Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
Abstract
C36H32ClN3O7, orthorhombic, P212121 (no. 19), a = 11.4608(5) Å, b = 12.1489(5) Å, c = 23.1684(7) Å, V = 3225.9(2) Å3, Z = 4, Rgt(F) = 0.0588, wRref(F2) = 0.1546, T = 293 K.
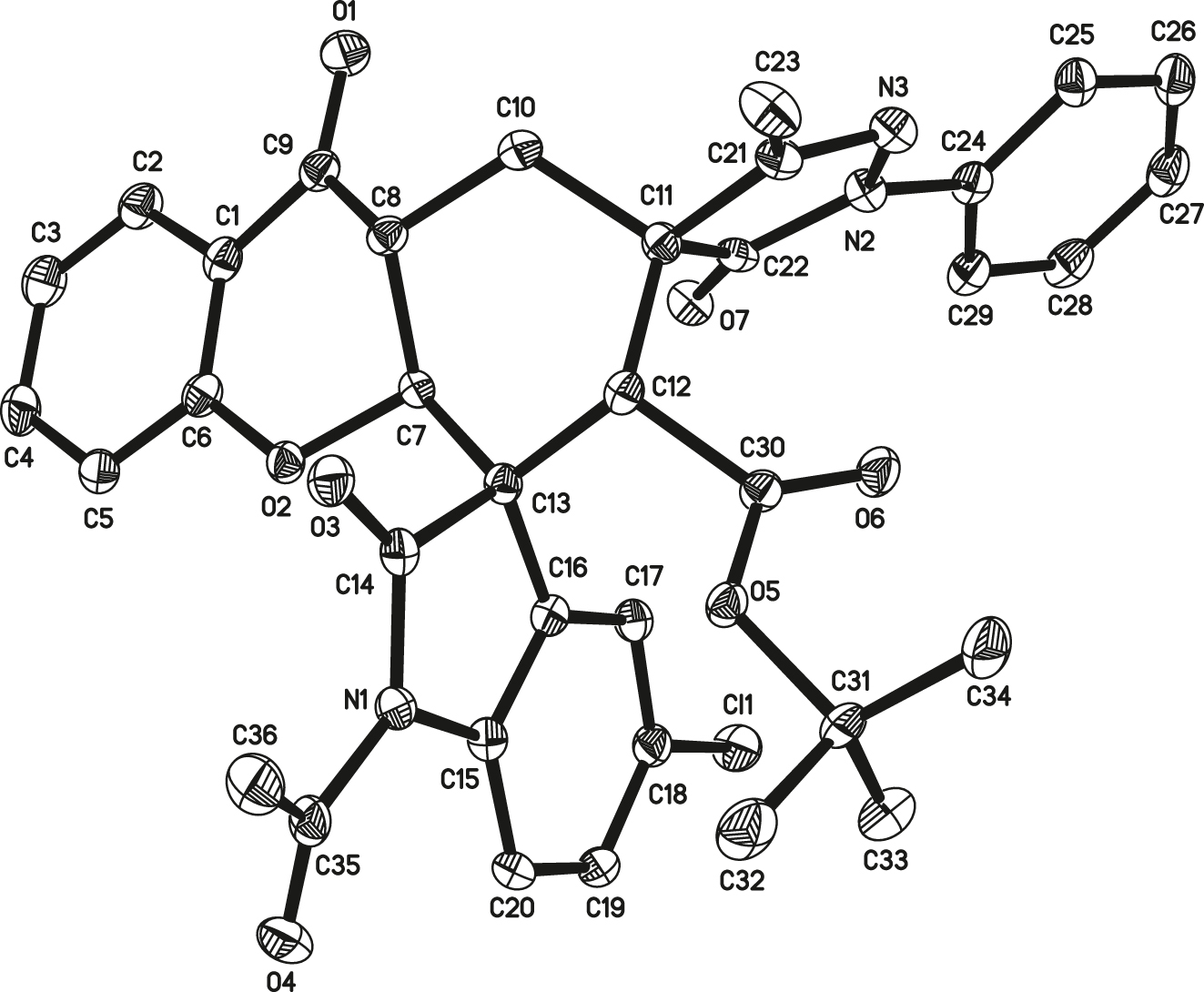
The molecular structure is shown in Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.11 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.51 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 74.3°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11,377, 5861, 0.035 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5154 |
| N(param)refined: | 429 |
| Programs: | SHELX [1], Bruker [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2794(5) | 0.4550(5) | 0.8815(2) | 0.0282(12) |
| C2 | 0.2686(5) | 0.5156(5) | 0.9330(2) | 0.0342(13) |
| H2 | 0.329590 | 0.560107 | 0.945511 | 0.041* |
| C3 | 0.1677(6) | 0.5088(6) | 0.9647(2) | 0.0410(15) |
| H3 | 0.159948 | 0.550031 | 0.998264 | 0.049* |
| C4 | 0.0769(5) | 0.4405(6) | 0.9470(2) | 0.0378(14) |
| H4 | 0.010625 | 0.433793 | 0.969845 | 0.045* |
| C5 | 0.0841(5) | 0.3824(5) | 0.8956(2) | 0.0308(12) |
| H5 | 0.022429 | 0.338770 | 0.883084 | 0.037* |
| C6 | 0.1858(5) | 0.3909(5) | 0.8633(2) | 0.0275(11) |
| C7 | 0.2816(4) | 0.3646(4) | 0.7739(2) | 0.0226(11) |
| H7 | 0.267463 | 0.440940 | 0.762147 | 0.027* |
| C8 | 0.3984(4) | 0.3598(4) | 0.8047(2) | 0.0239(11) |
| H8 | 0.403738 | 0.287983 | 0.823844 | 0.029* |
| C9 | 0.3928(5) | 0.4472(5) | 0.8516(2) | 0.0278(11) |
| C10 | 0.5056(5) | 0.3717(5) | 0.7656(2) | 0.0277(11) |
| H10A | 0.559169 | 0.312081 | 0.774155 | 0.033* |
| H10B | 0.544678 | 0.440105 | 0.775168 | 0.033* |
| C11 | 0.4796(4) | 0.3710(4) | 0.6996(2) | 0.0236(11) |
| C12 | 0.3868(4) | 0.2825(4) | 0.6868(2) | 0.0225(11) |
| H12 | 0.421713 | 0.212829 | 0.699179 | 0.027* |
| C13 | 0.2694(4) | 0.2911(4) | 0.7204(2) | 0.0220(10) |
| C14 | 0.2391(5) | 0.1713(5) | 0.7387(2) | 0.0252(11) |
