Abstract
C22H27ClN2O3, orthorhombic, P212121 (no. 19), a = 8.6797(3) Å, b = 9.8809(3) Å, c = 24.7824(8) Å, V = 2125.42(12) Å3, Z = 4, R gt (F) = 0.0478, wR ref (F2) = 0.1082, T = 170 K.
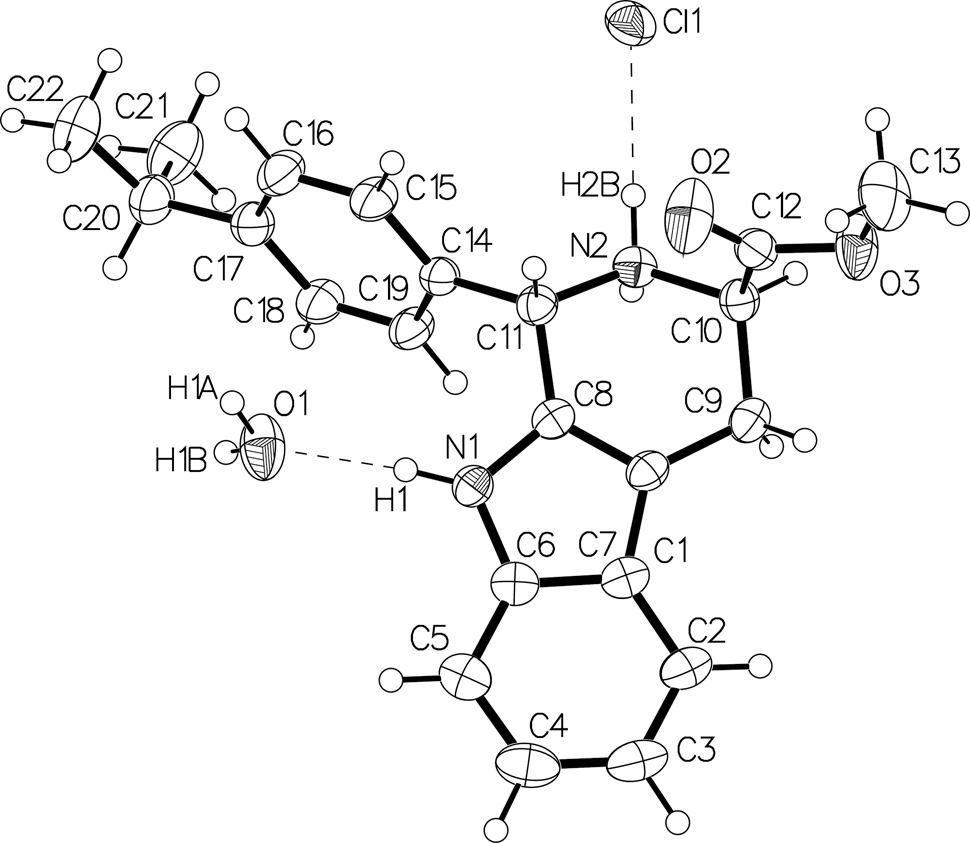
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.19 × 0.12 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.20 mm−1 |
| Diffractometer, scan mode: | D8 VENTURE, φ and ω |
| θmax, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 24639, 4345, 0.074 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3314 |
| N(param)refined: | 259 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.5727 (4) | 0.4381 (4) | 0.40848 (14) | 0.0297 (8) |
| C2 | 0.5681 (5) | 0.3964 (4) | 0.46242 (15) | 0.0372 (10) |
| H2 | 0.657926 | 0.400264 | 0.484247 | 0.045* |
| C3 | 0.4308 (6) | 0.3496 (4) | 0.48334 (17) | 0.0438 (11) |
| H3 | 0.426803 | 0.320925 | 0.519909 | 0.053* |
| C4 | 0.2971 (5) | 0.3434 (4) | 0.45167 (18) | 0.0451 (11) |
| H4 | 0.204387 | 0.310461 | 0.467203 | 0.054* |
| C5 | 0.2974 (5) | 0.3844 (4) | 0.39828 (16) | 0.0371 (10) |
| H5 | 0.206826 | 0.380409 | 0.376834 | 0.045* |
| C6 | 0.4360 (4) | 0.4315 (4) | 0.37734 (15) | 0.0302 (9) |
| C7 | 0.6904 (4) | 0.4907 (4) | 0.37379 (14) | 0.0282 (8) |
| C8 | 0.6232 (4) | 0.5120 (3) | 0.32466 (14) | 0.0264 (8) |
| C9 | 0.8587 (4) | 0.5133 (4) | 0.38437 (14) | 0.0324 (9) |
| H9A | 0.911517 | 0.424797 | 0.387332 | 0.039* |
| H9B | 0.871459 | 0.561587 | 0.419079 | 0.039* |
| C10 | 0.9328 (4) | 0.5963 (4) | 0.33900 (14) | 0.0284 (8) |
| H10 | 1.045464 | 0.575213 | 0.339044 | 0.034* |
| C11 | 0.6978 (4) | 0.5716 (4) | 0.27634 (14) | 0.0280 (8) |
| H11 | 0.673360 | 0.670473 | 0.275650 | 0.034* |
| C12 | 0.9162 (4) | 0.7469 (4) | 0.34691 (14) | 0.0311 (8) |
| C13 | 1.0128 (6) | 0.9353 (4) | 0.3941 (2) | 0.0607 (14) |
| H13A | 1.063084 | 0.982298 | 0.364050 | 0.091* |
| H13B | 1.068773 | 0.954057 | 0.427605 | 0.091* |
| H13C | 0.906330 | 0.967336 | 0.397521 | 0.091* |
| C14 | 0.6512 (4) | 0.5119 (4) | 0.22257 (14) | 0.0273 (8) |
| C15 | 0.6069 (5) | 0.5975 (4) | 0.18144 (15) | 0.0364 (10) |
| H15 | 0.609918 | 0.692631 | 0.186896 | 0.044* |
