Abstract
Objective
This study aims to identify superenhancer (SE)–transcriptional factor (TF) regulatory network related to eight common malignant tumors based on ChIP-seq data modified by histone H3K27ac in the enhancer region of the SRA database.
Methods
H3K27ac ChIP-seq data of eight common malignant tumor samples were downloaded from the SRA database and subjected to comparison with the human reference genome hg19. TFs regulated by SEs were screened with HOMER software. Core regulatory circuitry (CRC) in malignant tumor samples was defined through CRCmapper software and validated by RNA-seq data in TCGA. The findings were substantiated in bladder cancer cell experiments.
Results
Different malignant tumors could be distinguished through the H3K27ac signal. After SE identification in eight common malignant tumor samples, 35 SE-regulated genes were defined as malignant tumor-specific. SE-regulated specific TFs effectively distinguished the types of malignant tumors. Finally, we obtained 60 CRC TFs, and SMAD3 exhibited a strong H3K27ac signal in eight common malignant tumor samples. In vitro experimental data verified the presence of a SE–TF regulatory network in bladder cancer, and SE–TF regulatory network enhanced the malignant phenotype of bladder cancer cells.
Conclusion
The SE–TF regulatory network with SMAD3 as the core TF may participate in the carcinogenesis of malignant tumors.
1 Introduction
The superenhancer (SE) regulates gene expression and is characterized by high-density epigenetic modifications associated with transcription factors (TFs), and cofactors [1]. The abnormal gene transcription mediated by SEs is essential for maintaining the characteristics of tumor cells [2]. Tumor cells significantly promote the expression of various oncogenes by assembling SE, thereby enhancing their proliferation, invasion, and metastasis [3]. Therefore, the identification and analysis of core SEs and TFs in malignant tumors are of great value in tumor research [4].
Mechanisms of abnormal SE formation in malignant tumors have been demonstrated to exhibit a profound influence on both molecular pathogenesis and clinical management [5]. The transcriptional element, for example, bromodomain-containing protein 4 (BRD4) and cyclin-dependent kinase 7 (CDK7), has been reportedly the treatment target due to tumor-specific SEs [6]. Myeloid cell leukemia-1 gene (MCL1) and B-cell leukemia/lymphoma-xl (BCL-xl) are cell apoptosis regulators and express aberrantly in cancer, which plays an important role in chemoresistance [7]. By chromatin immunoprecipitation and sequencing (ChIP-seq) processing, Oldridge et al. found changed polymorphism within one SE element of LIM domain only 1 (LMO1) significantly affected neuroblastoma susceptibility by different binding of gamma-aminobutyrate (GATA) TF or regulation of LMO1 expression, which resulted in oncogenic dependency of neuroblastoma [8]. Lysine (K)-specific methyltransferase 2D (KMT2D) deficiency was reported to impair SEs to form glycolytic vulnerability in lung cancer, promote tumorigenesis in mice and upregulate protumorigenic progression, including glycolysis [9]. Oncogenic homeobox B8 (HOXB8) was driven by MYC-regulated SEs and enhanced colorectal cancer invasiveness through BTB and CNC homology 1 (BACH1) [10]. Increased expression of PPP1R15A and CDK7 is positively associated with undesirable clinical prognosis in anaplastic thyroid carcinoma. CDK7 and PPP1R15A are considered potential biomarkers and therapeutic targets for anaplastic thyroid carcinoma [11].
Although increasing reports have shown some SEs and TFs are correlated with malignancy, the common and specific SEs and TFs in various tumors, as well as complex regulatory networks, have largely been unknown. Furthermore, a majority of studies are only involved in one type of malignant tumor. Histone H3 Lys27 acetylation (H3K27ac) CHIP-seq and RNA-seq are recently hot tools for studying DNA, RNA, and protein [12,13]. Our study aims to identify and analyze core SEs and TFs in various malignant tumors such as liver cancer, lung cancer, and breast cancer based on H3K27ac ChIP-seq data in the sequence read archive (SRA) database as well as RNA-seq data in The Tumor Genome Atlas (TCGA) database.
2 Materials and methods
2.1 Data acquisition
The SRA database (https://www.ncbi.nlm.nih.gov/sra) is a database used to store the original data of second-generation sequencing. The ChIP-seq data of the differential histone modification regions of eight common malignant tumor samples were all from the SRA database. The TCGA project is a joint project initiated by the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) in 2006, which included 39 types of malignant tumors investigated from the very first glioblastoma multiforme to the present, involving 29 types of malignant tumor organs, and more than 10,000 tumor samples. The RNA-seq data used in the analysis were all from the TCGA database.
-
Informed consent: Informed consent was not applicable for this study.
-
Ethical approval: Ethics committee approval was not applicable for this study.
2.2 Data preprocessing
Sample sequencing volume and quality were first evaluated and the Cutadapt software (https://cutadapt.readthedocs.io/en/stable/) was adopted to remove the joint and low-quality bases, followed by data quality control using the FastQC software (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Clean reads were aligned to the human reference genome hg19 using the Bowtie2 software (ftp://igenome:G3nom3s4u@ussd-ftp.illumina.com/Homo_sapiens/UCSC/hg19/Homo_sapiens_UCSC_hg19.tar.gz). Unique alignment data were extracted from the obtained SAM files. The BAM files were deduplicated, and the data of the same cell line were merged and sorted using the Samtools [14]. With the input data as control, MACS2 software (https://pypi.org/project/MACS2/) [15] was applied to retrieve the significant H3K27ac peaks (p < 1 × 10−9 was considered significant). Significant H3K27ac peaks with a distance longer than 2.5 kb from the TSS sites were extracted as the enhancer. The obtained enhancers were extended upstream and downstream by 5 kb from the middle point of the corresponding peaks. Next, the enhancer sequence was segmented into 100 bp bin reads and aligned with the BED files converted from the BAM files generated before. The average number of peaks aligned to all the corresponding bins of an enhancer was regarded as the H3K27ac signal of the enhancer. bamCoverage in the deepTools [16] was applied to convert the processed BAM file of each sample into a corresponding Bigwig file, and the Integrated Genomics Viewer (IGV) was uploaded for visualization.
2.3 Histone H3K27ac modification analysis
The human reference genome hg19 was divided into small fragments with a length of 2 kb, and the number of reads of each sample mapped to each small fragment was calculated and normalized by the sequencing depth of the sample. The normalized H3K27ac signal files of the small fragments were then integrated into a matrix file according to tumor type and the correlation was calculated by an R algorithm.
2.4 Prediction of SEs in eight common malignant tumor samples
The SEs and TEs in all samples were calculated using the ROSE algorithm [6,17] combined with the significant H3K27ac peak found by MACS2. The SEs found in different cell lines of the same malignant tumor were merged through the merge module of Bedtools software [18], and then the frequency of occurrence of each merged SE in different cell lines was calculated. In each malignant tumor, the SEs appearing in at least two cell lines were selected for subsequent analysis.
2.5 Differential expression analysis of SEs and TEs
The SEs that appeared in at least two cell lines were annotated with HOMER software [19]. Subsequently, the SE-regulated target genes in each malignant tumor sample were extracted for functional enrichment analysis with DAVID software [20,21]. These genes were merged using the merge module of the Bedtools software, and the frequency of all SEs presented in eight common malignant tumors was calculated. The number of different SE-regulated target genes in eight common malignant tumor samples was counted and the conservative SE-regulated target gene and the malignant tumor-specific SE-regulated target gene were defined in sequence. Afterward, a malignant tumor conservative SE-regulated target gene and a malignant tumor-specific SE-regulated target gene were selected and displayed (H3K27ac signal) using IGV. Finally, expression profile data in the TCGA database was used for verification.
2.6 Identification of conservative and malignant tumor-specific TFs
Single significant H3K27ac peaks included in the SEs that appeared more than twice in eight common malignant tumors were first extracted. The nucleosome-free regions (NFRs) were then extracted from the bed file converted from the sorted BAM file with HOMER. The single significant H3K27ac peaks were overlapped with the extracted NFRs to identify the NFRs located on the SEs, and the results were saved as NFR bed files. The bed files were used as input to find the TFs regulated by SEs in corresponding malignant tumor samples with HOMER, and the p-value and motif graphs of the corresponding TFs were generated. The frequency of occurrence of TFs binding to SEs in eight common malignant tumor samples was counted, and conservative and malignant tumor-specific TFs were defined according to the significance of all the TFs in each malignant tumor sample (the significance was indicated by the p-value). The expression of all TFs in each malignant tumor sample was calculated through the expression profile in TCGA (the average value of FPKM in all samples obtained) for verification of the conservative and malignant tumor-specific TFs. Finally, a conservative TF gene and a malignant tumor-specific TF were selected and their expression in each malignant tumor sample was calculated and shown by the Beeswarm package (https://github.com/aroneklund/beeswarm).