| C15 | 0.0813(4) | 0.2389(4) | 0.6875(2) | 0.0238(10) |
| C16 | 0.1621(4) | 0.3225(4) | 0.6870(2) | 0.0244(11) |
| C17 | 0.1366(5) | 0.4232(5) | 0.6606(2) | 0.0265(11) |
| H17 | 0.190097 | 0.480696 | 0.660424 | 0.032* |
| C18 | 0.0288(5) | 0.4335(5) | 0.6347(2) | 0.0297(12) |
| C19 | −0.0512(5) | 0.3486(5) | 0.6331(2) | 0.0308(12) |
| H19 | −0.122251 | 0.358230 | 0.614380 | 0.037* |
| C20 | −0.0255(5) | 0.2480(5) | 0.6597(2) | 0.0293(12) |
| H20 | −0.077996 | 0.189599 | 0.658831 | 0.035* |
| C21 | 0.5894(4) | 0.3482(5) | 0.6662(2) | 0.0263(11) |
| C22 | 0.4518(4) | 0.4870(4) | 0.6776(2) | 0.0247(11) |
| C23 | 0.6564(5) | 0.2432(5) | 0.6687(3) | 0.0363(14) |
| H23A | 0.719699 | 0.246013 | 0.641559 | 0.055* |
| H23B | 0.605858 | 0.182851 | 0.659173 | 0.055* |
| H23C | 0.686938 | 0.233016 | 0.706900 | 0.055* |
| C24 | 0.5480(5) | 0.6067(4) | 0.6028(2) | 0.0266(11) |
| C25 | 0.6561(5) | 0.6313(5) | 0.5790(2) | 0.0319(13) |
| H25 | 0.720847 | 0.587944 | 0.587213 | 0.038* |
| C26 | 0.6659(6) | 0.7218(5) | 0.5427(2) | 0.0385(15) |
| H26 | 0.737748 | 0.738762 | 0.526347 | 0.046* |
| C27 | 0.5705(6) | 0.7866(5) | 0.5306(2) | 0.0402(15) |
| H27 | 0.578179 | 0.846566 | 0.505969 | 0.048* |
| C28 | 0.4637(6) | 0.7631(5) | 0.5549(3) | 0.0410(15) |
| H28 | 0.399698 | 0.807588 | 0.546869 | 0.049* |
| C29 | 0.4512(5) | 0.6716(5) | 0.5917(2) | 0.0338(13) |
| H29 | 0.379365 | 0.655189 | 0.608300 | 0.041* |
| C30 | 0.3621(5) | 0.2683(4) | 0.6226(2) | 0.0244(11) |
| C31 | 0.2764(5) | 0.1299(5) | 0.5567(2) | 0.0309(13) |
| C32 | 0.2283(7) | 0.0171(6) | 0.5721(3) | 0.0450(16) |
| H32A | 0.286159 | −0.023717 | 0.592997 | 0.068* |
| H32B | 0.208421 | −0.021689 | 0.537332 | 0.068* |
| H32C | 0.159826 | 0.025518 | 0.595537 | 0.068* |
| C33 | 0.1837(6) | 0.2035(6) | 0.5300(3) | 0.0422(15) |
| H33A | 0.117856 | 0.208175 | 0.555525 | 0.063* |
| H33B | 0.159334 | 0.173073 | 0.493729 | 0.063* |
| H33C | 0.215358 | 0.275727 | 0.523943 | 0.063* |
