Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
Abstract
C21H22NNaO12S, orthorhombic, P212121 (no. 19), a = 7.6850(1) Å, b = 10.8030(1) Å, c = 25.9262(2) Å, V = 2152.42(4) Å3, Z = 4, Rgt(F) = 0.0309, wRref(F2) = 0.0818, T = 100.0(4) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.19 × 0.15 × 0.13 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.20 mm−1 |
| Diffractometer, scan mode: | ROD, Synergy Custom DW system, ω |
| θmax, completeness: | 75.8°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 12,136, 4,386, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4,356 |
| N(param)refined: | 335 |
| Programs: | CrysAlisPRO [1], Diamond [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.57877 (8) | 1.08139 (6) | 0.58175 (3) | 0.02550 (15) |
| O1 | 0.7463 (3) | 0.47611 (19) | 0.23022 (7) | 0.0286 (4) |
| H1 | 0.787942 | 0.512351 | 0.204348 | 0.043* |
| O2 | 0.8515 (3) | 0.87833 (18) | 0.40909 (7) | 0.0276 (4) |
| O3 | 0.5893 (2) | 0.54869 (17) | 0.40125 (7) | 0.0239 (4) |
| O4 | 0.7040 (3) | 0.8824 (2) | 0.65896 (7) | 0.0294 (4) |
| H4 | 0.673745 | 0.956861 | 0.661412 | 0.044* |
| O5 | 0.6809 (3) | 1.1576 (2) | 0.54750 (11) | 0.0471 (6) |
| O6 | 0.3944 (2) | 1.0892 (2) | 0.57065 (9) | 0.0338 (5) |
| O7 | 0.6172 (3) | 1.1030 (2) | 0.63621 (9) | 0.0438 (6) |
| O8 | 0.6169 (3) | 0.1521 (2) | 0.24948 (7) | 0.0340 (5) |
| H8 | 0.676190 | 0.171327 | 0.223466 | 0.051* |
| O9 | 0.5580 (3) | 0.2432 (2) | 0.44122 (7) | 0.0351 (5) |
| O10 | 0.3575 (3) | 0.09911 (18) | 0.42274 (7) | 0.0282 (4) |
| O11 | 0.4541 (3) | 0.4166 (3) | 0.52979 (10) | 0.0468 (6) |
| H11A | 0.510741 | 0.399618 | 0.558412 | 0.070* |
| H11B | 0.413711 | 0.481578 | 0.546422 | 0.070* |
| N1 | 0.6433 (3) | 0.2934 (2) | 0.34493 (8) | 0.0215 (4) |
| H1A | 0.684130 | 0.323801 | 0.379306 | 0.035 (9)* |
| C1 | 0.7489 (3) | 0.5534 (3) | 0.27112 (10) | 0.0240 (5) |
| C2 | 0.8322 (4) | 0.6699 (3) | 0.26934 (10) | 0.0262 (5) |
| H2 | 0.883529 | 0.697815 | 0.238094 | 0.031* |
| C3 | 0.8392 (3) | 0.7429 (3) | 0.31259 (10) | 0.0251 (5) |
| H3 | 0.897340 | 0.820425 | 0.310994 | 0.030* |