2.7 Screening of the SE–TE regulatory network in eight common malignant tumors
Core regulatory circuitry (CRC), namely the SE–TE regulatory network, mainly refers to the regulatory loop composed of SEs and core TFs in cells [22]. Generally, the expression of a core TF gene was not only regulated by the corresponding SEs but also regulated by binding of the SEs with the TF itself. CRCmapper software [22] was applied to define the core TF regulatory circuits in each malignant tumor sample. The TFs with the highest score in the SE–TF regulatory network in each malignant tumor sample were counted and collated according to the frequency of their appearance in each malignant tumor sample. For further validation, the expression of all TF coding genes was displayed according to the tumor expression profile data in the TCGA database, and then a CRC TF that appeared several times in malignant tumor core TFs, as well as a specific TF that only appeared in one specific malignant tumor sample, was selected to separately display the H3K27ac signal of the SEs near the two loci by IGV.
2.8 Cell culture and transfection
Human gastric malignant tumor cell line MKN45, human renal malignant tumor cell line 786-M1A, human esophageal squamous cell line KYSE140, human colorectal malignant tumor cell line HCT116, human bladder malignant tumor cell line T24, and human breast malignant tumor cell line 76NF2V, small cell lung malignant tumor cell line COR-L311, and human liver malignant tumor cell line HepG2 were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). MKN45, 786-M1A, T24, and COR-L311 cells were cultured with the RPMI-1640 medium (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco), 10 μg/mL streptomycin, and 100 U/mL penicillin, while HCT116, 76NF2V, and HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich Chemical Company, St Louis, MO, USA) containing 10% FBS (Gibco), 10 μg/mL streptomycin, and 100 U/mL penicillin. KYSE140 cells were cultured with minimum essential medium (MEM) supplemented with 10% FBS (Gibco), 10 μg/mL streptomycin, and 100 U/mL penicillin. The aforementioned cells were incubated in a 5% CO2 incubator (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 37°C.
T24 cells in the logarithmic phase were trypsinized, seeded into a 6-well plate at a density of 1 × 105 cells per well, and cultured for 24 h. Upon reaching about 75% confluence, the cells were transfected using the Lipofectamine 2000 reagent (Invitrogen Inc., Carlsbad, CA, USA) with short hairpin RNA plasmids targeting NC (sh-NC), SMAD3 (sh-SMAD3), ETS1 (sh-ETS1), and HOXB2 (sh-HOXB2), and overexpression plasmids of NC (pcDNA3.1), SMAD3 (pcDNA3.1-SMAD3), ETS1 (pcDNA3.1-ETS1), and HOXB2 (pcDNA3.1-HOXB2), as well as sh-NC + pcDNA3.1, sh-SMAD3 + pcDNA3.1, sh-SMAD3 + pcDNA3.1-ETS1, sh-SMAD3 + pcDNA3.1-HOXB2, and sh-SMAD3 + pcDNA3.1-ETS1 + pcDNA3.1-HOXB2 plasmids. After 48 h of transfection, subsequent experiments were carried out, and each experiment was repeated 3 times. The concentration of plasmids used was 50 ng/mL. shRNA or overexpression plasmids were provided by Shanghai GenePharma Co., Ltd (Shanghai, China).
2.9 Chromatin immunoprecipitation (ChIP)
ChIP kit (Thermo Fisher Scientific) was used for this experiment. The treated cells were fixed with 1% formaldehyde and sonicated to trigger DNA strand breaks. The complex was immunoprecipitated by incubation with rabbit antibodies against IgG (1:100, ab6757, Abcam Inc., Cambridge, UK), H3K27ac (1:50, ab4727, Abcam), SMAD3 (1:50, ab208182, Abcam), ETS1 (1:50, ab124282, Abcam), and HOXB2 (1:50, ab220390, Abcam). Next, the complex was filtered from the DNA fragments through protein G–Sepharose beads. Cross-linking of the DNA complex was relieved and DNA strands were purified. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed to quantify ChIP products. The primer sequences are shown in Table S1.
2.10 RNA isolation and quantitation
Total RNA was extracted from cells using the TRIzol reagent (16096020, Thermo Fisher Scientific). For mRNA analysis, the extracted RNA was reversely transcribed into cDNA using a reverse transcription kit (RR047A, Takara, Tokyo, Japan). RT-qPCR was conducted using an SYBR® Premix Ex TaqTM II kit (DRR081, Takara) on an ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA), with three repeated wells for each sample. GAPDH served as the internal reference, and the fold changes were calculated using the method of 2−ΔΔCt. The primer sequences are shown in Table S2.
2.11 Western blot analysis
Total protein was extracted from cells with the radio-immunoprecipitation assay (RIPA) lysis buffer (C0481, Sigma-Aldrich) containing 1% protease inhibitor and 1% phosphorylase inhibitor (Shanghai Beyotime Biotechnology Co., Ltd., Shanghai, China). The protein concentration was then determined with a bicinchoninic acid kit (23227, Thermo Fisher Scientific). The protein was quantified in 5× loading buffer (P0015, Beyotime), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA). Next, the membrane was treated with 5% bovine serum albumin (BSA) at room temperature for 1 h and incubated overnight at 4°C with primary rabbit antibodies against SMAD3 (1:1,000, ab208182, Abcam), ETS1 (1:2,000, ab124282, Abcam), HOXB2 (1:5,000, ab220390, Abcam), and β-actin (1:5,000, ab8227, Abcam). The following day, the membrane was incubated with horseradish peroxidase-labeled secondary antibody goat anti-rabbit IgG (1: 20,000, ab205718, Abcam) at room temperature for 1.5 h. Afterward, the membrane was developed using the developing solution (NCI4106, Pierce, Rockford, IL, USA), after which the protein bands were quantified by ImageJ 1.48 u software (Bio-Rad, Inc., Hercules, CA, USA). The ratio of the gray value of the target band to that of β-actin was representative of the relative protein expression.
2.12 Transwell assay
Transwell chambers (8 μm pore size; Corning Incorporated, Corning, NY, USA) in 24-well plates were used for in vitro cell migration and invasion detection. In brief, 600 μL of 20% FBS-containing RPMI-1640 medium was preadded to the 8 μm pore-size Matrigel-coated Transwell chambers and the Matrigel-free Transwell chambers and equilibrated at 37°C for 1 h. T24 cells transfected for 48 h were resuspended in RPMI-1640 medium containing 10% FBS, and 100 μL of the cell suspension containing 1 × 109 cells/L was added to the upper chamber, and cultured at 37°C with 5% CO2 for 24 h. The Transwell chamber was removed and the cells in the inner layer of the Transwell chamber were wiped with a cotton swab. After washing with PBS, cells were fixed 4% methanol, and stained with 0.1% crystal violet before counting; they were photographed under an inverted microscope (Olympus IX73, Olympus Optical Co., Ltd, Tokyo, Japan) in five randomly selected fields of view, with three replicates for each specimen. The differences between the groups were analyzed and the histogram of migration and invasion was plotted.
2.13 Cell count kit-8 (CCK-8) assay
T24 cell proliferation was evaluated using a CCK-8 kit (K1018, Apexbio, USA). Cells (1 × 104 cells per well, 100 μL/well) were plated in a 96-well plate, and 10 μL of CCK-8 solution was added at each time point (0, 24, 48, 72, and 96 h) to incubate the cells at 37°C for 2 h. Next, the optical density (OD) value was measured at 450 nm with a microplate reader, and the obtained data were displayed in curve graphs.
2.14 Statistical analysis
All data were analyzed using SPSS 21.0 statistical software (IBM Corp. Armonk, NY, USA). The measurement data were described as mean ± standard deviation. Data obeying normal distribution and homogeneity of variance between two groups were compared by unpaired t-test. Differences among multiple groups were statistically analyzed using one-way analysis of variance (ANOVA) or repeated measures ANOVA, followed by Tukey’s post hoc tests with corrections for multiple comparisons. A value of p < 0.05 was statistically significant.
3 Results
3.1 Data information of cell lines in eight common malignant tumor samples in the SRA database
ChIP-seq data of differential histone modification regions in eight common malignant tumor cell lines were downloaded from the SRA database. All cell lines did not receive special treatment, and the data of different cell lines for each malignant tumor were merged and 71 types of cell lines were obtained. The data information of eight common malignant tumor cell lines in the SRA database is shown in Table S3.
