Abstract
C16H30O5, monoclinic, P21 (no. 4), a = 5.6814(5) Å, b = 13.0181(12) Å, c = 22.833(2) Å, β = 96.473(2)°, V = 1677.9(3) Å3, Z = 4, R gt (F) = 0.0508, wRref (F 2) = 0.1346, T = 296(2) K.
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
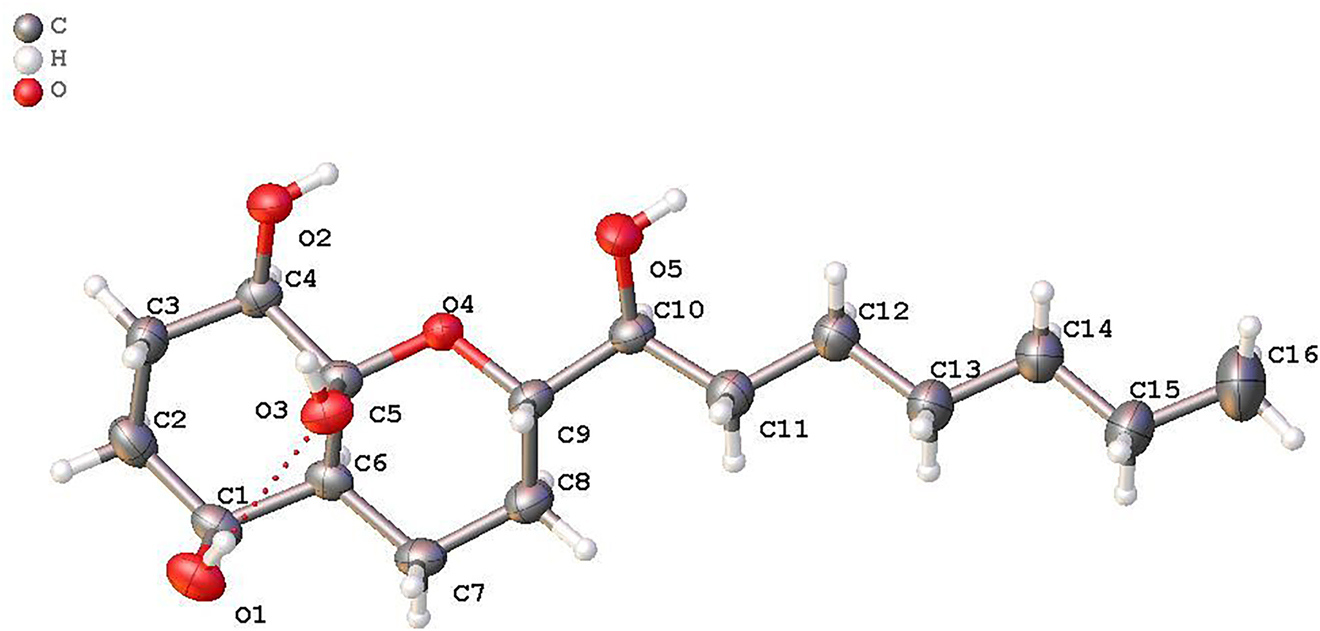
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | CCD, φ and ω |
| θ max, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 35,545, 5903, 0.084 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4005 |
| N(param)refined: | 381 |
| Programs: | SHELX [1], [2], [3], [4], Diamond [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | −0.1136 (7) | 0.0479 (3) | 0.75195 (19) | 0.0367 (11) |
| H1 | −0.0908 | −0.0106 | 0.7790 | 0.044* |
| C2 | −0.0993 (8) | 0.0069 (3) | 0.6903 (2) | 0.0454 (12) |
| H2A | −0.2378 | −0.0351 | 0.6786 | 0.054* |
| H2B | 0.0395 | −0.0366 | 0.6906 | 0.054* |
| C3 | −0.0855 (8) | 0.0929 (4) | 0.64492 (19) | 0.0404 (11) |
| H3A | −0.0645 | 0.0633 | 0.6069 | 0.049* |
| H3B | −0.2326 | 0.1315 | 0.6408 | 0.049* |
| C4 | 0.1175 (7) | 0.1640 (3) | 0.66384 (17) | 0.0318 (11) |
| H4 | 0.2659 | 0.1251 | 0.6658 | 0.038* |
| C5 | 0.0925 (7) | 0.2083 (3) | 0.72393 (18) | 0.0299 (10) |
| C6 | 0.0856 (7) | 0.1237 (3) | 0.76975 (18) | 0.0321 (10) |
| H6 | 0.2339 | 0.0853 | 0.7698 | 0.039* |
| C7 | 0.0889 (8) | 0.1710 (3) | 0.83109 (18) | 0.0386 (11) |
| H7A | −0.0593 | 0.2067 | 0.8340 | 0.046* |
| H7B | 0.1042 | 0.1170 | 0.8605 | 0.046* |
| C8 | 0.2949 (8) | 0.2462 (4) | 0.84296 (19) | 0.0414 (11) |
| H8A | 0.4433 | 0.2087 | 0.8460 | 0.050* |
| H8B | 0.2846 | 0.2808 | 0.8802 | 0.050* |
| C9 | 0.2914 (8) | 0.3259 (3) | 0.79368 (18) | 0.0336 (11) |
| H9 | 0.1455 | 0.3663 | 0.7928 | 0.040* |
| C10 | 0.5011 (8) | 0.3990 (3) | 0.79911 (17) | 0.0342 (11) |
| H10 | 0.6465 | 0.3580 | 0.8015 | 0.041* |
| C11 | 0.5095 (8) | 0.4641 (4) | 0.8543 (2) | 0.0420 (12) |
| H11A | 0.3653 | 0.5045 | 0.8522 | 0.050* |
| H11B | 0.5103 | 0.4186 | 0.8880 | 0.050* |
| C12 | 0.7189 (8) | 0.5364 (4) | 0.8653 (2) | 0.0456 (12) |
| H12A | 0.8643 | 0.4975 | 0.8651 | 0.055* |
| H12B | 0.7122 | 0.5863 | 0.8336 | 0.055* |
| C13 | 0.7233 (9) | 0.5926 (4) | 0.9235 (2) | 0.0518 (13) |
| H13A | 0.5775 | 0.6315 | 0.9231 | 0.062* |
| H13B | 0.7246 | 0.5419 | 0.9547 | 0.062* |
| C14 | 0.9286 (9) | 0.6649 (4) | 0.9387 (2) | 0.0549 (14) |
| H14A | 0.9272 | 0.7166 | 0.9081 | 0.066* |
| H14B | 1.0753 | 0.6266 | 0.9394 | 0.066* |
| C15 | 0.9228 (10) | 0.7176 (5) | 0.9974 (2) | 0.0668 (17) |
| H15A | 0.7758 | 0.7558 | 0.9964 | 0.080* |
| H15B | 0.9215 | 0.6654 | 1.0277 | 0.080* |