| C16 | 0.5576 (5) | 0.5461 (4) | 0.13199 (15) | 0.0418 (11) |
| H16 | 0.527547 | 0.606749 | 0.104170 | 0.050* |
| C17 | 0.5520 (5) | 0.4078 (4) | 0.12279 (15) | 0.0338 (9) |
| C18 | 0.5978 (5) | 0.3229 (4) | 0.16453 (16) | 0.0365 (9) |
| H18 | 0.596482 | 0.227767 | 0.159037 | 0.044* |
| C19 | 0.6454 (4) | 0.3734 (4) | 0.21387 (15) | 0.0336 (9) |
| H19 | 0.674287 | 0.312882 | 0.241891 | 0.040* |
| C20 | 0.4974 (5) | 0.3467 (4) | 0.06993 (15) | 0.0428 (10) |
| H20 | 0.416691 | 0.278213 | 0.079102 | 0.051* |
| C21 | 0.6281 (6) | 0.2705 (5) | 0.04117 (17) | 0.0572 (13) |
| H21A | 0.706663 | 0.335258 | 0.029290 | 0.086* |
| H21B | 0.586573 | 0.222530 | 0.009774 | 0.086* |
| H21C | 0.674537 | 0.205224 | 0.066105 | 0.086* |
| C22 | 0.4245 (6) | 0.4466 (5) | 0.03189 (17) | 0.0596 (14) |
| H22A | 0.337711 | 0.491159 | 0.049881 | 0.089* |
| H22B | 0.387448 | 0.399018 | −0.000288 | 0.089* |
| H22C | 0.500894 | 0.514624 | 0.021315 | 0.089* |
| N1 | 0.4704 (3) | 0.4756 (3) | 0.32606 (11) | 0.0283 (7) |
| H1 | 0.405566 | 0.479786 | 0.298803 | 0.034* |
| N2 | 0.8704 (3) | 0.5563 (3) | 0.28468 (11) | 0.0286 (7) |
| H2A | 0.895701 | 0.468152 | 0.278688 | 0.034* |
| H2B | 0.919335 | 0.606632 | 0.259151 | 0.034* |
| O2 | 0.8275 (4) | 0.8186 (3) | 0.32400 (13) | 0.0615 (10) |
| O3 | 1.0129 (4) | 0.7903 (3) | 0.38385 (12) | 0.0521 (8) |
| Cl1 | 1.02330 (11) | 0.75272 (11) | 0.20249 (4) | 0.0437 (3) |
| O1 | 0.2293 (4) | 0.5255 (3) | 0.25344 (12) | 0.0512 (8) |
| H1A | 0.198073 | 0.597013 | 0.236153 | 0.077* |
| H1B | 0.223748 | 0.455323 | 0.232290 | 0.077* |
Source of material
The reaction substrate D-Tryptophan methyl ester hydrochloride (10 mmol) was dissolved evenly in methanol (40 mL) with stirring, then 4-isopropylbenzaldehyde (20 mmol) was added and refluxed for 12 h. After the reaction was completed (monitored by TLC), the mixture was concentrated under vacuum and then purified by silica gel column chromatography with dichloromethane and ethyl acetate (v/v = 3/1) as the eluent. The product was light-yellow solid with a yield of 52%. The single crystal configuration of the title compound was obtained by recrystallization using the solvent volatilization method in four days.