3.2 Difference in the H3K27ac signal intensity in the enhancer region of eight common malignant tumor cell lines
Analysis of the histone H3K27ac modification changes in the enhancer region of each malignant tumor cell line by the MACS2 software indicated a large number of significant peaks in each malignant tumor cell line (Figure 1a). In addition, the Python script results showed the presence of a large number of enhancers in each malignant tumor cell line, which was consistent with the significant H3K27ac peak trend (Figure 1b). To ensure that the prediction of the enhancers was accurate, we selected a malignant tumor cell line for each malignant tumor (gastric malignant tumor: MKN45; renal malignant tumor: 786-M1A; esophageal squamous cell carcinoma: KYSE140; colorectal malignant tumor: HCT116; bladder malignant tumor: T24; breast malignant tumor: 76NF2V; small cell lung malignant tumor: COR-L311; hepatocellular carcinoma: HepG2) and displayed the H3K27ac signals in the predicted enhancer regions. A strong signal peak of H3K27ac was observed in the middle of the enhancer in each malignant tumor sample (Figure 1c). Furthermore, we displayed the H3K27ac signals on the neutrophil cytosolic factor 2 (NCF2) gene locus by IGV in the eight selected malignant tumor cell lines, as representative; and we found that H3K27ac signals were highly enriched in the enhancer regions (Figure 1d).
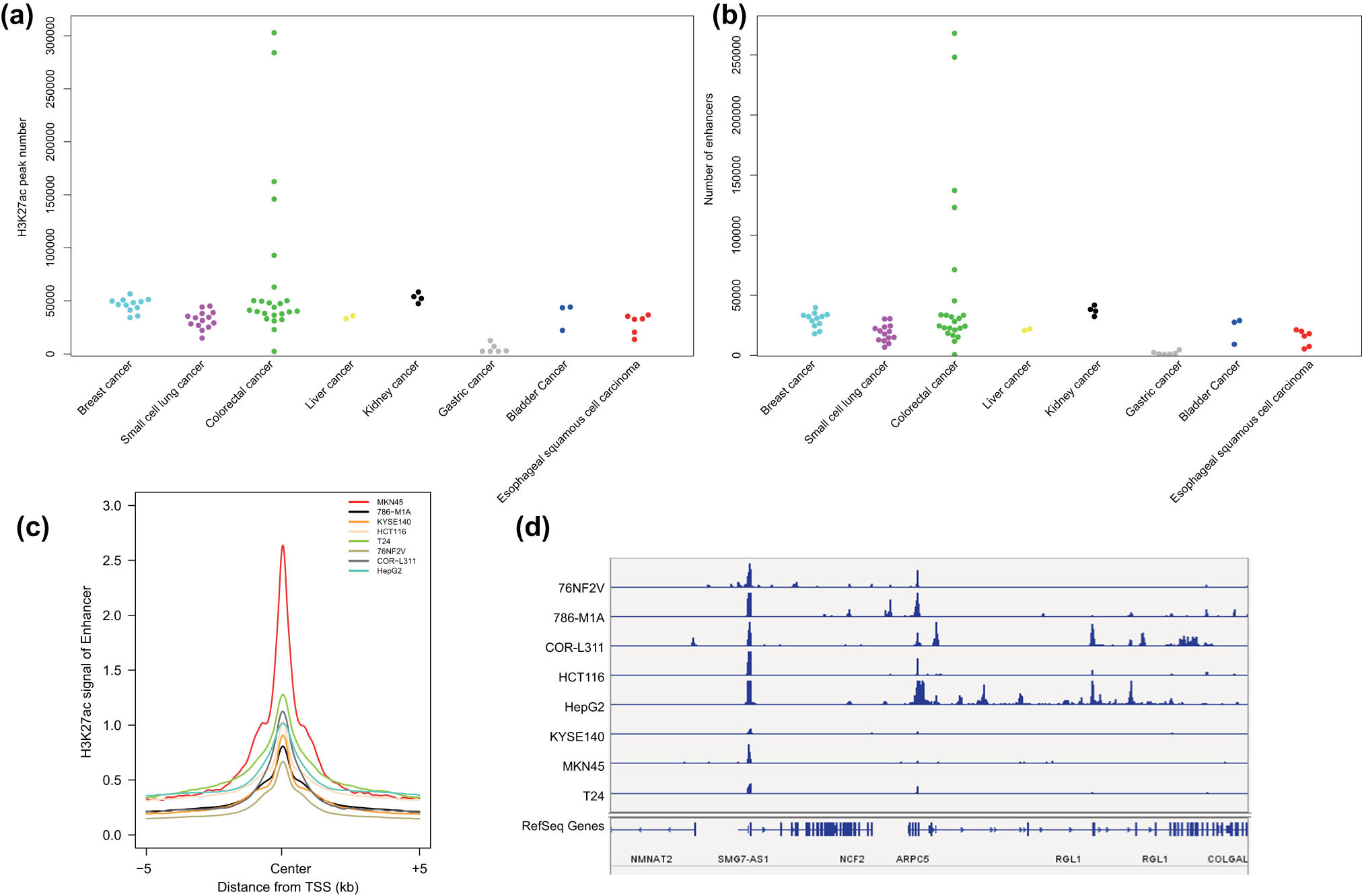
H3K27ac signal intensity on the enhancers of the eight common malignant tumor cell lines. (a) The number of significant H3K27ac signal peaks in eight common malignant tumor cell lines. (b) The number of enhancers in eight common malignant tumor cell lines. (c) H3K27ac signal intensity on the enhancers of the eight common malignant tumor cell lines. (d) H3K27ac signal intensity in the NCF2 gene region in the eight common malignant tumor cell lines.
3.3 The type of H3K27ac modification signal is different for each malignant tumor
Correlation analysis of H3K27ac signals among malignant tumor samples revealed the H3K27ac signals of the same type of malignant tumor cell lines could be clustered together, while the H3K27ac signals of different types of malignant tumor cell lines failed to be clustered. This finding indicated that the H3K27ac modification signal type of each malignant tumor was different and different malignant tumors could be thus distinguished by the H3K27ac signal type (Figure 2).
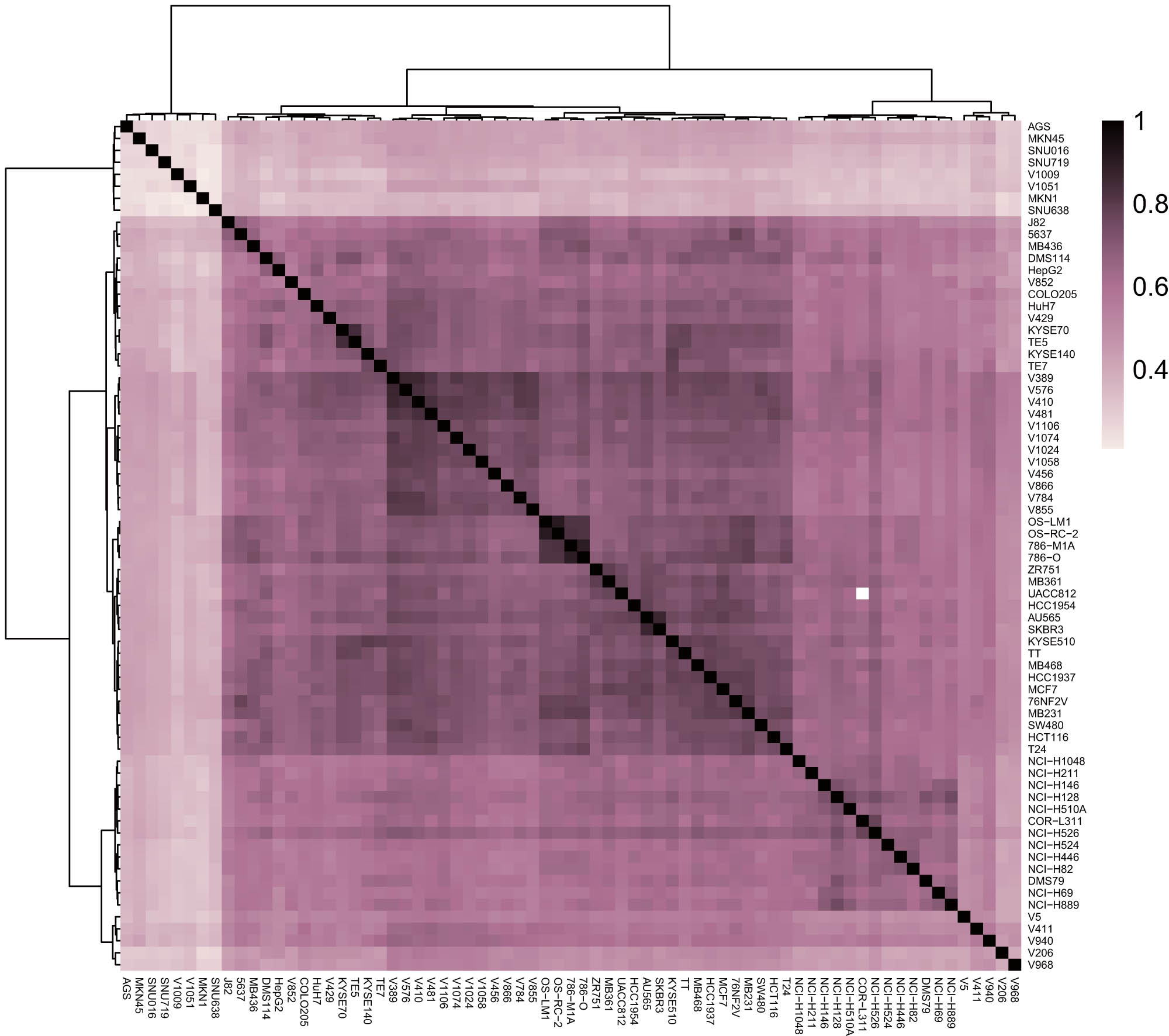
A heat map of the correlation analysis of the H3K27ac signals in different malignant tumor cell lines.
