Abstract
C19H15ClFeO, triclinic,
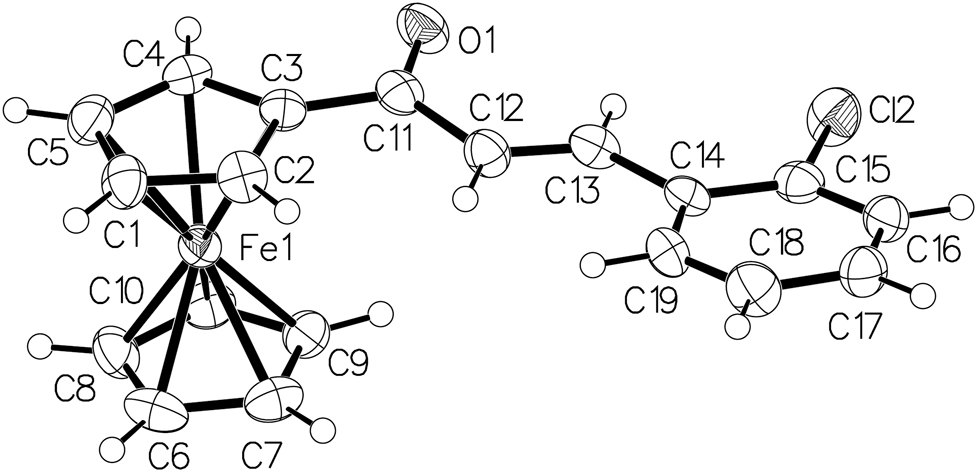
One of the three crystallographically independent molecules of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.14 × 0.11 × 0.06 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.15 mm−1 |
| Diffractometer, scan mode: | D8 VENTURE, φ and ω |
| θ max, completeness: | 26.4°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 26,628, 9450, 0.075 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 5797 |
| N(param)refined: | 596 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 1.0155 (4) | 0.4610 (4) | 0.6198 (2) | 0.0373 (11) |
| H1 | 0.960078 | 0.462717 | 0.588888 | 0.045* |
| C2 | 1.0073 (4) | 0.5422 (4) | 0.66520 (19) | 0.0328 (10) |
| H2 | 0.945593 | 0.607692 | 0.670343 | 0.039* |
| C3 | 1.1095 (4) | 0.5077 (4) | 0.70243 (19) | 0.0281 (9) |
| C4 | 1.1787 (4) | 0.4048 (4) | 0.67834 (19) | 0.0311 (10) |
| H4 | 1.251413 | 0.362770 | 0.693579 | 0.037* |
| C5 | 1.1198 (4) | 0.3768 (4) | 0.6279 (2) | 0.0377 (11) |
| H5 | 1.146038 | 0.312180 | 0.603505 | 0.045* |
| C6 | 0.7975 (4) | 0.2952 (4) | 0.7341 (2) | 0.0452 (12) |
| H6 | 0.739908 | 0.302203 | 0.703906 | 0.054* |
| C7 | 0.7985 (4) | 0.3716 (4) | 0.7808 (2) | 0.0405 (11) |
| H7 | 0.740970 | 0.439368 | 0.787733 | 0.049* |
| C8 | 0.8982 (4) | 0.2058 (4) | 0.7405 (2) | 0.0408 (12) |
| H8 | 0.919446 | 0.141657 | 0.715442 | 0.049* |
| C9 | 0.8997 (4) | 0.3306 (4) | 0.81551 (19) | 0.0347 (11) |
| H9 | 0.922170 | 0.365818 | 0.849766 | 0.042* |
| C10 | 0.9613 (4) | 0.2281 (4) | 0.7903 (2) | 0.0359 (11) |
| H10 | 1.032992 | 0.182115 | 0.804459 | 0.043* |
| C11 | 1.1288 (4) | 0.5564 (4) | 0.7604 (2) | 0.0313 (10) |
| C12 | 1.0310 (4) | 0.6468 (4) | 0.7865 (2) | 0.0326 (10) |
| H12 | 0.961178 | 0.672929 | 0.764049 | 0.039* |
| C13 | 1.0368 (4) | 0.6930 (4) | 0.8400 (2) | 0.0333 (10) |
| H13 | 1.102249 | 0.660014 | 0.863935 | 0.040* |
| C14 | 0.9513 (4) | 0.7905 (4) | 0.8655 (2) | 0.0304 (10) |
| C15 | 0.9658 (4) | 0.8380 (4) | 0.92081 (19) | 0.0326 (10) |
| C16 | 0.8944 (4) | 0.9384 (4) | 0.9400 (2) | 0.0357 (11) |
| H16 | 0.907633 | 0.969031 | 0.977684 | 0.043* |
| C17 | 0.8042 (4) | 0.9941 (4) | 0.9046 (2) | 0.0379 (11) |
| H17 | 0.755690 | 1.063612 | 0.917485 | 0.045* |
| C18 | 0.7845 (4) | 0.9485 (4) | 0.8502 (2) | 0.0438 (12) |
| H18 | 0.721330 | 0.986000 | 0.825976 | 0.053* |