Experimental details
All hydrogen atoms were added in their geometrically idealized positions and refined by the riding models on their parent atoms with Uiso = 1.2 Ueq. The anomalous scattering determined the absolute configuration of the title compound (Flack parameter 0.05(5)).
Comment
Tryptophan, also known as β-Indolyl alanine, whose molecular configuration is similar to 3-Indoleacetic acid, is a significant neurotransmitter in the human body as the precursor of serotonin [5]. It is also an essential precursor of auxin biosynthesis in plants [6]. It can be used as a nutritional supplement for pregnant women and young children. As a tranquilizer, it can adjust the mental rhythm and promote sleep. In addition, tryptophan scaffolds are widely found in bioactive natural products, including halogenated and non-halogenated indoles. Modification of these indoles may lead to novel pharmaceutically active compounds [7]. Some derivatives are commonly used to design antitumor and anti-inflammatory compounds [8, 9]. This paper reports a novel crystal structure of a tryptophan derivative, which formed a 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole skeleton through the ring-closing reaction. It has significant potential application for related drugs and drug intermediates, and similar structures have been reported abundantly [10], [11], [12], [13], [14], [15], [16], [17], [18], [19].
The molecule structure of the title compound is shown in the figure consisting of the protonated target molecule, a couter chlorid anion and a water molecule. The amino group on indole participates in the closed-loop to form a hexatomic ring, similar to the reported systems [10], [11], [12], whose one hydrogen atom is replaced by a cumene group, and the length of the C11–C14 bond is 1.512(6) Å. The isopropyl-phenyl ring plane on the cumene group has obvious torsion with the indole plane, and the dihedral angle is 73.17°. There are two hydrogen bonds formed between a bonded water molecule and the organic molecules in the title crystal. The hydrogen bond N1–H1⃛O1 is between the amino group on indole and the water molecule with bond length 1.952(4) Å and bond angle 162.6(3)°. The other hydrogen bond O1–H1B⃛O2 is between the water molecule and the ester group with bond length 1.992(4) Å and bond angle 166.9(3)°. In addition, the chloride anion Cl1 stabilizes the structure with two hydrogen bonds O1–H1A⃛Cl1 with bond length 2.3161(11) Å and bond angle 157.1(3)° and N2–H2B⃛Cl1 with bond length 2.2067(11) Å and bond angle 172.22(19)°. The results illustrate that the forces of these hydrogen bonds are the main factor in stabilizing the crystal structure.