| C4 | 0.7618 (3) | 0.7052 (2) | 0.35946 (10) | 0.0226 (5) |
| C5 | 0.6740 (3) | 0.5923 (3) | 0.35862 (10) | 0.0225 (5) |
| C6 | 0.6641 (3) | 0.5147 (3) | 0.31557 (10) | 0.0221 (5) |
| C7 | 0.7752 (3) | 0.7769 (3) | 0.40717 (10) | 0.0235 (5) |
| C8 | 0.6946 (3) | 0.7183 (3) | 0.45212 (10) | 0.0246 (5) |
| C9 | 0.6072 (3) | 0.6109 (3) | 0.44585 (10) | 0.0261 (6) |
| H9 | 0.553693 | 0.576261 | 0.475559 | 0.031* |
| C10 | 0.7030 (3) | 0.7688 (3) | 0.50536 (10) | 0.0237 (5) |
| C11 | 0.6487 (3) | 0.8873 (3) | 0.51764 (10) | 0.0250 (5) |
| H11 | 0.611995 | 0.941684 | 0.490995 | 0.030* |
| C12 | 0.6473 (3) | 0.9278 (3) | 0.56894 (9) | 0.0231 (5) |
| C13 | 0.7020 (3) | 0.8478 (3) | 0.60870 (10) | 0.0235 (5) |
| C14 | 0.7577 (4) | 0.7302 (3) | 0.59613 (10) | 0.0253 (5) |
| H14 | 0.796583 | 0.676006 | 0.622571 | 0.030* |
| C15 | 0.7575 (3) | 0.6905 (3) | 0.54538 (10) | 0.0245 (5) |
| H15 | 0.794889 | 0.608796 | 0.537510 | 0.029* |
| C16 | 0.5549 (3) | 0.3997 (3) | 0.31741 (10) | 0.0241 (5) |
| H16A | 0.526839 | 0.373901 | 0.281719 | 0.029* |
| H16B | 0.443935 | 0.418681 | 0.335143 | 0.029* |
| C17 | 0.5184 (3) | 0.1853 (3) | 0.35387 (9) | 0.0234 (5) |
| H17 | 0.409794 | 0.197456 | 0.333227 | 0.028* |
| C18 | 0.6173 (4) | 0.0720 (3) | 0.33422 (10) | 0.0260 (5) |
| H18A | 0.689775 | 0.035148 | 0.361808 | 0.035 (9)* |
| H18B | 0.536391 | 0.008292 | 0.320844 | 0.041* |
| C19 | 0.7296 (4) | 0.1249 (3) | 0.29125 (10) | 0.0265 (5) |
| H19 | 0.825174 | 0.067078 | 0.281034 | 0.032* |
| C20 | 0.7992 (3) | 0.2428 (3) | 0.31594 (10) | 0.0242 (5) |
| H20A | 0.896474 | 0.224312 | 0.339776 | 0.016 (7)* |
| H20B | 0.839792 | 0.302040 | 0.289414 | 0.020* |
| C21 | 0.4740 (3) | 0.1766 (3) | 0.41114 (10) | 0.0245 (5) |
| Na1a | 0.7198 (2) | 0.33397 (18) | 0.50240 (6) | 0.0272 (3) |
| Na1Ab | 0.8296 (4) | 0.2694 (3) | 0.49229 (9) | 0.0272 (3) |
| O12 | 0.8998 (3) | 0.53901 (19) | 0.14507 (7) | 0.0305 (4) |
| H12A | 0.822199 | 0.562859 | 0.123928 | 0.046* |
| H12B | 0.955649 | 0.484429 | 0.128218 | 0.046* |
-
aOccupancy: 0.605 (2). bOccupancy: 0.395 (2).