| C19 | 0.8561 (4) | 0.8492 (4) | 0.8313 (2) | 0.0355 (11) |
| H19 | 0.841250 | 0.818792 | 0.793790 | 0.043* |
| Cl2 | 1.07745 (11) | 0.77116 (11) | 0.96854 (5) | 0.0466 (3) |
| Fe1 | 0.97948 (6) | 0.37285 (5) | 0.71595 (3) | 0.02744 (16) |
| O1 | 1.2249 (3) | 0.5230 (3) | 0.78557 (14) | 0.0403 (8) |
| C20 | 0.9865 (4) | 1.1423 (4) | 0.54650 (19) | 0.0285 (10) |
| C21 | 1.1182 (4) | 1.1056 (4) | 0.5347 (2) | 0.0370 (11) |
| H21 | 1.183095 | 1.153643 | 0.502557 | 0.044* |
| C22 | 1.1568 (4) | 0.9992 (4) | 0.5695 (2) | 0.0430 (12) |
| H22 | 1.247855 | 0.973656 | 0.561463 | 0.052* |
| C23 | 1.0610 (4) | 0.9299 (4) | 0.6164 (2) | 0.0412 (12) |
| H23 | 1.086541 | 0.857123 | 0.641055 | 0.049* |
| C24 | 0.9295 (4) | 0.9668 (4) | 0.6270 (2) | 0.0348 (10) |
| H24 | 0.864572 | 0.916814 | 0.657899 | 0.042* |
| C25 | 0.8876 (4) | 1.0757 (4) | 0.59371 (19) | 0.0282 (10) |
| C26 | 0.7478 (4) | 1.1173 (4) | 0.60747 (19) | 0.0302 (10) |
| H26 | 0.723246 | 1.182520 | 0.577036 | 0.036* |
| C27 | 0.6534 (4) | 1.0723 (4) | 0.65834 (19) | 0.0312 (10) |
| H27 | 0.675838 | 1.008159 | 0.689980 | 0.037* |
| C28 | 0.5135 (4) | 1.1179 (4) | 0.66806 (19) | 0.0277 (9) |
| C29 | 0.4128 (4) | 1.0457 (3) | 0.71899 (19) | 0.0266 (9) |
| C30 | 0.2730 (4) | 1.0696 (4) | 0.73013 (19) | 0.0302 (10) |
| H30 | 0.229766 | 1.132473 | 0.705908 | 0.036* |
| C31 | 0.2100 (4) | 0.9835 (4) | 0.7834 (2) | 0.0414 (12) |
| H31 | 0.116440 | 0.978203 | 0.801212 | 0.050* |
| C32 | 0.4358 (4) | 0.9435 (4) | 0.76660 (19) | 0.0334 (10) |
| H32 | 0.519906 | 0.907585 | 0.770895 | 0.040* |
| C33 | 0.3085 (4) | 0.9064 (4) | 0.8061 (2) | 0.0449 (12) |
| H33 | 0.292546 | 0.840643 | 0.841696 | 0.054* |
| C34 | 0.3406 (7) | 1.0847 (6) | 0.9093 (3) | 0.084 (2) |
| H34 | 0.330805 | 1.016347 | 0.943366 | 0.100* |
| C35 | 0.4615 (6) | 1.1296 (8) | 0.8686 (4) | 0.099 (3) |
| H35 | 0.548392 | 1.097618 | 0.870448 | 0.118* |
| C36 | 0.4308 (6) | 1.2304 (6) | 0.8247 (3) | 0.079 (2) |
| H36 | 0.493395 | 1.278366 | 0.791218 | 0.094* |
| C37 | 0.2935 (5) | 1.2477 (4) | 0.8386 (2) | 0.0506 (13) |
| H37 | 0.245483 | 1.309811 | 0.816460 | 0.061* |
| C38 | 0.2378 (5) | 1.1580 (4) | 0.8908 (2) | 0.0532 (14) |
| H38 | 0.145249 | 1.148599 | 0.910401 | 0.064* |
| Cl3 | 0.94509 (10) | 1.27827 (10) | 0.50079 (5) | 0.0379 (3) |
| Fe3 | 0.34247 (6) | 1.08104 (6) | 0.81255 (3) | 0.03316 (17) |
| O3 | 0.4824 (3) | 1.2107 (3) | 0.63423 (14) | 0.0410 (8) |
