Abstract
C22H24I2N2, orthorhombic, Pbca (no. 33), a = 21.069(4) Å, b = 8.7963(17) Å, c = 23.757(5) Å, V = 4402.8(15) Å3, Z = 8, R gt (F) = 0.0530, wR ref (F2) = 0.1569, T = 296(2) K.
CCDC no.: 2153325
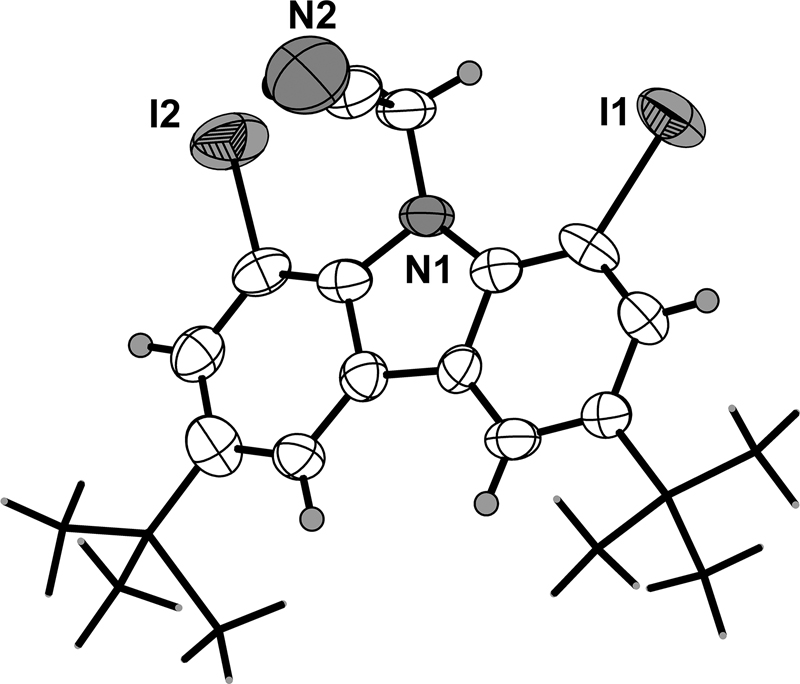
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.38 × 0.32 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.87 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 47,153, 9974, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7966 |
| N(param)refined: | 463 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2988 (5) | 1.1112 (12) | 0.7439 (5) | 0.049 (2) |
| C2 | 0.2633 (5) | 1.0092 (12) | 0.7744 (4) | 0.049 (2) |
| H2 | 0.260475 | 1.021876 | 0.813220 | 0.059* |
| C3 | 0.2311 (5) | 0.8869 (11) | 0.7495 (4) | 0.045 (2) |
| C4 | 0.2383 (5) | 0.8681 (11) | 0.6921 (5) | 0.048 (2) |
| H4 | 0.218779 | 0.786719 | 0.674079 | 0.058* |
| C5 | 0.2749 (5) | 0.9706 (10) | 0.6608 (4) | 0.043 (2) |
| C6 | 0.3049 (4) | 1.0954 (10) | 0.6865 (4) | 0.043 (2) |
| C7 | 0.1918 (6) | 0.7711 (12) | 0.7837 (5) | 0.058 (3) |
| C8 | 0.2316 (8) | 0.6221 (18) | 0.7847 (8) | 0.102 (5) |
| H8A | 0.249009 | 0.603819 | 0.747959 | 0.153* |
| H8B | 0.204834 | 0.538378 | 0.795208 | 0.153* |
| H8C | 0.265421 | 0.632047 | 0.811468 | 0.153* |
| C9 | 0.1306 (8) | 0.739 (2) | 0.7526 (9) | 0.091 (4) |
| H9A | 0.110003 | 0.832883 | 0.743365 | 0.136* |
| H9B | 0.103089 | 0.678903 | 0.776020 | 0.136* |
| H9C | 0.139734 | 0.683806 | 0.718618 | 0.136* |
| C10 | 0.1759 (11) | 0.828 (2) | 0.8428 (7) | 0.114 (5) |
| H10A | 0.214278 | 0.858473 | 0.861571 | 0.170* |
| H10B | 0.155693 | 0.748771 | 0.863824 | 0.170* |
| H10C | 0.147830 | 0.914084 | 0.840176 | 0.170* |
| C11 | 0.2931 (4) | 0.9717 (10) | 0.6017 (4) | 0.043 (2) |
| C12 | 0.3323 (4) | 1.1001 (10) | 0.5927 (4) | 0.045 (2) |
| C13 | 0.3576 (5) | 1.1217 (11) | 0.5404 (5) | 0.048 (2) |
| C14 | 0.3443 (5) | 1.0189 (12) | 0.4976 (5) | 0.053 (2) |
| H14 | 0.361740 | 1.036449 | 0.462257 | 0.064* |
| C15 | 0.3070 (6) | 0.8930 (14) | 0.5048 (5) | 0.061 (3) |
| C16 | 0.2809 (5) | 0.8706 (11) | 0.5581 (4) | 0.047 (2) |
| H16 | 0.255072 | 0.786705 | 0.564513 | 0.056* |
| C17 | 0.2941 (12) | 0.784 (3) | 0.4593 (9) | 0.118 (3) |
| C18 | 0.2229 (9) | 0.747 (2) | 0.4519 (10) | 0.118 (3) |
| H18A | 0.207875 | 0.692613 | 0.484231 | 0.177* |
| H18B | 0.217185 | 0.685928 | 0.418808 | 0.177* |
| H18C | 0.199494 | 0.840114 | 0.447947 | 0.177* |
| C19 | 0.3202 (9) | 0.609 (2) | 0.4821 (8) | 0.118 (3) |
| H19A | 0.341881 | 0.620579 | 0.517377 | 0.177* |
| H19B | 0.348851 | 0.567049 | 0.454740 | 0.177* |
| H19C | 0.284636 | 0.542248 | 0.486785 | 0.177* |
| C20 | 0.3283 (10) | 0.795 (2) | 0.4052 (8) | 0.118 (3) |
| H20A | 0.304058 | 0.856031 | 0.379426 | 0.177* |
| H20B | 0.334138 | 0.695400 | 0.389696 | 0.177* |