3.4 Screening of the number of SEs in eight common malignant tumor samples
SEs are a large cluster of transcriptionally active enhancers that drive the expression of genes that control cell identity [23]. To identify the SEs in all the eight common malignant tumors, we applied a method consisting of three steps. The first step was to determine the active enhancer sites, which are described in Figure 1. The next step was to stitch the enhancers we obtained by their distribution and distance between each other. Within the genome range, if the distance between two enhancer annotations were within 12.5 kb, then they were merged into a single entity called the stitched enhancer. Finally, the threshold between the SEs and the TEs was determined. We sorted the stitched enhancers and the remaining single enhancers according to the intensity of the H3K27ac signal level (from low to high) and drafted a graph of the ranking result. On this graph of H3K27ac signal density vs density ranking, we identified the tangent point by a tangent line with a slope of 1, which was considered as the threshold. That is, the points with higher density (to the right and above the tangent point) were SEs, while the rest were TEs (Figure 3a). The significant H3K27ac peak found by the ROSE algorithm combined with the screening results of MACS2 software was used to calculate the SEs and TEs in all samples. The SEs found in different cell lines of the same type of malignant tumor were merged using the merge module of Bedtools software, and the SEs that appeared in at least 2 cell lines in the eight common malignant tumor samples were calculated. The results indicated that eight common malignant tumor samples had a large number of SEs, and colorectal malignant tumor samples had the largest number (2500) (Figure 3b). To further confirm that our prediction of SEs was correct, we selected the PADI1 gene, which was reported to be regulated by an adjacent estrogen receptor alpha-dependent SE [24] and visualized by IGV. The results showed that, except for the COR-L311, HepG2, and KYSE140 cell lines, other cell lines all had strong PADI1 gene enhancer region H3K27ac signal expression, suggesting SE expression (Figure 3c).
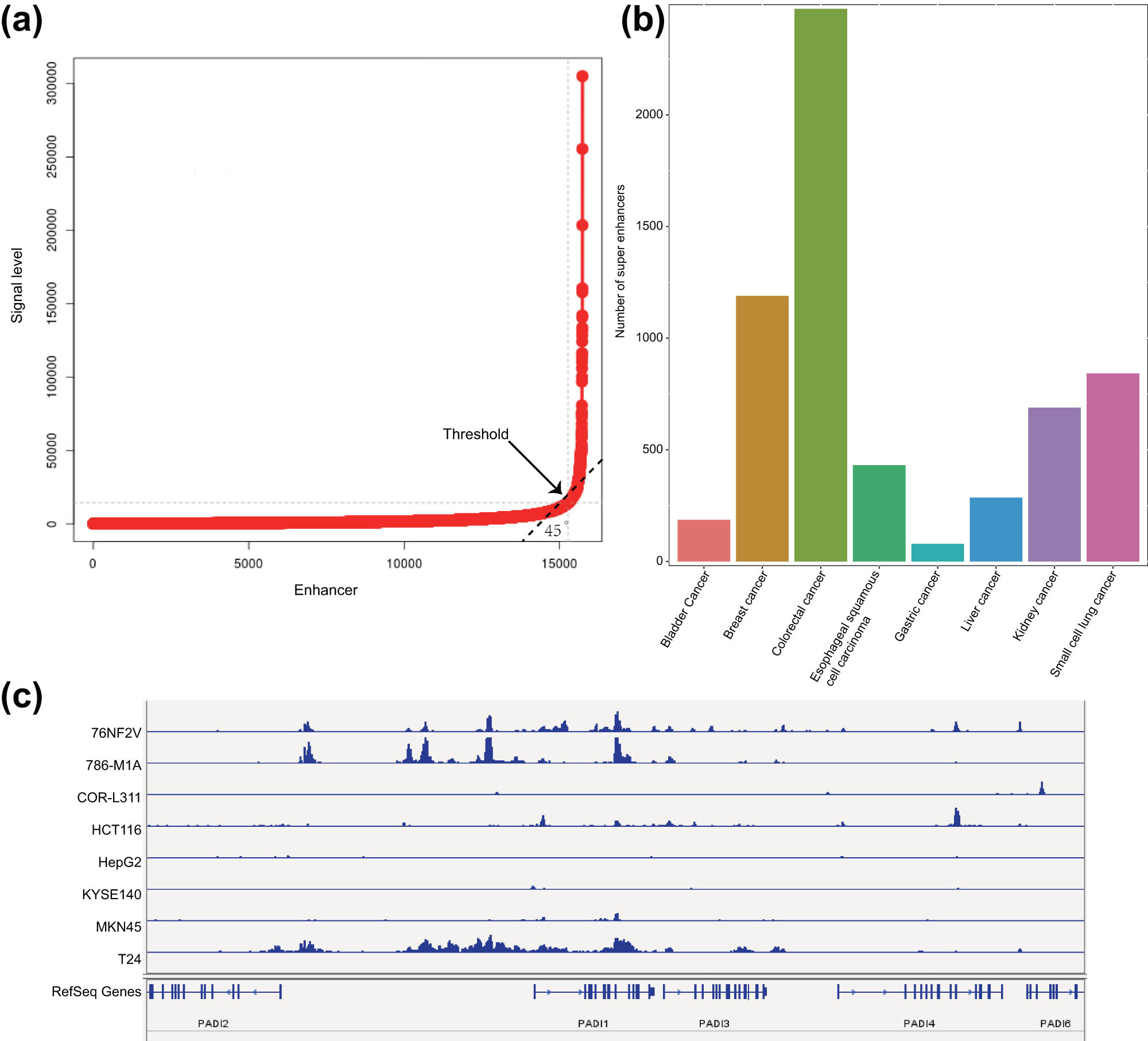
Prediction of SEs in eight common malignant tumor cell lines. (a) Ranking result of the H3K27ac signal intensity of the stitched enhancers and the remaining single enhancers. The arrow indicates the threshold point. (b) The number of SEs that occurred in eight common malignant tumor cell lines. The abscissa represents the type of malignant tumor, and the ordinate represents SEs appearing at least twice in each malignant tumor cell line. (c) The difference in the signal intensity of H3K27ac in the enhancer region of the PADI1 gene in eight common malignant tumor samples.
3.5 Analysis of conservative and malignant tumor-specific target genes regulated by SEs in eight common malignant tumor samples
Functional enrichment analysis of the SE-regulated genes in malignant tumor samples through DAVID software revealed that these genes were significantly enriched in pathways including cell junction organization, cell junction assembly, and cell–cell junction organization (Figure 4a and b). We collated all SE-regulated genes in eight common malignant tumor samples and obtained 5,923 target genes (Figure 4c and d). As shown in Table S4, there were 2154 SE-regulated genes (conservative target genes) occurring more than 6 times in eight common malignant tumor samples, while 35 genes (specific target genes) occurring only once. We selected the malignant tumor-specific SE-regulated gene A2M and the conservative SE-regulated gene ABALON as representatives and displayed the H3K27ac signal distribution on the two loci by IGV in the eight common malignant tumor cell lines. The results showed that H3K27ac signal peaks on A2M were only observed in a few malignant tumor cell lines (Figure 4e), while the peaks on ABALON was clearly observed in eight common malignant tumor cell lines (Figure 4f). Analysis of the SE-regulated target gene expression data in the eight common malignant tumor samples from the TCGA database revealed that the results were consistent with the H3K27ac ChIP-seq results (Figure 4g).