| C34 | 0.3847(6) | 0.1202(6) | 0.5199(2) | 0.0441(16) |
| H34A | 0.409591 | 0.192216 | 0.508167 | 0.066* |
| H34B | 0.367734 | 0.076592 | 0.486403 | 0.066* |
| H34C | 0.445624 | 0.085562 | 0.541826 | 0.066* |
| C35 | 0.0680(5) | 0.0449(5) | 0.7250(2) | 0.0321(13) |
| C36 | 0.1404(6) | −0.0537(5) | 0.7378(3) | 0.0436(15) |
| H36A | 0.173319 | −0.046966 | 0.775736 | 0.065* |
| H36B | 0.202048 | −0.059320 | 0.709899 | 0.065* |
| H36C | 0.092595 | −0.118522 | 0.736037 | 0.065* |
| O1 | 0.4761(4) | 0.5057(3) | 0.86414(16) | 0.0363(9) |
| O2 | 0.1892(3) | 0.3315(3) | 0.81252(14) | 0.0257(8) |
| O3 | 0.3027(3) | 0.1105(3) | 0.76560(16) | 0.0310(9) |
| O4 | −0.0364(4) | 0.0428(4) | 0.71914(19) | 0.0448(11) |
| O5 | 0.3083(3) | 0.1728(3) | 0.61473(14) | 0.0256(8) |
| O6 | 0.3864(4) | 0.3352(3) | 0.58597(15) | 0.0358(10) |
| O7 | 0.3726(3) | 0.5478(3) | 0.69324(16) | 0.0310(9) |
| N1 | 0.1276(4) | 0.1462(4) | 0.71786(18) | 0.0253(9) |
| N2 | 0.5377(4) | 0.5122(4) | 0.63875(18) | 0.0239(9) |
| N3 | 0.6207(4) | 0.4275(4) | 0.63294(19) | 0.0268(10) |
| Cl1 | −0.00963(13) | 0.56086(13) | 0.60524(6) | 0.0416(4) |
Source of material
To a mixture of 3-methyl-4-((4-oxo-4H-chromen-3-yl)methyl)-1-phenyl-1H-pyrazol-5(4H)-one (0.1 mmol) and quinine-derived thiourea (20 mol%) in 1.0 mL of freshly distilled Et2O was added tert-butyl 2-(1-acetyl-5-chloro-2-oxoindolin-3-ylidene)acetate. The reaction mixture was stirred at room temperature for 4 days and the progress of the reaction was monitored by TLC. Upon completion, the solvent was removed under reduced pressure and the residue was taken in water and extracted with ethyl acetate. Finally, the organic layer was washed with water and then dried over anhydrous sodium sulfate. The solvent was evaporated. The crude product was purified by column chromatography using hexane/ethylacetate (10/1, v/v) as an eluent. Crystals were obtained from slow evaporation of its ethyl acetate solution.
