, E. Design synthesis and cytotoxic activity of spiro (oxindole-3-3′- pyrrolidine) derivatives. Lett. Drug Des. Discov. 2018, 15, 37–45; https://doi.org/10.2174/1570180814666170810120634.Search in Google Scholar
10. Meena, K., Kumari, S., Khurana, J.-M., Malik, A., Sharma, C., Panwar, H. One pot three component synthesis of spiro [indolo-3,10′-indeno[1,2-b] quinolin]- 2,4,11′-triones as a new class of antifungal and antimicrobial agents. Chin. Chem. Lett. 2017, 28, 136–142; https://doi.org/10.1016/j.cclet.2016.06.025.Search in Google Scholar
11. Wang, S.-Z., Chen, S.-Q., Guo, Z.-J., He, S.-P., Zhang, F., Liu, X.-Y., Chen, W.-P., Zhang, S.-Y., Sheng, C.-Q. Synthesis of spiro- tetrahydrothiopyran-oxindoles by michael- aldol cascade reactions: discovery of potential P53–MDM2 inhibitors with good antitumor activity. Chin. Chem. Lett. 2018, 16, 625–634; https://doi.org/10.1039/c7ob02726e.Search in Google Scholar PubMed
12. Mani, K.-S., Kaminsky, W., Rajendran, S.-P. A facile atom economic one pot multicomponent synthesis of bioactive spiro-indenoquinoxaline pyrrolizines as potent antioxidants and anti-cancer agents. Chin. Chem. Lett. 2018, 42, 301–310; https://doi.org/10.1039/c7nj02993d.Search in Google Scholar
13. Gupta, N.-K., Haque, J., Salghi, R., Lgaz, H., Mukherjee, A.-K., Quraishi, M.-A. Spiro [indoline-3,4′-pyrano [2,3-c]pyrazole] derivatives as novel class of green corrosion inhibitors for mild steel in hydrochloric acid medium: theoretical and experimental approach. J. Bio- Tribo-Corros. 2018, 4, 16; https://doi.org/10.1007/s40735-018-0132-5.Search in Google Scholar
14. Sun, M.-L., Zhang, F., Qian, Y., Ou, C.-J., Liu, B., Xie, L.-H., Wei, Y., Ren, B.-Y., Huang, W. Catalyst-free photocyclization for the synthesis of spirofusedaromatic organic semiconductor based on SFX. Tetrahedron 2018, 74, 2063–2067; https://doi.org/10.1016/j.tet.2018.03.013.Search in Google Scholar
15. Sun, W., Zhou, N.-L., Xiao, Y., Wang, S.-R., Li, X.-G. Novel carbazolyl- substituted spiro [acridine-9,9′-fluorene] derivatives as deep-blue emitting materials for OLED applications. Dyes Pigments 2018, 154, 30–37; https://doi.org/10.1016/j.dyepig.2018.02.041.Search in Google Scholar
16. Zeng, W., Li, Y., Guo, H. Syntheses and crystal structures of 1,5-dioxaspiro[5.5]undecane-2,4-dione derivatives. J. Chem. Crystallogr. 2013, 43, 223–227; https://doi.org/10.1007/s10870-013-0408-z.Search in Google Scholar
17. Zeng, W., Cai, X., Guo, H. Synthesis, and experimental and theoretical characterization of 3-(4-(dimethylamino) benzylidene)-1,5-dioxaspiro[5.5] undecane-2,4-dione. Chin. J. Struct. Chem. 2013, 32, 1603–1610.Search in Google Scholar
18. Zeng, W., Wang, X., Jiang, J. Design and crystal structures of two new compounds fused with 3, 4, 5-trimethoxybenzyl group and 6, 10-dioxaspiro group. Crystals 2018, 8, 146; https://doi.org/10.3390/cryst8040146.Search in Google Scholar
19. Zeng, W., Wang, X., Zhang, Y. Crystal structure, thermodynamic properties and DFT studies of 5,6-dimethyl-1H-benzo[d]imidazol-3-ium 3-((2,4-dioxo-1,5-dioxaspiro[5.5]undecan-3-ylidene)methyl)-2,4-dioxo-1,5-dioxaspiro[5.5]undecane hydrate. Crystals 2021, 11, 1393; https://doi.org/10.3390/cryst11111393.Search in Google Scholar
© 2022 Xia Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