Source of material
The title compound was synthesized via a Mannich reaction. Formaldehyde solution (10 mL, 37%), water (20 mL), trans-4-hydroxy-l-proline (1.97 g, 0.015 mol) and sodium 2-hydroxy-5-(7-hydroxy-4-oxo-4H-chromen-3-yl)benzenesulfonate (3.56 g, 0.01 mol) were added to ethanol (150 mL, 99.5%) and stirred for 24 h at 338 K. Then the mixture was filtered and the residue was collected. Then the residue was dried at 373 K. Sodium 5-(8-(((2S,4R)-2-carboxy-4-hydroxypyrrolidin-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate (3.02 g) was obtained. 1H-NMR (400 MHz, D2O) δ: 7.98 (s, 1H, H9), 7.77 (d, J = 8.9 Hz, 1H, H3), 7.67 (s, 1H, H11), 7.17 (d, J = 8.4 Hz, 1H, H15), 6.86 (d, J = 8.9 Hz, 1H, H2), 6.83 (d, J = 8.4 Hz, 1H, H14), 4.58 (m, 1H, H19), 4.50 (s, 2H, H16A, H16B), 4.24 (m, 1H, H17), 3.77 (m, 1H, H20A), 3.28 (m, 1H, H20B), 2.43 (m, 1H, H18A), 2.12 (m, 1H, H18B). 13C-NMR (100 MHz, D2O) δ:176.53 (C7), 172.64 (C21), 161.64 (C1), 155.80 (C13), 153.81 (C5), 153.15 (C9), 132.93 (C15), 128.74 (C11), 127.64 (C12), 127.58 (C3), 122.25 (C10), 121.97 (C8), 117.03 (C4), 115.91 (C14), 114.69 (C2), 103.90 (C6), 69.60 (C17), 68.28 (C19), 60.89 (C20), 48.71 (C16), 37.87 (C18). A mixture of sodium salt described before (0.050 g), water (1 mL) and saturated sodium chloride solution (14 mL) was sealed in a 20 mL vial and sonicated for 5 min. Then the mixture was heated at 358 K for 6 h. Colourless block crystals of the title compound were obtained after 7 days. IR spectra (potassium bromide pellet) were recorded on a Nicolet 6700. IR (v/cm−1): 3,460, 2,068, 1,627, 1,491, 1,441, 1,402, 1,352, 1,329, 1,288, 1,249, 1,163, 1,096, 1,022, 1,001, 958, 906, 844, 831, 794, 781, 725, 713, 636, 541, 478, 466.
Experimental details
Carbon-bound H atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 Ueq(C). The oxygen-bound and nitrogen-bond H atoms were located on a difference Fourier map. The sodium atoms were disordered. The absolute structure was established by refinement of the Flack parameter (0.020(9) from 1795 selected quotients) using the Parsons–Flack method [5].
Comment
Trans-4-hydroxy-l-proline is an important amino acid that is a valuable chiral building block for the synthesis of pharmaceutical intermediates [6], [7], [8]. In the past decade, trans-4-hydroxy-l-proline derivatives have been gradually recognized as chiral catalysts for many asymmetric reactions, such as Aldol reaction and Michael reaction [9], [10], [11], [12], [13], [14]. Our previous investigations showed that sodium 2-hydroxy-5-(7-hydroxy-4-oxo-4H-chromen-3-yl)benzenesulfonate (DSS) can react with amino acids by Mannich reaction [15], [16], [17], [18]. In this paper, we report a trans-4-hydroxy-l-proline derivative of DSS.
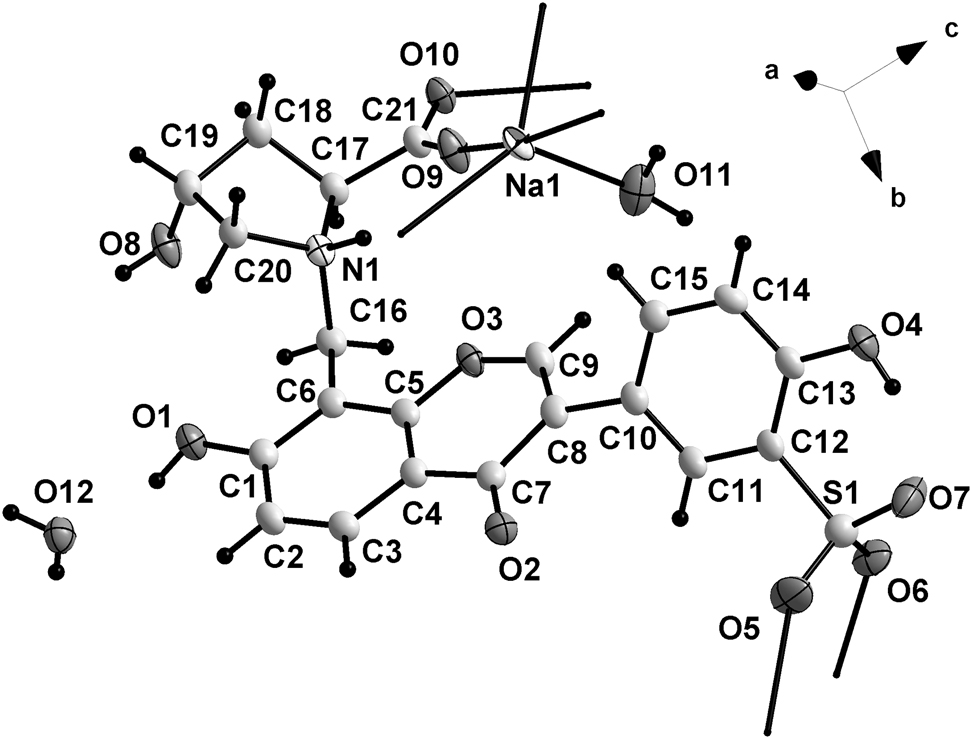