Experimental details
All hydrogen atoms were placed in geometrically idealized positions. The Uiso values of the hydrogen atoms of methyl groups were set to 1.5 Ueq(C) and the Uiso values of all other hydrogen atoms were set to 1.2 Ueq(C).
Comment
Oxindoles are part of many natural and biological compounds isolated from plant, marine sources [3], [4], [5] and can be converted in to advanced intermediates for complex and natural molecules [6], [,7]. The synthetic indolin-2-ones are reported for anticancer [8], [,9], antimicrobial, anticonvulsant [10], spermicidal [11] properties. Chromones are widely distributed in nature [12] and exhibit low toxicity along with a wide range of biological and pharmacological activities including antiinflammatory [13], anti–HIV [14], anticancer [15], antibacterial [16], antimalarial [17], and antitumor [18], as well as treatment of Alzheimer’s disease [19]. In addition, pyrazole derivatives are reported to have various biological activities including antidiabetic [20], anaesthetic [21], antimicrobial and antioxidant [22]. Herein we combine the oxindoles, chromones and pyrazole moieties in the same molecules (hybrid drug concept) via Michael cycloaddition reaction.
As shown in the figure (cf. the figure; the hydrogen atom is omitted for clarity), there is one crystallographically independent molecule in the asymmetric unit. The structure of the title compound has one dihydrochromone skeleton, a cyclohexane ring, a indolin-2-one skeleton, one 1-phenyl-pyrazolone ring and one t-butyloxy carbonyl. Chromone skeleton and cyclohexane ring form a condensed ring structure through C7 and C8, pyrone ring adopts an envelope conformation with the C8 atom deviating from the plane of the remaining five atoms [23], cyclohexane ring exhibits a chair conformation. The spiro atom C13 connects the cyclohexane ring and the indolin-2-one ring system, indolin-2-one ring is almost planar [24] and the dihedral angle between the dihydrochromone ring and the indolin-2-one ring is 88°. The 1-phenyl-pyrazolone ring and cyclohexane ring form a spiro structure through C11, the 1-phenyl-pyrazolone ring is almost in a vertical position with the dihydrochromone ring which is evident by the dihedral angle value of 88°. The structure of the molecule is stabilized by the hydrogen bonds of the type C–H⋯O, adjacent molecules are connected via hydrogen bonds C3–H3⋯O6, C23–H23B⋯O1 and C34–H34C⋯O1 into a two-dimensional plane running along the crystallographic [100] direction.
Funding source: Education Department of Shaanxi Province
Award Identifier / Grant number: 18JK0837
Funding source: Xianyang Normal University
Award Identifier / Grant number: XSYK18006
Award Identifier / Grant number: XSYQL201904
Funding source: Natural Science Basic Research Plan Funded by Shaanxi Province of China
Award Identifier / Grant number: 2018JM2045
Funding source: Science and Technology Projects of Xianyang City
Award Identifier / Grant number: 2017k02-19
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This research was supported by Scientific Research Program Funded by Shaanxi Provincial Education Department (No. 18JK0837), Natural Science Basic Research Plan Funded by Shaanxi Province of China (No. 2018JM2045), Science and Technology Projects of Xianyang City (No. 2017k02-19), Scientific Research Project Funded by Xianyang Normal University (No. XSYK18006) and Qing-Lan Talents Project Funded by Xianyang Normal University (No. XSYQL201904).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