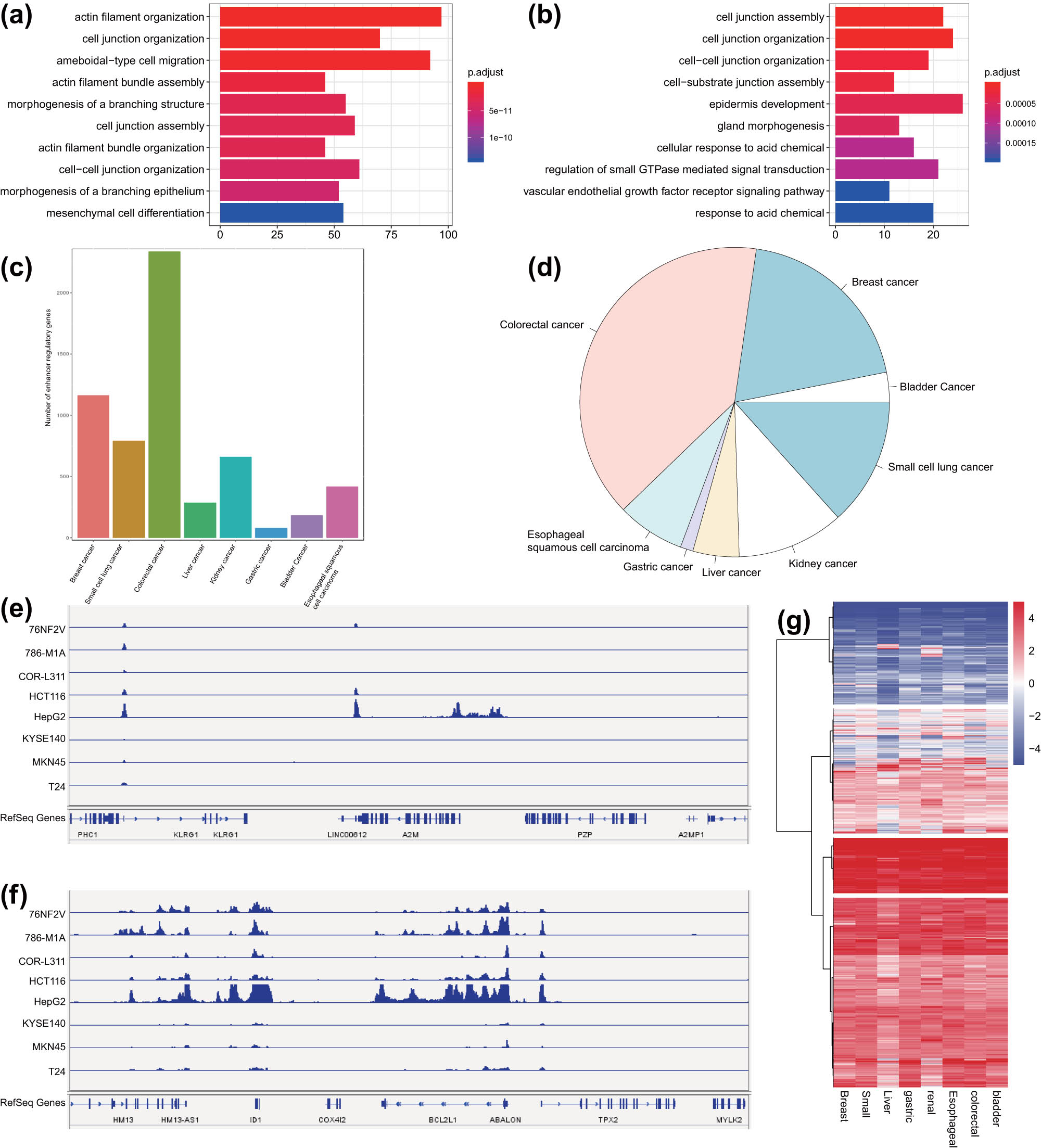
Analysis of conservative and malignant tumor-specific SEs in eight common malignant tumor cell lines. (a) GO functional enrichment analysis of SE-regulated genes in colorectal cancer samples. The ordinate represents the enriched pathway, and the abscissa represents the number of genes enriched in the pathway. The color represents the significance, with deep red indicating more significance. (b) GO functional enrichment analysis of SE-regulated genes in bladder cancer samples. The ordinate shows the enriched pathway, and the abscissa indicates the number of genes enriched in the pathway. The color represents the significance of the pathway, with deep red indicating more significance. (c) The number of SE-regulated genes in eight common malignant tumor cell lines. (d) The frequency of SE-regulated target genes in eight common malignant tumor cell lines following consolidation. (e) H3K27ac signal distribution on the specific SE-regulated target gene A2M in the eight common malignant tumor cell lines. (f) H3K27ac signal distribution on the conservative SE-regulated target gene ABALON in the eight common malignant tumor cell lines. (g) Expression of SE-regulated target genes in the eight common malignant tumor cell lines in TCGA database.
3.6 Identification of malignant tumor-specific and conservative TFs regulated by SEs in eight common malignant tumor samples
The TFs regulated by SEs in each malignant tumor sample were identified using HOMER software, and the top 10 TFs with the smallest p-value were screened. The results displayed that some TFs existed in multiple malignant tumor samples, and some TFs only in specific malignant tumor samples (Figure 5a). The differential expression of all TFs in each malignant tumor sample is shown in Figure 5b. The TF occurring more than 4 times was defined as a conservative TF and that occurring only once was defined as a specific TF. Based on TCGA RNA-seq data of eight common malignant tumor samples, we calculated the expression of all the identified TFs in each malignant tumor type (Figure 5c). Finally, we selected a conservative TF kruppel-like factor 5 (KLF5) and malignant tumor-specific TF POU class 2 homeobox 2 (POU2F2) and analyzed their expression in each type of malignant tumor samples. The data exhibited that the expression of KLF5 was almost similar in 4–5 malignant tumor samples (Figure 5d), while that of POU2F2 was upregulated only in small cell lung cancer samples (Figure 5e). These results indicated that the specific TFs regulated by SEs could distinguish the malignant tumor types effectively.
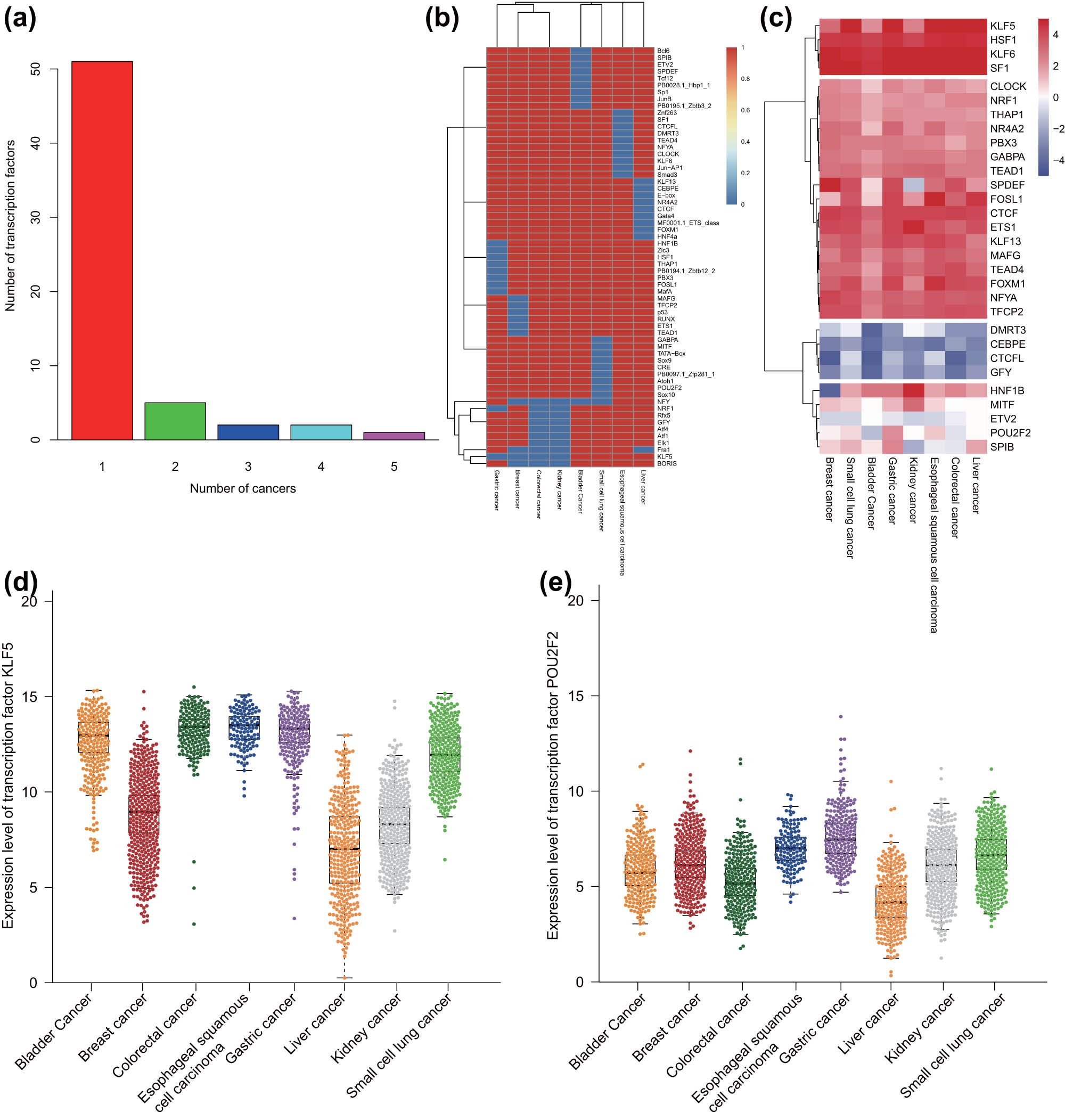
Identification of SE-related malignant tumor-specific and -conservative TFs in eight common malignant tumor samples. (a) The frequency and number of TFs regulated by SEs in eight common malignant tumor samples. (b) A heat map of all the identified TFs in each malignant tumor sample. (c) The expression of all the identified TFs in TCGA RNA-seq data of each malignant tumor sample. The expression was evaluated by taking the average value of FPKM of all samples in TCGA RNA-seq data. (d) The expression of the conservative TF KLF5 in TCGA RNA-seq data of each malignant tumor sample. (e) The expression of the malignant tumor-specific TF POU2F2 in TCGA RNA-seq data of each malignant tumor sample.