| C39 | 0.5045 (5) | 0.8046 (5) | 0.5223 (3) | 0.0551 (15) |
| H39 | 0.564827 | 0.822707 | 0.480342 | 0.066* |
| C40 | 0.5044 (5) | 0.8554 (4) | 0.5770 (3) | 0.0540 (14) |
| H40 | 0.564738 | 0.914693 | 0.579522 | 0.065* |
| C41 | 0.4022 (5) | 0.8065 (4) | 0.6284 (2) | 0.0503 (14) |
| H41 | 0.379970 | 0.826266 | 0.672050 | 0.060* |
| C42 | 0.3371 (4) | 0.7224 (4) | 0.6043 (3) | 0.0509 (14) |
| H42 | 0.263011 | 0.674663 | 0.628543 | 0.061* |
| C43 | 0.4027 (5) | 0.7225 (5) | 0.5376 (3) | 0.0522 (14) |
| H43 | 0.380916 | 0.674609 | 0.508220 | 0.063* |
| C44 | 0.6925 (4) | 0.5675 (4) | 0.5647 (2) | 0.0432 (12) |
| H44 | 0.739090 | 0.568845 | 0.519796 | 0.052* |
| C45 | 0.5810 (5) | 0.4954 (4) | 0.5981 (2) | 0.0422 (12) |
| H45 | 0.539773 | 0.440223 | 0.579333 | 0.051* |
| C46 | 0.7221 (4) | 0.6369 (4) | 0.60951 (19) | 0.0351 (11) |
| H46 | 0.791959 | 0.693528 | 0.600000 | 0.042* |
| C47 | 0.6293 (4) | 0.6075 (4) | 0.67183 (19) | 0.0268 (9) |
| C48 | 0.5417 (4) | 0.5193 (4) | 0.6639 (2) | 0.0327 (10) |
| H48 | 0.469530 | 0.483114 | 0.697084 | 0.039* |
| C49 | 0.6258 (4) | 0.6647 (4) | 0.73057 (19) | 0.0268 (9) |
| C50 | 0.5211 (4) | 0.6247 (3) | 0.79105 (19) | 0.0269 (9) |
| H50 | 0.448838 | 0.579188 | 0.787123 | 0.032* |
| C51 | 0.5247 (4) | 0.6498 (3) | 0.85000 (19) | 0.0299 (10) |
| H51 | 0.594528 | 0.700316 | 0.851886 | 0.036* |
| C52 | 0.4309 (4) | 0.6072 (4) | 0.91309 (19) | 0.0288 (10) |
| C53 | 0.3495 (4) | 0.5080 (4) | 0.9195 (2) | 0.0352 (11) |
| H53 | 0.352028 | 0.470118 | 0.881845 | 0.042* |
| C54 | 0.2655 (4) | 0.4644 (4) | 0.9796 (2) | 0.0416 (12) |
| H54 | 0.210028 | 0.397868 | 0.982867 | 0.050* |
| C55 | 0.2622 (4) | 0.5173 (4) | 1.0349 (2) | 0.0426 (12) |
| H55 | 0.205722 | 0.485907 | 1.076325 | 0.051* |
| C56 | 0.3398 (4) | 0.6149 (4) | 1.0305 (2) | 0.0404 (11) |
| H56 | 0.336586 | 0.651961 | 1.068528 | 0.048* |
| C57 | 0.4229 (4) | 0.6589 (4) | 0.97017 (19) | 0.0314 (10) |
| Cl1 | 0.51792 (13) | 0.78416 (11) | 0.96709 (6) | 0.0520 (3) |
| Fe2 | 0.53173 (6) | 0.67378 (5) | 0.59881 (3) | 0.02652 (15) |
| O2 | 0.7091 (3) | 0.7394 (3) | 0.73009 (13) | 0.0400 (8) |
Source of material