2. Bruker. APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
3. Peddibhotla, S. 3-Substituted-3-hydroxyl-2-oxindole, an emerging new scaffold for drug discovery with potential anticancer and other biological activities. Curr. Bioact. Compd. 2009, 5, 20–38; https://doi.org/10.2174/157340709787580900.Search in Google Scholar
4. Nagamine, J., Nagata, R., Seki, H., Nomura-Akimaru, N., Ueki, Y., Kumagai, K., Taiji, M., Noguchi, H. J. 3-Pharmacological profile of a new orally active growth hormone secretagogue, SM-130686. J. Endocrinol. 2001, 171, 481–489; https://doi.org/10.1677/joe.0.1710481.Search in Google Scholar
5. Hewawasam, P., Meanwell, N. A., Gribkoff, V. K., Dworetzky, S. I., Boissard, C. G. Discovery of a novel class of BK channel openers: enantiospecific synthesis and BK channel opening activity of 3-(5-chloro-2-hydroxyphenyl)-1,3-dihydro-3-hydroxy-6-trifluoromethyl-2H-indol-2-one. Bioorg. Med. Chem. Lett. 1997, 7, 1255–1260; https://doi.org/10.1016/s0960-894x(97)00202-3.Search in Google Scholar
6. Babu, K. N., Kariyandi, N. R., Saheeda, M. K. S., Kinthada, L. K., Bisai, A. Lewis acid-catalyzed malonate addition onto 3-hydroxy-2-oxindoles: mechanistic consideration and synthetic approaches to the pyrroloindoline alkaloids. J. Org. Chem. 2018, 83, 12664–12682. https://doi.org/10.1021/acs.joc.8b02017.s001.Search in Google Scholar
7. Kinthada, L. K., Medisetty, S. R., Parida, A., Babu, K. N., Bisai, A. FeCl∼3∼–Catalyzed allylation reactions onto 3-hydroxy-2-oxindoles: formal total syntheses of bis-cyclotryptamine alkaloids, (+-)-chimonanthine, and (+-)-folicanthine. J. Org. Chem. 2017, 82, 8548–8567; https://doi.org/10.1021/acs.joc.7b01232.Search in Google Scholar
8. Natarajan, A., Fan, Y. H., Chen, H., Guo, Y., Iyasere, J., Harbinski, F., Christ, W. J., Aktas, H., Halperin, J. A. 3,3-Diaryl-1,3-dihydroindol-2-ones as antiproliferatives mediated by translation initiation inhibition. J. Med. Chem. 2004, 47, 1882–1885; https://doi.org/10.1021/jm0499716.Search in Google Scholar
9. Kamal, A., Srikanth, Y. V. V., Khan, M. N. A., Shaik, T. B., Ashraf, M. Synthesis of 3,3-diindolyl oxyindoles efficiently catalysed by FeCl∼3∼ and their in vitro evaluation for anticancer activity. Bioorg. Med. Chem. Lett. 2010, 20, 5229–5231; https://doi.org/10.1016/j.bmcl.2010.06.152.Search in Google Scholar
10. Praveen, C., Ayyanar, A., Perumal, P. T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4072–4077; https://doi.org/10.1016/j.bmcl.2011.04.117.Search in Google Scholar
11. Paira, P., Hazra, A., Kumar, S., Paira, R., Sahu, K. B., Naskar, S., Saha, P., Mondal, S., Maity, A., Banerjee, S., Mondal, N. B. Efficient synthesis of 3,3-diheteroaromatic oxindole analogues and their in vitro evaluation for spermicidal potential. Bioorg. Med. Chem. Lett. 2009, 19, 4786–4789; https://doi.org/10.1016/j.bmcl.2009.06.049.Search in Google Scholar
12. Sasnovskikh, V. Y., Irgashev, R. A. Uncatalyzed addition of indoles and N-methylpyrrole to 3-formylchromones: synthesis of (chromon-3-yl) bis(indol-3-yl)methanes and E-2-hydroxy-3-(1-methylpyrrol-2-ylmethylene)chroman-4-ones under solvent-free conditions. Tetrahedron Lett. 2007, 48, 7436–7439. https://doi.org/10.1016/j.tetlet.2007.08.078.Search in Google Scholar