3.7 Screening of the SE–TF regulatory network in eight common malignant tumor cell lines
The screening of the SE–TF regulatory network was divided into three steps: the first step was to find all the TFs regulated by the SEs; the second step was to find the TFs that could bind to the SEs to regulate the expression of their own genes from the TFs regulated by SEs, which is the so-called autoregulated; the third step was to merge all autoregulated TFs. These TFs could regulate the expression of each other as well as themselves, and they together formed a SE–TF regulatory network (Figure 6a).
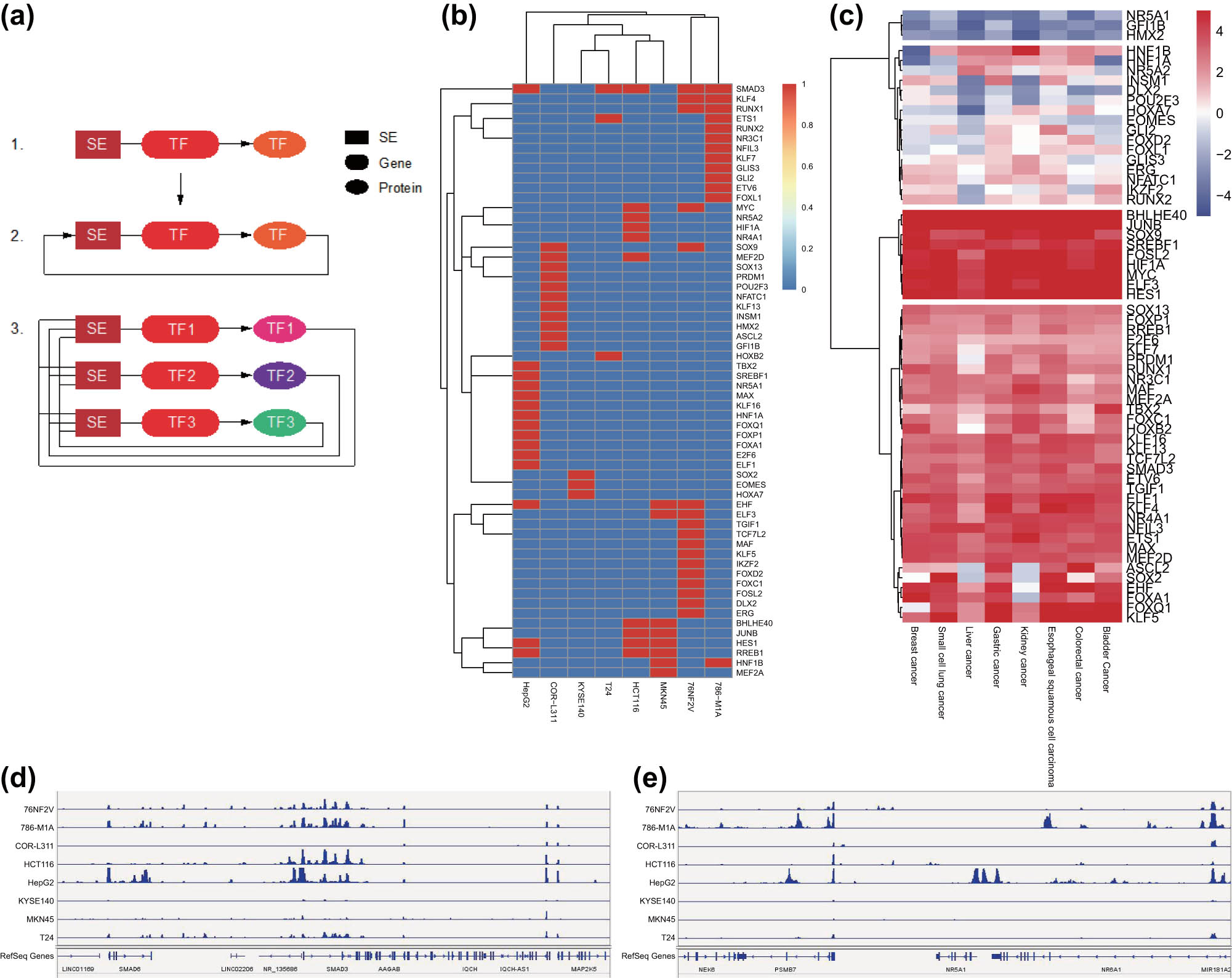
Screening of the SE–TF regulatory network in eight common malignant tumor cell lines. (a) Schematic diagram of SE–TF network. (b) A heat map of the top 10 core TFs in each malignant tumor sample. (c) A heat map of the expression of core TFs in TCGA RNA-seq data. (d) H3K27ac signal intensity of SMAD3 in eight common malignant tumor cell lines. (e) H3K27ac signal intensity of NR5A1 in eight common malignant tumor cell lines.
Prediction results of the core TFs in eight common malignant tumor cell lines by CRCmapper software are shown in Table S5. The core TFs with the highest scores in each malignant tumor sample were merged, with a total of 60 core TFs obtained (Figure 6b). To verify the prediction, we analyzed TCGA RNA-seq data of eight common malignant tumor samples and found consistent results with those of CRCmapper software (Figure 6c). We further validated the results by selecting TFs SMAD3 and NR5A1 and employed IGV to analyze the H3K27ac signal in the gene enhancer region. The results showed that SMAD3 had strong H3K27ac signals in eight common malignant tumor samples, while the NR5A1 only had H3K27ac signals in liver cancer samples (Figure 6d and e). The above results indicated that the SE–TF regulatory network with the TF SMAD3 as the core may be involved in the occurrence and development of a variety of malignant tumors.
3.8 Validation of the identified SE–TF regulatory network in bladder cancer cells
Next, we aimed to verify the H3K27ac signal of the TF SMAD3 in tumor samples by culturing eight kinds of malignant tumor cell lines. The results of ChIP-PCR showed SMAD3 had a strong H3K27ac signal in eight common malignant tumors (Figure 7a), suggesting that the SE–TF regulatory network with SMAD3 as the core may be involved in the occurrence and development of a variety of malignant tumors.