| H20C | 0.368944 | 0.841729 | 0.411285 | 0.177* |
| C21 | 0.6037 (5) | 0.4129 (12) | 0.4676 (4) | 0.050 (2) |
| C22 | 0.5900 (5) | 0.5201 (12) | 0.5081 (5) | 0.054 (3) |
| H22 | 0.608171 | 0.508104 | 0.543478 | 0.065* |
| C23 | 0.5504 (6) | 0.6463 (13) | 0.4995 (5) | 0.056 (2) |
| C24 | 0.5234 (5) | 0.6644 (12) | 0.4459 (4) | 0.050 (2) |
| H24 | 0.498147 | 0.748274 | 0.437672 | 0.060* |
| C25 | 0.5354 (5) | 0.5529 (11) | 0.4051 (4) | 0.046 (2) |
| C26 | 0.5740 (5) | 0.4277 (11) | 0.4147 (4) | 0.046 (2) |
| C27 | 0.5395 (7) | 0.7673 (14) | 0.5481 (5) | 0.060 (3) |
| C28 | 0.5405 (11) | 0.9226 (19) | 0.5213 (8) | 0.120 (5) |
| H28A | 0.581225 | 0.940026 | 0.504471 | 0.180* |
| H28B | 0.532619 | 0.998444 | 0.549509 | 0.180* |
| H28C | 0.508142 | 0.928108 | 0.492896 | 0.180* |
| C29 | 0.4729 (11) | 0.757 (2) | 0.5635 (11) | 0.124 (5) |
| H29A | 0.447246 | 0.760968 | 0.530118 | 0.186* |
| H29B | 0.462030 | 0.840130 | 0.587768 | 0.186* |
| H29C | 0.465411 | 0.662613 | 0.582737 | 0.186* |
| C30 | 0.5948 (11) | 0.761 (2) | 0.5866 (9) | 0.108 (5) |
| H30A | 0.593987 | 0.667740 | 0.607360 | 0.163* |
| H30B | 0.592980 | 0.845445 | 0.612290 | 0.163* |
| H30C | 0.633291 | 0.766948 | 0.565050 | 0.163* |
| C31 | 0.5167 (4) | 0.5462 (11) | 0.3459 (4) | 0.044 (2) |
| C32 | 0.4813 (5) | 0.6454 (11) | 0.3143 (4) | 0.046 (2) |
| H32 | 0.461978 | 0.728906 | 0.331102 | 0.055* |
| C33 | 0.4745 (5) | 0.6193 (12) | 0.2555 (5) | 0.049 (2) |
| C34 | 0.5051 (5) | 0.4937 (11) | 0.2333 (5) | 0.049 (2) |
| H34 | 0.500751 | 0.474853 | 0.194982 | 0.059* |
| C35 | 0.5416 (5) | 0.3945 (11) | 0.2648 (4) | 0.046 (2) |
| C36 | 0.5462 (4) | 0.4195 (11) | 0.3226 (4) | 0.045 (2) |
| C37 | 0.4390 (6) | 0.7355 (12) | 0.2171 (5) | 0.052 (2) |
| C38 | 0.4766 (8) | 0.866 (2) | 0.2061 (9) | 0.123 (8) |
| H38A | 0.496572 | 0.898783 | 0.240379 | 0.185* |
| H38B | 0.450178 | 0.945986 | 0.191839 | 0.185* |
| H38C | 0.508586 | 0.841316 | 0.178830 | 0.185* |
| C39 | 0.3751 (7) | 0.7773 (19) | 0.2487 (7) | 0.080 (4) |
| H39A | 0.348842 | 0.688361 | 0.251544 | 0.120* |
| H39B | 0.352953 | 0.854518 | 0.227960 | 0.120* |
| H39C | 0.384727 | 0.814245 | 0.285736 | 0.120* |
| C40 | 0.4159 (12) | 0.670 (3) | 0.1626 (8) | 0.141 (10) |
| H40A | 0.451459 | 0.635656 | 0.140890 | 0.212* |
| H40B | 0.393403 | 0.747088 | 0.141960 | 0.212* |
| H40C | 0.387994 | 0.586430 | 0.170118 | 0.212* |
| C41 | 0.5865 (6) | 0.1775 (13) | 0.3654 (6) | 0.058 (3) |
| H41A | 0.612961 | 0.146757 | 0.396781 | 0.070* |
| H41B | 0.606632 | 0.142933 | 0.330991 | 0.070* |
| C42 | 0.5218 (7) | 0.1029 (13) | 0.3709 (5) | 0.065 (3) |
| C43 | 0.3427 (6) | 1.3408 (11) | 0.6461 (6) | 0.055 (3) |
| H43A | 0.374995 | 1.373885 | 0.619711 | 0.066* |
| H43B | 0.355638 | 1.373824 | 0.683393 | 0.066* |
| C44 | 0.2832 (8) | 1.4134 (14) | 0.6319 (6) | 0.074 (4) |
| I1 | 0.35550 (5) | 1.25975 (11) | 0.79165 (4) | 0.0791 (3) |