The asymmetric unit of the title structure contains one sodium ion, one ligand, one coordinated water molecule and one uncoordinated water molecule (cf. the figure). The dihedral angle between planar rings B (C10–C15) and C (C7–C9/O3/C5/C4) is 53.46°. There exist various O–H⃛O and N–H⃛O hydrogen bonds. The nitrogen atom N1 is protonated. The sodium atoms were disordered. The Na1 ion is five-coordinated. The Na1A ion is six-coordinated. The bond lengths are Na1–O9 = 2.241 (3) Å, Na1–O11 = 2.339 (3) Å, Na1–O5A = 2.255 (3) Å, Na1–O6A = 2.465 (3) Å, Na1–O10A = 2.326 (3) Å, Na1A–O9 = 2.485 (4) Å, Na1A–O5A = 2.194 (3), Na1A–O6A = 2.290 (3) Å, Na1A–O9A = 2.464 (3) Å, Na1A–O10A = 2.630 (3) Å, Na1A–O11A = 2.298 (4) Å, respectively, which is in the normal range [19]. The sodium coordination polymer is extended to a two dimensional layer along the ab plane. The two-dimensional layers form three-dimensional framework structure by hydrogen bonds. It is obvious that the hydrogen bonds play important roles in the self-assembly and enhance stability of the resultant title structure.
Funding source: the Foundation of Hechi University
Award Identifier / Grant number: XJ2018GKQ012
Funding source: Guangxi Natural Science Foundation of China
Award Identifier / Grant number: 2020GXNSFBA297138
Funding source: 2021 High-level Talents Scientific Research Startup Fund of Hechi University
Award Identifier / Grant number: 2021GCC021
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by the Foundation of Hechi University (No. XJ2018GKQ012), Guangxi Natural Science Foundation of China (No. 2020GXNSFBA297138) and 2021 High-level Talents Scientifific Research Startup Fund of Hechi University (No. 2021GCC021).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. CrysAlisPro; Rigaku Corporation: Yarnton, Oxfordshire, England, 2020.Search in Google Scholar
2. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 3.2; Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL- integrated space-group andcrystal-structure determination. Acta Crystallogr. 2015, A71, 3–8, https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Parsons, S., Flack, H. D., Wagner, T. Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. 2013, B69, 249–259, https://doi.org/10.1107/s2052519213010014.Search in Google Scholar PubMed PubMed Central
6. Comesse, S., Daïch, A. An unprecedented straightforward synthesis of chiral pyrrolo[3,4-b]quinolone and pyrrolo[3,2-b]quinolone backbones starting from trans-4-hydroxy-L-proline. J. Chem. 2016, 2016, 1504682, https://doi.org/10.1155/2016/1504682.Search in Google Scholar
7. Zhang, Y., Hubbard, J. W., Akhmedov, N. G., Petersen, J. L., Söderberg, B. C. G. Total synthesis of the tetracyclic indole alkaloid ht-13-B. J. Org. Chem. 2015, 80, 4783–4790, https://doi.org/10.1021/acs.joc.5b00433.Search in Google Scholar PubMed
8. Singh, R., Panda, G. l-Proline derived nitrogenous steroidal systems: an asymmetric approach to 14-azasteroids. RSC Adv. 2013, 3, 19533–19544, https://doi.org/10.1039/c3ra42272k.Search in Google Scholar