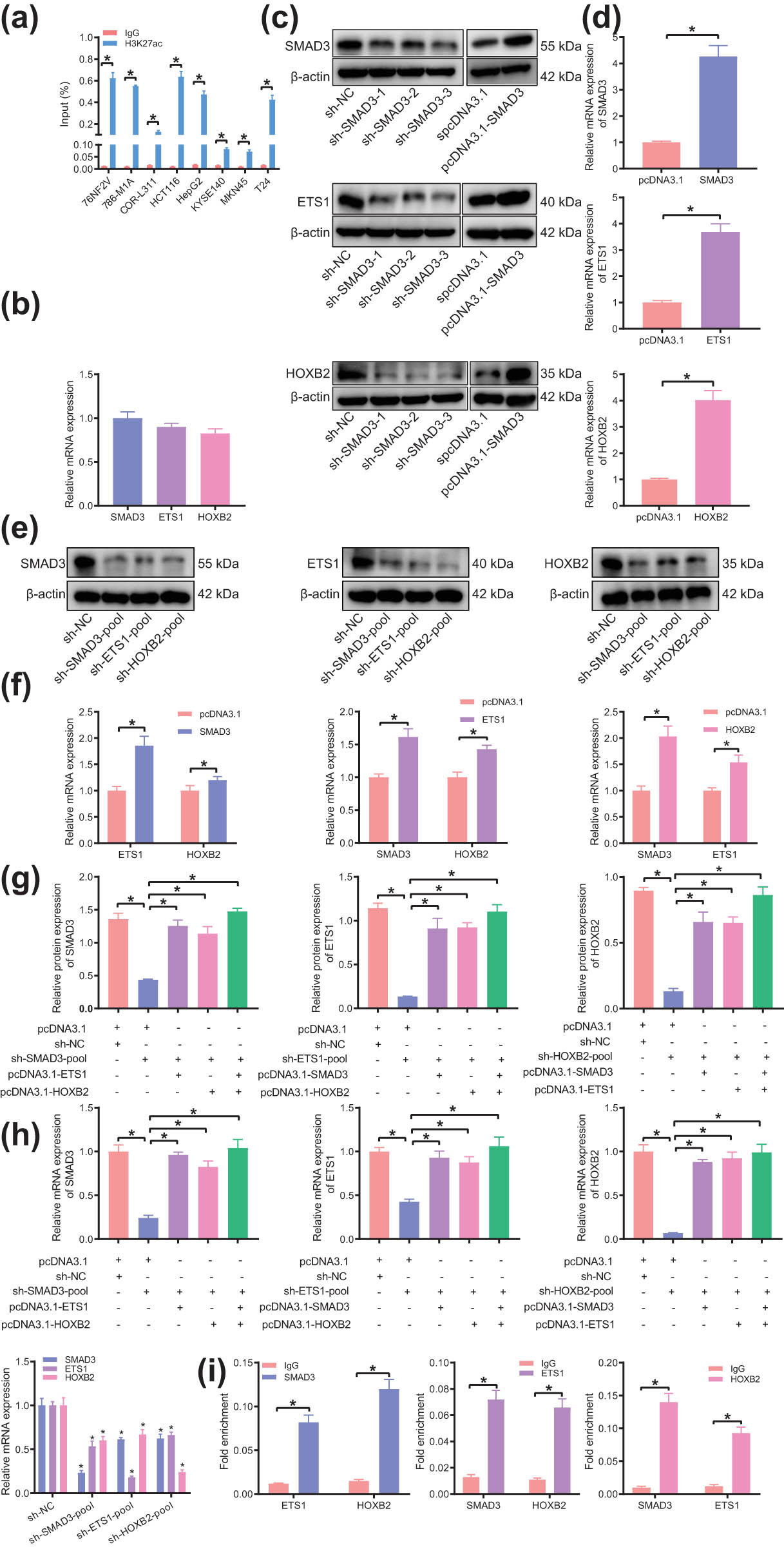
The presence of SE–TF regulatory network consisting of SMAD3, ETS1, and HOXB2 in T24 cells. (a) H3K27ac intensity of SMAD3 in eight common malignant tumor cell lines analyzed by ChIP-PCR. (b) Expression of core TFs of bladder cancer (SMAD3, ETS1, and HOXB2) in T24 cells determined by RT-qPCR. (c) Silencing and overexpression efficiency of SMAD3, ETS1, and HOXB2 in T24 cells determined by western blot analysis. (d) Overexpression efficiency of SMAD3, ETS1, and HOXB2 in T24 cells determined by RT-qPCR. (e) Expression of other two TFs in T24 cells following silencing of any bladder cancer TFs determined by western blot analysis. (f) Expression of other two TFs in T24 cells following overexpression of any bladder cancer TFs determined by RT-qPCR. (g) Expression of other TFs in T24 cells following intervention with two types of bladder cancer TFs determined by western blot analysis. (h) Expression of other TFs in T24 cells following intervention with two types of bladder cancer TFs determined by RT-qPCR. (i) The binding of each TF to the promoter regions of the other two TFs analyzed by ChIP-PCR. *p < 0.05. The experiment was conducted three times independently.
Further, we took bladder cancer as an example for verification. The core TFs of bladder cancer were SMAD3, ETS1, and HOXB2. The results of RT-qPCR showed (Figure 7b) these TFs were expressed at an average level in the bladder cancer cell line T24. T24 cells were transfected with shRNA and overexpression plasmids targeting these TFs, the efficiency of which was confirmed by RT-qPCR and western blot analysis (Figure 7c and d). In order to achieve the best silencing efficiency, the siRNA-pool was transfected into T24 cells where the expression of TFs was determined by RT-qPCR and western blot analysis. The results presented that silencing the expression of any TFs would result in a decrease in the expression of other TFs, and meanwhile, overexpression of any TFs would also lead to an increase of other TFs (Figure 7e and f). In addition, silencing any TFs and overexpressing other TFs simultaneously in T24 cells could partially reverse the above results (Figure 7g and h). These results indicated that SMAD3, ETS1, and HOXB2 in bladder cancer interacted with each other to construct a SE–TF regulatory network.
For further verification of the direct transcriptional regulation between these TFs, we used ChIP-PCR to detect the binding of each TF to the promoter regions of the other two TFs. The results displayed that each TF could bind to the promoter regions other than itself (Figure 7i), indicating that all TFs were connected to each other to form a network. The above results demonstrated the presence of a SE–TF regulatory network in bladder cancer.
3.9 SE–TF regulatory network enhances the malignant phenotype of bladder cancer cells
Finally, we attempted to elucidate the effect of the SE–TF regulatory network on the function of T24 cells. The results of Transwell and CCK-8 assays showed that overexpression of TFs stimulated the migration, invasion of T24 cells, accompanied by enhanced proliferation while silencing of TFs resulted in opposite results (Figure 8a). Furthermore, weakening of the malignant phenotype of T24 cells caused by silencing of SMAD3 could be partially reversed by overexpression of other one or two TFs (Figure 8b). The aforementioned findings indicated the promoting effect of the SE–TF regulatory network on the malignant phenotype of bladder cancer cells.