| I2 | 0.42659 (5) | 1.29108 (11) | 0.52107 (5) | 0.0867 (3) |
| I3 | 0.67336 (5) | 0.25233 (10) | 0.48930 (5) | 0.0813 (3) |
| I4 | 0.59634 (5) | 0.23584 (11) | 0.22030 (4) | 0.0786 (3) |
| N1 | 0.3395 (4) | 1.1771 (9) | 0.6453 (4) | 0.0471 (19) |
| N2 | 0.2381 (8) | 1.4726 (18) | 0.6200 (8) | 0.134 (7) |
| N3 | 0.5816 (4) | 0.3413 (9) | 0.3645 (4) | 0.0441 (18) |
| N4 | 0.4727 (8) | 0.0498 (15) | 0.3744 (7) | 0.105 (5) |
Source of material
Five grams of 3,6-di-tert-butylcarbazole was dissolved in 60 ml of chloroform, and after stirring for half an hour, 5 g iodine and 3 ml of concentrated sulfuric acid were added, and the mixture was stirred at room temperature for 24 h. The reaction solution was quenched by adding 100 ml of water, and then dichloromethane (DCM) was added to extract and separate the layers. The retained organic layer (lower layer) was placed in a 100 ml round-bottomed flask, and then spin-dried using a rotary evaporator to obtain 3,6-di-tert-butyl-1,8-diiodo-9H-carbazole as white solid. At room temperature 0.5 ml of KOH (50%, 8.95 mmol) was added to a stirred solution of 1.9 g of 3,6-di-tert-butyl-1,8-diiodo-9H-carbazole (3.58 mmol) and 0.115 g of TBAB in 14 ml of DMSO. After stirred for half an hour, 0.7 ml of BrCH2CN (5.37 mmol) was added dropwise. The mixture was warmed to 80 °C and stirred for 5 h. The reaction was quenched by ice water, and extracted by DCM (3 × 50 ml). The organic layer was dried with anhydride Na2SO4. The solvent was removed in vacuo. The organic layer was dried with anhydride Na2SO4. The residue was purified by purified by recrystallization using ethanol to yield 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile as a brown solid. Crystals of the title compound were obtained by slow evaporation of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile in CH2Cl2 within one weeks.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
Carbazole-based compounds have been widely utilized in prominent optoelectronic applications such as organic light emitting diodes (OLEDs) and photovoltaic cells, because of their excellent thermal and electronic properties [3], [4], [5], [6], [7], [8]. Biologically active carbazole alkaloids have been isolated from diverse natural sources and exhibit a broad range of different frameworks and functional groups. Therefore, a large number of classical and nonclassical methods have been developed for their synthesis [9, 10]. The synthesis of carbazole derivatives is a hot topic in organic chemistry. Previously, Zhang reported the synthesis and crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole [11]. Herein, we reported the synthesis of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, which may enriched the application of carbazoles in materials chemistry.
