Abstract
C10H6Cl3NO, monoclinic, P21/n (no. 14), a = 9.7097(10) Å, b = 10.6037(11) Å, c = 10.1735(10) Å, β = 96.580(4)°, V = 1040.55(18) Å3, Z = 4, R gt (F) = 0.0214, wR ref (F2) = 0.0574, T = 101 K.
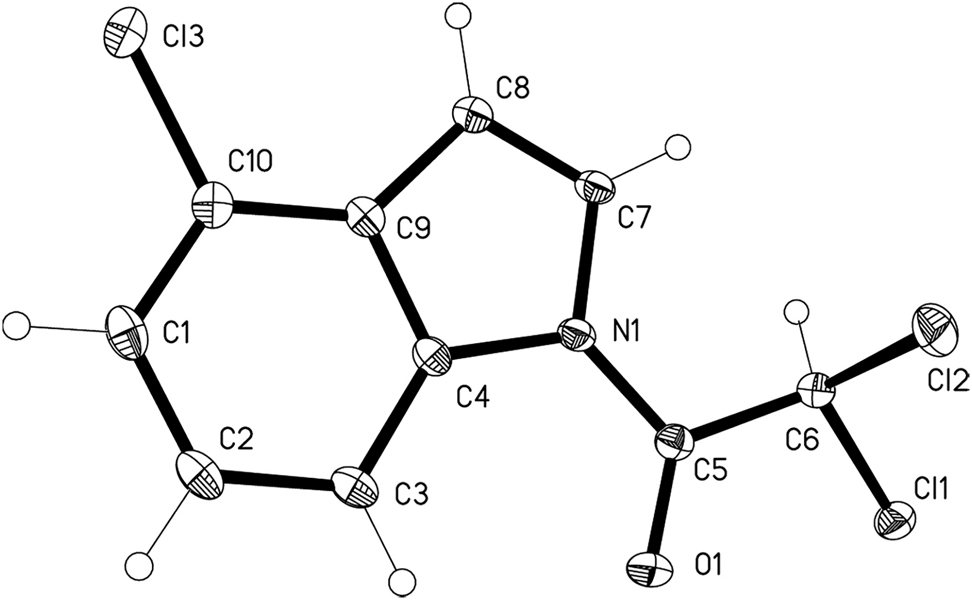
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.21 × 0.19 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.85 mm−1 |
| Diffractometer, scan mode: | Bruker SMART, φ and ω-scans |
| θmax, completeness: | 31.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 25,606, 3460, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3271 |
| N(param)refined: | 137 |
| Programs: | Bruker programs [1], OLEX2 [2, 3], SHELX [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| x | y | z | Uiso*/Ueq | |
|---|---|---|---|---|
| Cl1 | 0.66735 (2) | 0.44793 (2) | 0.93686 (2) | 0.01812 (6) |
| Cl2 | 0.50769 (3) | 0.58554 (2) | 0.72222 (3) | 0.02354 (7) |
| Cl3 | 0.97674 (2) | 0.57050 (2) | 0.16074 (2) | 0.01820 (6) |
| O1 | 0.69760 (7) | 0.31633 (6) | 0.69040 (7) | 0.01742 (13) |
| N1 | 0.76683 (8) | 0.48313 (7) | 0.57268 (7) | 0.01227 (13) |
| C1 | 0.90682 (10) | 0.33479 (9) | 0.23380 (9) | 0.01738 (17) |
| H1 | 0.9402 | 0.3061 | 0.1548 | 0.021* |
| C2 | 0.85569 (10) | 0.24892 (9) | 0.32038 (10) | 0.01780 (17) |
| H2 | 0.8553 | 0.1617 | 0.2991 | 0.021* |
| C3 | 0.80533 (10) | 0.28680 (8) | 0.43660 (9) | 0.01566 (16) |
| H3 | 0.7706 | 0.2276 | 0.4947 | 0.019* |
| C4 | 0.80815 (9) | 0.41569 (8) | 0.46412 (9) | 0.01235 (15) |
| C5 | 0.71410 (9) | 0.42902 (8) | 0.67961 (9) | 0.01283 (15) |
| C6 | 0.67366 (9) | 0.52315 (8) | 0.78359 (9) | 0.01435 (15) |
| H6 | 0.7431 | 0.5931 | 0.7941 | 0.017* |
| C7 | 0.79327 (9) | 0.61296 (8) | 0.55275 (9) | 0.01327 (15) |
| H7 | 0.7748 | 0.6791 | 0.6113 | 0.016* |
| C8 | 0.84864 (9) | 0.62818 (8) | 0.43817 (9) | 0.01327 (15) |
| H8 | 0.8759 | 0.7059 | 0.4025 | 0.016* |
| C9 | 0.85910 (9) | 0.50510 (8) | 0.37950 (8) | 0.01250 (15) |
| C10 | 0.90847 (9) | 0.46241 (9) | 0.26412 (9) | 0.01451 (16) |
Source of material
The title compound was synthesized according to the literature [5]. About 0.61 g 4-chloroindole (4.0 mmol) was dissolved in dichloroethane (40 mL) at room temperature. About 0.59 g dichloracetyl chloride (4.0 mmol) was added dropwise to this stirred solution. Then, the mixture was heated to reflux for 4 h. The crude product was purified by column chromatography (petroleum ether: EtOAc = 50:1, v/v) to give the title compound. Crystals were grown using a mixed solvent of EtOAc and petroleum ether at room temperature.
Experimental details
The structures was solved by direct methods with the SHELXS program [4]. All hydrogen atoms were placed in geometrically calculated positions and constrained to ride on their parent atoms (Table 2).
Comment