A mixed solvent system comprising acetylferrocene (10 mmol) and 2-(4-chlorophenyl)acetaldehyde (10 mmol) were added to a flask. Then ethanol (25 mL) was added and stirred at room temperature for 20 min until all the solids are dissolved. To this solution 10 mL KOH (20%) was added and continue stirring until all raw materials disappear (monitored by thin layer chromatography). The reaction products were translated into 50 mL water with some solids separated out. The solids were obtained by suction filtration and washed with 30% ethanol aqueous solution. The single crystal of the title compound was obtained within seven days at room temperature by recrystallization.
Experimental details
All hydrogen atoms were placed in theoretical positions and refined as riding atoms. Their U iso values were set to 1.2U eq of the parent atoms.
Comment
Ferrocene is known as a widely applicable organometallic framework structure for the development of various functional derivatives that are applicated in drug design, catalysis, material science, agriculture, and electrochemistry [5], [6], [7]. The wide application of ferrocene derivatives is attributable to the unique properties of ferrocene scaffold, such as native excellent air stability, solubility, thermal, and photochemical stability [8, 9]. Substituting the benzene ring of a bioactive molecule by ferrocene group will dramatically change hydrophobicity, lipophilicity, solubility, and stability [10]. Various ferrocene derivatives were designed to exhibit a wide range of biological properties, like anti-cancer [11], anti-oxidant [12], anti-HIV, analgesic, anti-infective [13], anti-viral [14], anti-microbial [15], anti-convulsant effects [5, 16]. Therefore, in this work, one ferrocene derivative, i.e., (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, was synthesized and crystallized.
As displayed in the figure, the title compound adopts the s-trans configuration in all three crystallographically independent molecules. The bulky substituent is incorporated at C3 position of ferrocene unit, which makes the bond distances of C2–C3 (1.442(7) Å) and C3–C4 (1.431(7) Å) more elongate. Other bond length in the ferrocenyl group ranges from 1.404(7) Å to 1.418(6) Å. Compared with 3-ferrocenylprop-2-enal, the introducing of 2-chlorophenyl leads to bond lengths of C–C and C=C closer, which are closer to the typical single and double bonds [17]. However, similar to 3-ferrocenylprop-2-enal, the crystal structure of the title compound is also stabilized mainly by intermolecular C–H···O interactions [17].