Funding source: Key Scientific Research Projects of Colleges and Universities in Henan Province http://dx.doi.org/10.13039/501100013066
Award Identifier / Grant number: 22A430032
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by the Key Scientific Research Projects of Colleges and Universities in Henan Province (22A430032).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SMART APEX-II CCD; Bruker AXS Inc.: Madison, WI, USA, 2006.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Höglund, E., Øverli, Ø., Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front. Endocrinol. 2019, 10, 158.10.3389/fendo.2019.00158Suche in Google Scholar PubMed PubMed Central
6. Mustafa, A., Imran, M., Ashraf, M., Mahmood, K. Perspectives of using l-tryptophan for improving productivity of agricultural crops: a review. Pedosphere 2018, 28, 16–34; https://doi.org/10.1016/s1002-0160(18)60002-5.Suche in Google Scholar
7. Gruß, H., Sewald, N. Late-stage diversification of tryptophan-derived biomolecules. Chem. Eur J. 2020, 26, 5328–5340; https://doi.org/10.1002/chem.201903756.Suche in Google Scholar PubMed PubMed Central
8. Meng, D., Sommella, E., Salviati, E., Campiglia, P., Ganguli, K., Djebali, K., Zhu, W., Walker, W. A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217; https://doi.org/10.1038/s41390-019-0740-x.Suche in Google Scholar PubMed PubMed Central
9. Schramme, F., Crosignani, S., Frederix, K., Hoffmann, D., Pilotte, L., Stroobant, V., Preillon, J., Driessens, G., Van den Eynde, B. J. Inhibition of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol. Res. 2020, 8, 32–45; https://doi.org/10.1158/2326-6066.cir-19-0041.Suche in Google Scholar
10. Mondal, A., Chowdhury, C. Palladium-catalyzed synthesis of 1-vinyltetrahydro-β-carbolines and aza-spiroindolenines: access to the syntheses of 1-vinyl-β-carbolines and Eudistomins Y1 and Y2. J. Org. Chem. 2021, 86, 3810–3825; https://doi.org/10.1021/acs.joc.0c02651.Suche in Google Scholar PubMed
11. Liang, L., Zhou, S., Zhang, W., Tong, R. Catalytic asymmetric alkynylation of 3,4-dihydro-β-carbolinium ions enables collective total syntheses of indole alkaloids. Angew. Chem. Int. Ed. 2021, 60, 25135–25142; https://doi.org/10.1002/anie.202112383.Suche in Google Scholar PubMed
12. Gurram, R., Nanubolu, J. B., Menon, R. S. Rapid synthesis of azepinoindole derivatives from tryptamine sulfonamides and bromoallyl sulfones via an acid-mediated cyclization and rearrangement. Chem. Commun. 2021, 57, 635–638; https://doi.org/10.1039/d0cc06991d.Suche in Google Scholar PubMed
13. Chan, Y.-C., Sak, M. H., Frank, S. A., Miller, S. J. Tunable and cooperative catalysis for enantioselective Pictet–Spengler reaction with varied nitrogen-containing heterocyclic carboxaldehydes. Angew. Chem. Int. Ed. 2021, 60, 24573–24581; https://doi.org/10.1002/anie.202109694.Suche in Google Scholar PubMed PubMed Central
14. Alvarez-Rodríguez, N. V., Islas-Jácome, A., Rentería-Gómez, A., Cárdenas-Galindo, L. E., Unnamatla, M. V. B., Gámez-Montaño, R. Synthesis of 1’-tetrazolylmethyl-spiro[pyrrolidine-3,3’-oxindoles] via two coupled one-pot processes Ugi-azide/Pictet–Spengler and oxidative spiro-rearrangement. New J. Chem. 2018, 42, 1600–1603.10.1039/C7NJ03829ASuche in Google Scholar
15. Chauhan, J., Luthra, T., Gundla, R., Ferraro, A., Holzgrabe, U., Sen, S. A diversity oriented synthesis of natural product inspired molecular libraries. Org. Biomol. Chem. 2017, 15, 9108–9120; https://doi.org/10.1039/c7ob02230a.Suche in Google Scholar PubMed
16. Drew, M. A., Arndt, S., Richardson, C., Rudolph, M., Hashmi, A. S. K., Hyland, C. J. T. Divergent gold-catalysed reactions of cyclopropenylmethyl sulfonamides with tethered heteroaromatics. Chem. Commun. 2019, 55, 13971–13974; https://doi.org/10.1039/c9cc06241f.Suche in Google Scholar PubMed
17. Lynch-Colameta, T., Greta, S., Snyder, S. A. Synthesis of aza-quaternary centers via Pictet–Spengler reactions of ketonitrones. Chem. Sci. 2021, 12, 6181–6187.10.1039/D1SC00882JSuche in Google Scholar PubMed PubMed Central
18. Xia, Q., Li, Y., Cheng, L., Liang, X., Cao, C., Dai, P., Deng, H., Zhang, W., Wang, Q. Electron donor–acceptor complex-initiated photochemical cyanation for the preparation of α-amino nitriles. Org. Lett. 2020, 22, 9638–9643; https://doi.org/10.1021/acs.orglett.0c03703.Suche in Google Scholar PubMed
19. Colucci, W. J., Tilstra, L., Sattler, M. C., Fronczek, F. R., Barkley, M. D. Conformational studies of a constrained tryptophan derivative: implications for the fluorescence quenching mechanism. J. Am. Chem. Soc. 1990, 112, 9182–9190; https://doi.org/10.1021/ja00181a022.Suche in Google Scholar
© 2022 Jingxiao Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