| C16 | 1.1256 (13) | 0.7900 (6) | 1.0149 (3) | 0.102 (3) |
| H16A | 1.2729 | 0.7549 | 1.0126 | 0.153* |
| H16B | 1.1180 | 0.8134 | 1.0545 | 0.153* |
| H16C | 1.1151 | 0.8478 | 0.9886 | 0.153* |
| C17 | −0.3180 (8) | 0.5036 (4) | 0.4719 (2) | 0.0434 (12) |
| H17 | −0.3572 | 0.5537 | 0.4403 | 0.052* |
| C18 | −0.5337 (8) | 0.4342 (5) | 0.4738 (2) | 0.0548 (14) |
| H18A | −0.5517 | 0.3914 | 0.4388 | 0.066* |
| H18B | −0.6744 | 0.4766 | 0.4733 | 0.066* |
| C19 | −0.5144 (8) | 0.3654 (4) | 0.52838 (19) | 0.0452 (12) |
| H19A | −0.6612 | 0.3277 | 0.5293 | 0.054* |
| H19B | −0.3879 | 0.3160 | 0.5262 | 0.054* |
| C20 | −0.4650 (7) | 0.4281 (4) | 0.58418 (18) | 0.0359 (11) |
| H20 | −0.5985 | 0.4748 | 0.5870 | 0.043* |
| C21 | −0.2427 (7) | 0.4916 (3) | 0.58211 (19) | 0.0335 (11) |
| C22 | −0.2662 (7) | 0.5634 (3) | 0.52925 (18) | 0.0374 (11) |
| H22 | −0.4060 | 0.6061 | 0.5328 | 0.045* |
| C23 | −0.0549 (8) | 0.6369 (4) | 0.5323 (2) | 0.0451 (12) |
| H23A | 0.0874 | 0.5987 | 0.5267 | 0.054* |
| H23B | −0.0812 | 0.6873 | 0.5009 | 0.054* |
| C24 | −0.0216 (10) | 0.6914 (4) | 0.5915 (2) | 0.0531 (14) |
| H24A | 0.1204 | 0.7333 | 0.5941 | 0.064* |
| H24B | −0.1556 | 0.7362 | 0.5951 | 0.064* |
| C25 | −0.0007 (8) | 0.6131 (3) | 0.64125 (19) | 0.0400 (12) |
| H25 | 0.1398 | 0.5707 | 0.6380 | 0.048* |
| C26 | 0.0117 (8) | 0.6542 (4) | 0.7037 (2) | 0.0434 (12) |
| H26 | −0.1327 | 0.6936 | 0.7076 | 0.052* |
| C27 | 0.2246 (9) | 0.7224 (4) | 0.7214 (2) | 0.0497 (13) |
| H27A | 0.3681 | 0.6833 | 0.7186 | 0.060* |
| H27B | 0.2230 | 0.7794 | 0.6940 | 0.060* |
| C28 | 0.2296 (9) | 0.7644 (4) | 0.7835 (2) | 0.0555 (14) |
| H28A | 0.0836 | 0.8019 | 0.7862 | 0.067* |
| H28B | 0.2327 | 0.7070 | 0.8106 | 0.067* |
| C29 | 0.4351 (9) | 0.8343 (4) | 0.8033 (2) | 0.0559 (14) |
| H29A | 0.4370 | 0.8906 | 0.7755 | 0.067* |
| H29B | 0.5817 | 0.7963 | 0.8028 | 0.067* |
| C30 | 0.4248 (10) | 0.8779 (5) | 0.8644 (2) | 0.0618 (15) |
| H30A | 0.2769 | 0.9151 | 0.8646 | 0.074* |
| H30B | 0.4222 | 0.8211 | 0.8918 | 0.074* |
| C31 | 0.6232 (11) | 0.9479 (5) | 0.8862 (3) | 0.0713 (17) |
| H31A | 0.6306 | 1.0033 | 0.8581 | 0.086* |
| H31B | 0.7709 | 0.9101 | 0.8880 | 0.086* |
| C32 | 0.6017 (14) | 0.9936 (6) | 0.9461 (3) | 0.094 (2) |
| H32A | 0.4631 | 1.0362 | 0.9441 | 0.141* |
| H32B | 0.7396 | 1.0343 | 0.9583 | 0.141* |
| H32C | 0.5888 | 0.9394 | 0.9741 | 0.141* |
| O1 | −0.3429 (5) | 0.0896 (2) | 0.75762 (13) | 0.0463 (8) |
| H1A | −0.3465 | 0.1501 | 0.7476 | 0.069* |
| O2 | 0.1230 (5) | 0.2441 (2) | 0.62126 (11) | 0.0357 (7) |
| H2 | 0.2453 | 0.2774 | 0.6284 | 0.054* |
| O3 | −0.1206 (5) | 0.2655 (2) | 0.72367 (12) | 0.0343 (7) |