N-dichloroacetamides herbicide safeners have generated considerable commercial interest and research activity for their application to crops to alleviate the injury from chloroacetanilide, thiocarbamate and imidazolinone herbicides [6, 7]. Indole derivatives have significant physiological activity and occupy an important position in agriculture, medicine and materials science [8], [9], [10], [11], [12], [13]. In view of the principle of fragment splicing, the indole derivative and dichloroacetyl were spliced together to design novel potential N-dichloroacetyl indole derivatives herbicide safeners [14].
There is one molecule in the asymmetric unit (see the Figure). Bond lengths and angles are all in normal ranges [15], [16], [17], [18]. Obviously, there is π–π conjugation effect in the amide moiety, which causes a short N(1)–C(5) (1.3792(11) Å) bond. The torsion angle of C(4)–N(1)–C(5)–O(1) and C(7)–N(1)–C(5)–O(1) are −0.23(14)° and −178.22(9)° respectively, which indicate the amide moiety and indole ring are almost coplanar. Weak intermolecular C–H⋯O and C–H⋯Cl hydrogen bonds are observed, which connect the molecules into a three dimensional network.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 30900953
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This research was supported by the National Natural Science Foundation of China (No. 30900953).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Bourhis, L. J., Dolomanov, O. V., Gildea, R. J., Howard, J. A. K., Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment-Olex2 dissected. Acta Crystallogr. A. 2015, 71, 59–75; https://doi.org/10.1107/s2053273314022207.Suche in Google Scholar
4. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
5. Rajeswaran, W. G., Cohen, L. A. A convenient method of protecting oxindoles. Tetrahedron Lett. 1997, 38, 7813–7814; https://doi.org/10.1016/s0040-4039(97)10090-9.Suche in Google Scholar
6. Jonathan, D. W., John, E. C. Specific Binding of a Dichloroacetamide herbicide safener in maize at asSite that also binds thiocarbamate and chloroacetanilide herbicides. Plant Physiol. 1995, 109, 213–219.10.1104/pp.109.1.213Suche in Google Scholar PubMed PubMed Central
7. Bolyard, K., Gresens, S. E., Ricko, A. N., Sivey, J. D., Salice, C. J. Assessing the toxicity of the “inset” safener benoxacor toward, hironomus riparius: effects of agrochemical mixtures. Environ. Toxicol. Chem. 2017, 36, 2660–2670; https://doi.org/10.1002/etc.3814.Suche in Google Scholar PubMed
8. Kaczmarek, D. K., Kleiber, T., Wenping, L., Niemczak, M., Chrzanowski, L., Pernak, J. Transformation of indole-3-butyric acid (IBA) into ionic liquids as a sustainable strategy leading to highly efficient plant growth stimulators. ACS Sustain. Chem. Eng. 2020, 8, 1591–1598; https://doi.org/10.1021/acssuschemeng.9b06378.Suche in Google Scholar