Funding source: Key Scientific Research Projects of Colleges and Universities in Henan Province http://dx.doi.org/10.13039/501100013066
Award Identifier / Grant number: 22A430032
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by the Key Scientific Research Projects of Colleges and Universities in Henan Province (22A430032).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. Smart Apex-II CCD; Bruker AXS Inc.: Madison, WI, USA, 2006.Suche in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Suche in Google Scholar PubMed PubMed Central
5. Larik, F. A., Saeed, A., Fattah, T. A., Muqadar, U., Channar, P. A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives. Appl. Organomet. Chem. 2017, 31, e3664; https://doi.org/10.1002/aoc.3664.Suche in Google Scholar
6. Gao, D.-W., Yin, Q., Gu, Q., You, S.-L. Enantioselective synthesis of planar chiral ferrocenes via Pd(0)-catalyzed intramolecular direct C–H bond arylation. J. Am. Chem. Soc. 2014, 136, 4841–4844; https://doi.org/10.1021/ja500444v.Suche in Google Scholar PubMed
7. Tong, R., Zhao, Y., Wang, L., Yu, H., Ren, F., Saleem, M., Amer, W. A. Recent research progress in the synthesis and properties of burning rate catalysts based on ferrocene-containing polymers and derivatives. J. Organomet. Chem. 2014, 755, 16–32; https://doi.org/10.1016/j.jorganchem.2013.12.052.Suche in Google Scholar
8. Lal, B., Badshah, A., Altaf, A. A., Khan, N., Ullah, S. Miscellaneous applications of ferrocene-based peptides/amides. Appl. Organomet. Chem. 2011, 25, 843–855; https://doi.org/10.1002/aoc.1843.Suche in Google Scholar
9. Ebadi-Dehaghani, H., Mehdipour-Ataei, S. Novel ferrocene-based organometallic poly(ether sulfone amide imide)s: preparation, characterization, and properties. J. Inorg. Organomet. Polym. Mater. 2012, 22, 223–234.10.1007/s10904-011-9555-xSuche in Google Scholar
10. Ferreira, C. L., Ewart, C. B., Barta, C. A., Little, S., Yardley, V., Martins, C., Polishchuk, E., Smith, P. J., Moss, J. R., Merkel, M., Adam, M. J., Orvig, C. Synthesis, structure, and biological activity of ferrocenyl carbohydrate conjugates. Inorg. Chem. 2006, 45, 8414–8422; https://doi.org/10.1021/ic061166p.Suche in Google Scholar PubMed
11. Ornelas, C. Application of ferrocene and its derivatives in cancer research. New J. Chem. 2011, 35, 1973–1985; https://doi.org/10.1039/c1nj20172g.Suche in Google Scholar
12. Khelef, A., Lanez, T. In vitro assays of the antioxidant activities of ferrocene derivatives bearing amine, amide or hydrazine groups. Der Pharma Chem. 2015, 7, 318–323.Suche in Google Scholar
13. Ludwig, B. S., Correia, J. D. G., Kühn, F. E. Ferrocene derivatives as anti-infective agents. Coord. Chem. Rev. 2019, 396, 22–48; https://doi.org/10.1016/j.ccr.2019.06.004.Suche in Google Scholar
14. Asghar, F., Badshah, A., Lal, B., Butler, I. S., Tabassum, S., Tahir, M. N. Bioactivity of new ferrocene incorporated N,N′- disubstituted ureas: synthesis, structural elucidation and DFT study. Inorg. Chim. Acta. 2016, 439, 82–91; https://doi.org/10.1016/j.ica.2015.10.007.Suche in Google Scholar
15. Yagnam, S., Trivedi, R., Krishna, S., Giribabu, L., Praveena, G., Prakasham, R. S. Bioactive isatin (oxime)-triazole-thiazolidinedione ferrocene molecular conjugates: design, synthesis and antimicrobial activities. J. Organomet. Chem. 2021, 937, 121716; https://doi.org/10.1016/j.jorganchem.2021.121716.Suche in Google Scholar
16. Fouda, M. F. R., Abd-Elzaher, M. M., Abdelsamaia, R. A., Labib, A. A. On the medicinal chemistry of ferrocene. Appl. Organomet. Chem. 2007, 21, 613–625; https://doi.org/10.1002/aoc.1202.Suche in Google Scholar
17. Imhof, W. 3-Ferrocenyl prop-2-enal. Acta Crystallogr. 2004, E60, m1234–m1236; https://doi.org/10.1107/s1600536804019129.Suche in Google Scholar
© 2022 Lilei Zhang and Mengru Xie, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