| H3 | −0.1512 | 0.2936 | 0.6916 | 0.051* |
| O4 | 0.2920 (5) | 0.2739 (2) | 0.73800 (11) | 0.0308 (7) |
| O5 | 0.4909 (6) | 0.4626 (2) | 0.74689 (12) | 0.0438 (8) |
| H5 | 0.6027 | 0.5028 | 0.7506 | 0.066* |
| O7 | −0.1194 (5) | 0.4453 (3) | 0.45705 (14) | 0.0514 (9) |
| H7 | −0.0573 | 0.4156 | 0.4865 | 0.077* |
| O8 | −0.0430 (4) | 0.4282 (2) | 0.57679 (12) | 0.0375 (8) |
| H8 | −0.0502 | 0.3768 | 0.5973 | 0.056* |
| O9 | −0.2074 (5) | 0.5470 (2) | 0.63589 (12) | 0.0390 (8) |
| O10 | 0.0225 (6) | 0.5695 (3) | 0.74382 (13) | 0.0530 (9) |
| H10A | 0.1055 | 0.5240 | 0.7321 | 0.079* |
| O6 | −0.4460 (5) | 0.3606 (2) | 0.63424 (12) | 0.0386 (7) |
| H6A | −0.4306 | 0.3948 | 0.6646 | 0.058* |
Source of material
Stilbella buquetii was cultured on slants of potato dextrose agar (PDA) at 25 °C for 15 days. Agar plugs were cut into small pieces (about 0.5 × 0.5 × 0.5 cm3) under aseptic conditions and 15 of these pieces were used to inoculate in three Erlenmeyer flasks (1 L), each containing 400 mL of media (1% protein, 4% glucose) [6]; 15 flasks of the inoculated media were incubated at 25 °C on a rotary shaker at 170 rpm for five days to prepare the seed culture. Fermentation was carried out in 500 Fernbach flasks (500 mL), each containing 2 g of protein, 8 g of glucose. Distilled H2O (200 mL) was added to each flask, and autoclaving at 15 psi for 30 min. After cooling to room temperature, each flask was inoculated with 5 mL of the spore inoculum and incubated at 25 °C for 60 days. The culture was filtered to separate the residue (mycelia) and the filtrate (broth). The broth was extracted with EtOAc (3 × 10 L) and concentrated to a brown gum (33 g; extract A). The mycelium was macerated in EtOAc (1.5 L, rt, three days) and filtered. The filtrate was partially concentrated to a brown gum (130 g; Extract B). Combine extract A and extract B, and perform column chromatography on silica gel (10 × 150 cm), using PE/EtOAc as the eluent (gradient from 100:0 to 0:100) to obtain 11 fractions. Fraction 6 (8 g) was repeatedly ODS (manually assembled C18 reverse column chromatography) column using a step gradient elution with hexane/EtOAc (from 100:0 to 60:40) to obtain nine fractions. The product (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol) (33 mg) was obtained using ethyl acetate and methyl alcohol recrystallization in the fraction 6–6.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C–H = 0.97 Å (methylene), C–H = 0.96 Å, (methyl), and O–H = 0.82 Å(–OH)). The absolute configuration was derived from the configuration of the educts.