13. Huo, H. X., Gu, Y. F., Sun, H., Zhang, Y. F., Liu, W. J., Zhu, Z. X., Shi, S. P., Song, Y. L., Jin, H. W., Zhao, Y. F., Tu, P. F., Li, J. Anti-inflammatory 2-(2-phenylethyl)chromone derivatives from Chinese agarwood. Fitoterapia 2017, 118, 49–55; https://doi.org/10.1016/j.fitote.2017.02.009.Search in Google Scholar
14. Yu, D., Chen, C., Brossi, A., Lee, K. Anti-AIDS agents. 60. substituted 3′R,4′R-di-O-(-)-camphanoyl-2′-2′, 2′-dimethyl dihydropyrano[2,3-f]chromone(DCP) analogues as potent anti-HIV agents. J. Med. Chem. 2004, 47, 4072–4082; https://doi.org/10.1021/jm0400505.Search in Google Scholar
15. Venkateswararao, E., Sharma, V. K., Manickam, M., Yun, J., Jung, S. H. Synthesis and SAR studies of bis-chromenone derivatives for anti-proliferative activity against human cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 5256–5259; https://doi.org/10.1016/j.bmcl.2014.09.057.Search in Google Scholar
16. Babu, K. S., Babu, T. H., Srinivas, P., Kishore, K., Murthy, U., Rao, J. Synthesis and biological evaluation of novel C (7) modified chrysin analogues as antibacterial agents. Bioorg. Med. Chem. Lett. 2006, 16, 221–224. https://doi.org/10.1016/j.bmcl.2005.09.009.Search in Google Scholar
17. Lerdsirisuk, P., Maicheen, C., Ungwitayatorn, J. Antimalarial activity of HIV-1 protease inhibitor in chromone series. Bioorg. Chem. 2014, 57, 142–147; https://doi.org/10.1016/j.bioorg.2014.10.006.Search in Google Scholar
18. Nawrot-Modranka, J., Nawrot, E., Graczyk, J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur. J. Med. Chem. 2006, 41, 1301–1309; https://doi.org/10.1016/j.ejmech.2006.06.004.Search in Google Scholar
19. Li, F., Wu, J. J., Wang, J., Yang, X. L., Cai, P., Liu, Q. H., Kong, L. Y., Wang, X. B. Synthesis and pharmacological evaluation of novel chromone derivatives asbalanced multifunctional agents against Alzheimer’s disease. Bioorg. Med. Chem. 2017, 25, 3815–3826; https://doi.org/10.1016/j.bmc.2017.05.027.Search in Google Scholar
20. Amir, M., Kumar, H., Khan, S. A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new antiinflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922; https://doi.org/10.1016/j.bmcl.2007.12.043.Search in Google Scholar
21. Shivarama Holla, B., Mahalinga, M., Boja, P., Mithun, A. Synthesis of pyrazolines promoted by amberlyst-15 catalyst. Eur. J. Med. Chem. 1980, 15, 567–570. https://doi.org/10.1002/chin.200623120.Search in Google Scholar
22. Renuka, N., Ajay Kumar, K. Synthesis and biological evaluation of novel formyl-pyrazoles bearing coumarin moiety as potent antimicrobial and antioxidant agents. Bioorg. Med. Chem. Lett. 2013, 23, 6406–6409; https://doi.org/10.1016/j.bmcl.2013.09.053.Search in Google Scholar
23. Li, W. W., Zheng, M. Y., Li, X. B., Zhang, Z. T. Synthesis, crystal structure and antineoplasmic activity of 3,6,8-tribromo-5-hydroxy-2,7-dimethoxy-2-phenyl-2,3-dihydrochromen-4-one. Chin. J. Struct. Chem. 2018, 37, 1071–1077. https://doi.org/10.14102/j.cnki.0254-5861.2011-1948.Search in Google Scholar
24. Li, W. W., Zheng, M. Y., Guo, Y., Jiang, Y. P., Wang, Q., Wang, M., Ji, M. X., Zhang, Y. T., Zhang, Z. T. Synthesis and crystal structure of (+-)-ethyl 5′-(difluoromethyl)-2-oxo-4′-5′-dihydrospiro[indoline-3,3′-pyrazole]-4′,5′-carboxylate, C14H13F2N3O3. Z. Kristallogr. NCS 2019, 234, 845–847; https://doi.org/10.1515/ncrs-2019-0188.Search in Google Scholar
© 2020 Wu-Wu Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3