9. Mustafa, A., Imran, M., Ashraf, M., Mahmood, K. Perspectives of using L-Tryptophan for improving productivity of agricultural crops: a Review. Pedosphere 2018, 28, 16–34; https://doi.org/10.1016/s1002-0160(18)60002-5.Suche in Google Scholar
10. Rajasekharan, S. K., Kim, S., Kim, J. C., Lee, J. Nematicidal activity of 5-iodoindole against root-knot nematodes. Pestic. Biochem. Physiol. 2020, 163, 76–83; https://doi.org/10.1016/j.pestbp.2019.10.012.Suche in Google Scholar PubMed
11. Verardi, L. M., Fiori, J., Andrisano, V., Locatelli, A., Morigi, R., Naldi, M., Bertucci, C., Strocchi, E., Boga, C., Micheletti, G., Calonghi, N. Indole derivative interacts with estrogen receptor beta and inhibits human ovarian cancer cell growth. Molecules 2020, 25, 4438–4452; https://doi.org/10.3390/molecules25194438.Suche in Google Scholar PubMed PubMed Central
12. Kumari, P., Mishra, V. S., Narayana, C., Khanna, A., Chakrabarty, A., Sagar, R. Design and efficient synthesis of pyrazoline and isoxazole bridged indole C-glycoside hybrids as potential anticancer agents. Sci. Rep. 2020, 10, 6660–6675; https://doi.org/10.1038/s41598-020-63377-x.Suche in Google Scholar PubMed PubMed Central
13. Li, A. L., Hao, Y., Wang, W. Y., Liu, Q. S., Sun, Y., Gu, W. Design, synthesis, and anticancer evaluation of novel indole derivatives of ursolic acid as potential topoisomerase II inhibitors. Int. J. Mol. Sci. 2020, 21, 2876–2895; https://doi.org/10.3390/ijms21082876.Suche in Google Scholar PubMed PubMed Central
14. Fu, Y., Zhang, D., Kang, T., Guo, Y. Y., Chen, W. G., Gao, S., Ye, F. Fragment splicing-based design, synthesis and safener activity of novel substituted phenyl oxazole derivatives. Bioorg. Med. Chem. Lett 2019, 29, 570–576; https://doi.org/10.1016/j.bmcl.2018.12.061.Suche in Google Scholar PubMed
15. Zhang, W., Zhang, Y., Feng, S.-H., Wang, J.-J. The crystal structure of 4-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 1155–1156; https://doi.org/10.1515/ncrs-2021-0256.Suche in Google Scholar
16. Almutairi, M. S., Ghabbour, H. A., Attia, M. I. Crystal structure of methyl 1H-indole-2-carboxylate, C10H9NO2. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 431–432; https://doi.org/10.1515/ncrs-2016-0303.Suche in Google Scholar
17. Wang, W. Z., Fan, N. T. Synthesis and structure of 4-(Dichloroacetyl)-1-oxa-4-azaspiro[4.5]decane. Chin. J. Struct. Chem. 2002, 21, 634–636.Suche in Google Scholar
18. Sivy, P., Koren, B., Valach, F., Krutosikova, A., Pavelcik, F. Structure of an indole derivative. Acta Crystallogr. C 1988, 44, 1855–1856; https://doi.org/10.1107/s0108270188006523.Suche in Google Scholar
© 2022 Zhao-Yu Ma et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