Comment
A series of complex pyranones discovered from Trichoderma corning are called koninginin. The main types of koninginin discovered so far are koninginin A–E and Koninginin G [7], [8], [9]. Koninginin G is also named (2S,4aR,5R,8S, 8aS)-2-((S)-1-hydroxyheptyl)octahydro-2H-chromene-5. Although its molecular structure has been discovered [10], its crystal structure has not yet been reported. It should be noted that 1,4,5,6-tetra-epi-koninginin G was extracted from Stilbella buquetii for the first time.
The structure solution shows that the title compound is a derivative of koninginin A. There are two crystallographically independent molecules in the asymmetric unit. The bond length in both molecules are within the expected range.
We speculate that the C7–O–C10 position of koninginin A is hydrolyzed by hydrolase to form two hydroxyl groups to form koninginin G (C5–OH, C10–OH).
Funding source: The Science and Technology Foundation of Guizhou Province
Award Identifier / Grant number: No. [2019]2451-3 & No. [2019]2333
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financed by The Science and Technology Foundation of Guizhou Province (No. [2019]2451-3 & No. [2019]2333).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. Shelxs-2014/7. Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014.Search in Google Scholar
2. Sheldrick, G. M. Shelxl-2014/7. Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Brandenburg, K. Diamond. Visual Crystal Structure Information System (version 3.2i); Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
6. Cutler, H. G., Himmelsbach, D. S., Jacyno, J. M., Cole, P. D., Yagen, B., Arrendale, R. F., Cox, R. H. Koninginin B: a biologically active congener of Koninginin A from Trichoderma koningii. J. Agric. Food Chem. 1991, 39, 977–980; https://doi.org/10.1021/jf00005a035.Search in Google Scholar
7. Cutler, H. G., Himmelsbach, D. S., Arrendale, R. F., Cole, P. D., Cox, R. H. Koninginin A: a novel plant growth regulator from Trichoderma koning. Agric. Biol. Chem. 1989, 53, 2605–2611; https://doi.org/10.1080/00021369.1989.10869746.Search in Google Scholar
8. Parker, S. R., Horace, G. C., Schreiner, P. R. Koninginin C: a biologically active natural product from Trichoderma koningii. Biosci. Biotechnol. Biochem. 1995, 59, 1126–1127; https://doi.org/10.1271/bbb.59.1126.Search in Google Scholar PubMed
9. Song, F., Dai, H., Tong, Y., Ren, B., Chen, C., Sun, N., Liu, X., Bian, J., Liu, M., Gao, H., Liu, H., Chen, X., Zhang, L. Trichodermaketones A–D and 7-O-methylkoninginin D from the marine fungus Trichoderma koningii. J. Nat. Prod. 2010, 73, 806–810; https://doi.org/10.1021/np900642p.Search in Google Scholar PubMed
10. Cutler, H. G., Cutler, S. J., Ross, S. A., El Sayed, K., Dugan, F. M., Bartlett, M. G., Hill, A. A., Hill, R. A., Parker, S. R. Koninginin G: a new metabolite from Trichoderma aureoviride. J. Nat. Prod. 1999, 62, 137–139; https://doi.org/10.1021/np9801817.Search in Google Scholar PubMed
© 2022 Guangdi Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co