The title compound, built up by the C22H24I2N2 molecules, has been synthesized. There are two crystallographically independent molecules in the asymmetric unit. Both molecules are very similar. The single-crystal structure verifies that all bond lengths are in normal ranges [11].
Funding source: National Natural Science Foundation of China http://dx.doi.org/10.13039/501100001809
Award Identifier / Grant number: 21602055
Funding source: Natural Science Foundation of Hunan Province
Award Identifier / Grant number: 2017 J J3094
Funding source: Undergraduate Research Study and Innovative Experiment of Hunan Provincial
Award Identifier / Grant number: 2016-636
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The work was supported by National Natural Science Foundation of China (No. 21602055); Natural Science Foundation of Hunan Province (No. 2017 J J3094) and Undergraduate Research Study and Innovative Experiment of Hunan Provincial (2016-636).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar
3. Yang, X., Zhou, G., Wong, W.-Y. Functionalization of phosphorescent emitters and their host materials by main-group elements for phosphorescent organic light-emitting devices. Chem. Soc. Rev. 2015, 44, 8484–8575; https://doi.org/10.1039/c5cs00424a.Search in Google Scholar PubMed
4. Hung, W.-Y., Chi, L.-C., Chen, W.-J., Chen, Y.-M., Chou, S.-H., Wong, K.-T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluorescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 45, 10113–10119; https://doi.org/10.1039/c0jm02143a.Search in Google Scholar
5. Grazulevicius, J. V., Strohriegl, P., Pielichowski, J., Pielichowski, K. Carbazole-containing polymers: synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353; https://doi.org/10.1016/s0079-6700(03)00036-4.Search in Google Scholar
6. Wakim, S., Aeich, B.-R., Tao, Y., Leclerc, M. Charge transport, photovoltaic, and thermoelectric properties of poly(2,7-carbazole) and poly(indolo[3,2-b]carbazole) derivatives. Polym. Rev. 2008, 48, 432–462; https://doi.org/10.1080/15583720802231726.Search in Google Scholar
7. Morin, J.-F., Leclerc, M. Syntheses of conjugated polymers derived from N-alkyl-2,7-carbazoles. Macromolecules 2001, 34, 4680–4682; https://doi.org/10.1021/ma010152u.Search in Google Scholar
8. Michinobu, T., Osako, H., Shigehara, K. Synthesis and properties of conjugated poly(1,8-carbazole)s. Macromolecules 2009, 42, 8172–8180; https://doi.org/10.1021/ma901502b.Search in Google Scholar
9. Knölker, H.-J., Reddy, K. R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002, 102, 4303–4428; https://doi.org/10.1021/cr020059j.Search in Google Scholar PubMed
10. Schmidt, A. W., Reddy, K. R., Knölker, H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012, 112, 3193–3328; https://doi.org/10.1021/cr200447s.Search in Google Scholar PubMed
11. Zhang, W.-J., Zeng, T. The crystal structure of 3,6-di-tert-butyl-1,8-diiodo-9-methyl-9H-carbazole, C21H25I2N. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 923–924; https://doi.org/10.1515/ncrs-2020-0103.Search in Google Scholar
© 2022 Lin-Li Tang and Fei Zeng, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