9. Bacha, K., Aguibi, K., Mbakidi, J.-P., Bouquillon, S. Beneficial contribution of biosourced ionic liquids and microwaves in the Michael reaction. Catalysts 2020, 10, 814, https://doi.org/10.3390/catal10080814.Search in Google Scholar
10. Patel, H. A., Gutal, A., Sahoo, S. K., Soni, H. P. Asymmetric direct Aldol reaction in confined space: molecular conformations of organocatalyst affect chiral induction. ChemistrySelect 2019, 4, 13210–13218, https://doi.org/10.1002/slct.201903032.Search in Google Scholar
11. Gurka, A. A., Szőri, K., Bartók, M., London, G. Dual stereocontrol in aldol reactions catalysed by hydroxyproline derivatives in the presence of a large amount of water. Tetrahedron: Asymmetry 2016, 27, 936–942, https://doi.org/10.1016/j.tetasy.2016.08.009.Search in Google Scholar
12. Yadav, G. D., Singh, S. Direct asymmetric aldol reactions catalysed by trans-4-hydroxy-(S)-prolinamide in solvent-free conditions. Tetrahedron: Asymmetry 2015, 26, 1156–1166, https://doi.org/10.1016/j.tetasy.2015.09.003.Search in Google Scholar
13. Gauchot, V., Gravel, J., Schmitzer, A. R. Asymmetric Michael addition induced by the anion of an imidazolium salt. Eur. J. Org. Chem. 2012, 2012, 6280–6284, https://doi.org/10.1002/ejoc.201201068.Search in Google Scholar
14. Yang, H., Li, S., Wang, X., Zhang, F., Zhong, X., Dong, Z., Ma, J. Core–shell silica magnetic microspheres supported proline as a recyclable organocatalyst for the asymmetric aldol reaction. J. Mol. Catal. Chem. 2012, 363–364, 404–410, https://doi.org/10.1016/j.molcata.2012.07.017.Search in Google Scholar
15. Chen, H.-L., Huang, X. X., Yao, D. M., Wang, Y. P., Lu, X. B. Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1371–1373, https://doi.org/10.1515/ncrs-2021-0346.Search in Google Scholar
16. Chen, H.-L., Pan, L.-W., Qin, Y.-L., Xie, Y.-J., Zhang, P. Synthesis and crystal structure of trans-tetraaqua-bis(3-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)propanoato-κO)zinc(II) tetrahydrate, C38H48N2O26S2Zn. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 217–219, https://doi.org/10.1515/ncrs-2018-0250.Search in Google Scholar
17. Chen, H.-L., Yin, X.-J. Synthesis and crystal structure of 5-(8-(((2-carboxyethyl)ammonio)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate trihydrate, C19H23NO12S. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 265–267, https://doi.org/10.1515/ncrs-2018-0307.Search in Google Scholar
18. Chen, H.-L., Lai, H.-F., Wei, L.-Q., Jiang, L.-R., Su, Y.-X. Synthesis and crystal structure of trans-tetraqua-bis(m32-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 1025–1027, https://doi.org/10.1515/ncrs-2017-0153.Search in Google Scholar
19. Tai, X.-S., Wang, L.-H., Xia, Y.-P. The crystal structure of poly[μ2-aqua- aqua-(μ3-(E)-2-(4-((2-carbamothioylhydrazineylidene)methyl)phenoxy)acetato-κ3O:S:S)sodium(I)], C10H14N3O5SNa. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 277–279, https://doi.org/10.1515/ncrs-2022-0006.Search in Google Scholar
© 2022 Hai-Lin Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