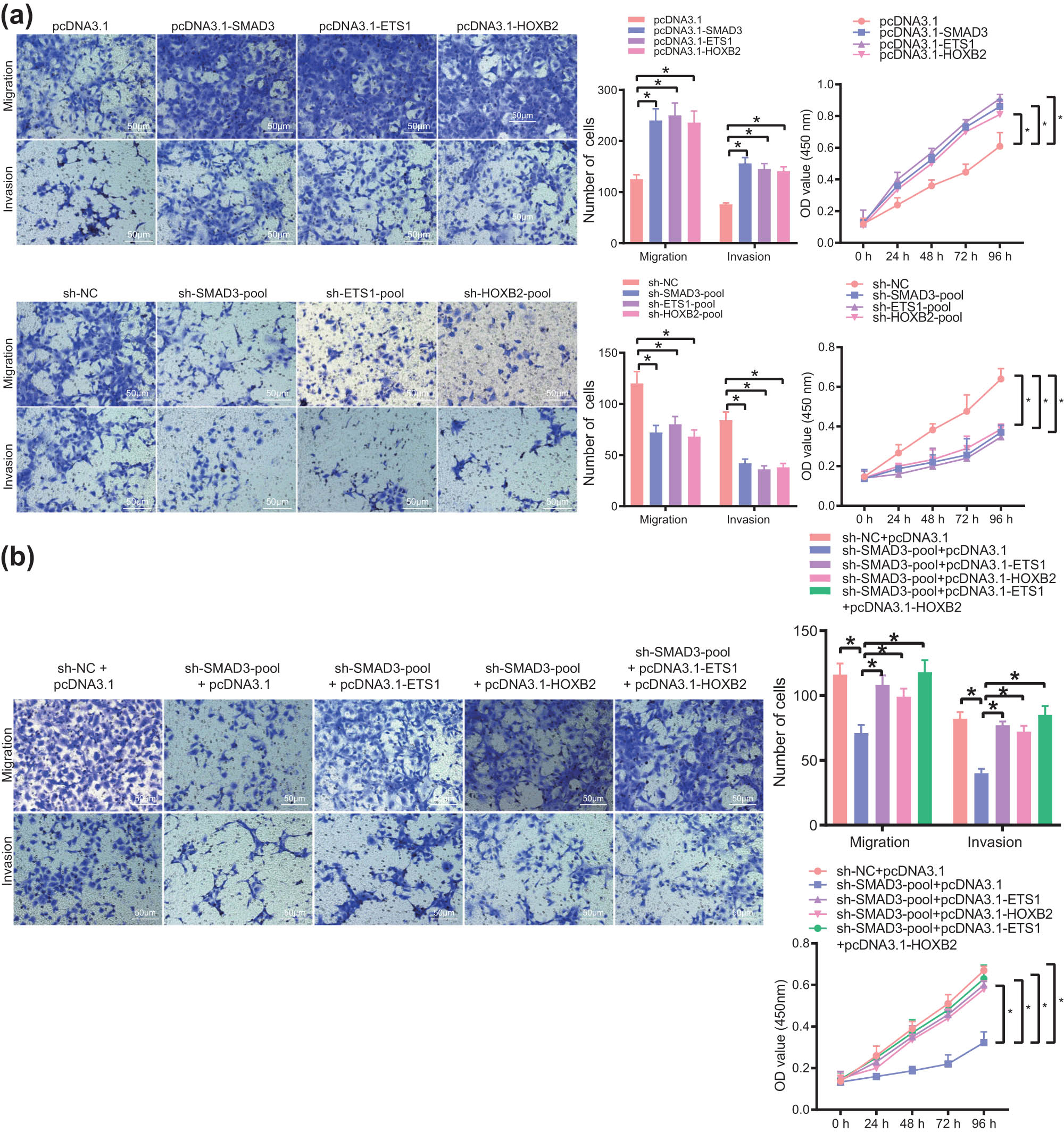
The SE–TF regulatory network consisting of SMAD3, ETS1, and HOXB2 promotes the malignant phenotype of bladder cancer cells. (a) Migration, invasion, and proliferation of T24 cells following overexpression or silencing of SMAD3, ETS1, and HOXB2 measured by Transwell and CCK-8 assays. (b) Migration, invasion, and proliferation of T24 cells following transfection with sh-SMAD3, sh-SMAD3 + pcDNA3.1-ETS1, sh-SMAD3 + pcDNA3.1-HOXB2, or sh-SMAD3 + pcDNA3.1-ETS1 + pcDNA3.1-HOXB2 measured by Transwell and CCK-8 assays. *p < 0.05. The experiment was conducted three times independently.
4 Discussion
SEs and TFs are associated with many genes involved in cancer pathogenesis [5,25]. Primary oncogene promoters of tumor cells are controlled by SE, rendering selective activation of transcription [26]. Mechanisms of abnormal SE formation in malignant tumors have been demonstrated to exhibit profound influence on both molecular pathogenesis and clinical management [5]. Our study is to identify and validate core SEs and TFs in eight common malignant tumors based on H3K27ac ChIP-seq in the SRA database as well as RNA-seq data in the TCGA database, followed by verifications in bladder cancer cell experiments.
First, we obtained H3K27ac and Input ChIP-seq sequencing data of eight common malignant tumor cell lines including gastric cancer through the SRA database and downloaded FPKM data of all RNA-seq from the TCGA database for verification. Similarly, other research works also downloaded H3K27ac and Input ChIP-seq sequencing data of tumor cell lines from the SRA database [27,28,29]. Unique to our result, FPKM data of all RNA-seq were downloaded from the TCGA database for verification; ChIP-seq sequencing data were not specially processed and from the same malignant tumor cell lines before merging. This study targets eight common malignant tumors, whereas the former reports only refer to a kind of malignant tumor [3,30]. Then, MACS2 software was used to find the H3K27ac peak in each malignant tumor cell line. Taking the significant H3K27ac peak at more than 2.5 kb from the TSS site as the enhancer, we found that abundant enhancers existed in each malignant tumor cell line, which was consistent with the tendency of the significant H3K27ac peak. The NCF2 gene was selected and IGV was used to show the H3K27ac signal on the enhancer of eight common malignant tumors. Generally, Bowtie2 is used for comparison in the preliminary processing of ChIP-seq sequencing data, and the only comparison data are obtained by Samtools to deduplicate [31,32,33]. Differently, this study merged data from the same tumor cell line and identified the significant H3K27ac peak through the MACS2 software. The significant H3K27ac peak at more than 2.5 kb from the TSS site was used as the enhancer. Then the significant H3K27ac peak was compared with the identified enhancer, and finally, all data analyzed were displayed as a whole. Next, we found that H3K27ac signals of same malignant tumor cell line could be clustered together by analyzing the correlation of H3K27ac signals, indicating that H3K27ac pattern is different and the H3K27ac signal can distinguish malignant tumors. We used the ROSE algorithm to predict SEs. A single enhancer entity within the range of 12.5 kb was merged and SEs that occurred in at least two cell lines were calculated so that the range was diminished and the stability of the prediction results was improved.
In addition, we examined all SE-regulated genes in eight common malignant tumors. Thirty-five genes that occurred more than six times in all malignant tumors were defined as conservative SE-regulated genes, and 2,154 genes were specific for each malignant tumor sample. The data analyzed in this study were only for SEs that occurred in at least two cell lines, and the specific and conservative SEs were defined differently. The defined SEs were verified by IGV, and the SE-regulated genes in all malignant tumors were obtained from the TCGA database. Finally, the expression data in eight common malignant tumors were compared with the analysis results of H3K27ac ChIP-seq data. Generally, the core TFs that bound to SEs in each malignant tumor sample were found with HOMER software [34,35,36]. Importantly, the TFs that appeared more than four times in malignant tumors were defined as malignant tumor-conservative TFs, while TFs that appeared only once were defined as malignant tumor-specific TFs. Based on TCGA RNA-seq data, we calculated the expression of all TFs in each type of malignant tumor, which can be distinguished according to the defined specific and conservative TFs. Conservative TF KLF5 and specific TF POU2F2 were chosen to exhibit the expression of all malignant tumors through a beeswarm package, which also could distinguish malignant tumor-specific TFs from conservative TFs. Finally, CRC TFs were predicted in eight common malignant tumor cell lines through the CRCmapper software, which is the same as other reports [37,38]. This study combined with the expression profile data of patients in the TCGA database, and also showed some of the identified CRC TFs near SE H3K27ac signal through the IGV. Furthermore, the in vitro experimental data demonstrated the presence of a SE–TF regulatory network in bladder cancer, and the SE–TF regulatory network enhanced the malignant phenotype of bladder cancer cells.
In conclusion, we integrated the H3K27ac ChIP-seq of eight common malignant tumor cell lines and RNA-seq from cancer patients in the TCGA database and identified core SEs and TFs. In all malignant tumor samples, there were 35 SE-regulated genes that occurred more than six times, while 2,154 SE-regulated genes occurred only once, which were defined as conservative SE-regulated genes and specific SE-regulated genes, respectively. We found the core TFs bound to SEs in each malignant tumor sample with HOMER. The TFs that appeared more than four times in tumor samples were defined as malignant tumor-conservative TFs including Fral, KLF5, and NFY, while TFs that appeared only once were defined as specific TFs such as POU2F2. Finally, eight common malignant tumor cell lines including 76NF2V and 786-M1A were selected to predict CRC TFs with CRCmapper software. A total of 60 CRC TFs were obtained, among which SMAD3 were present in five types of common malignant tumors, while 46 CRC TFs including NR5A1 were only present in one type of malignant tumor. Taken together, our study provides new ideas for the research of these malignant tumors and experimental validations in cancer cells. However, identified conservative and specific SEs and TFs need to be verified by basic experiments, which might indicate a promising method to improve malignant tumor therapy.
Acknowledgements
The authors would like to acknowledge the helpful suggestions concerning this study received from their colleagues.
-
Funding information: None.
-
Author contributions: L.L.L., T.X., and B.R.L. designed the study. Y.L. and D.L.Z. collated the data, carried out data analyses, and produced the initial draft of the manuscript. L.L.L. and D.L.Z. contributed to drafting the manuscript. All authors have read and approved the final submitted manuscript.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Data availability statement: The datasets generated/analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
Primer sequences for ChIP-PCR
| Gene | Sequence |
|---|---|
| SMAD3 (human) | Forward: 5ʹ-CTCCTGTCTTGCCCCACTTT-3ʹ |
| Reverse: 5ʹ-GGTTGGACTCGCAGCAAGTA-3ʹ | |
| ETS1 (human) | Forward: 5ʹ-GGTCGTGGGAGGGTTGTTAG-3ʹ |
| Reverse: 5ʹ-CCGTCTGATTCTCCACGCAT-3ʹ | |
| HOXB2 (human) | Forward: 5ʹ-AGCCTCTTTCGACTCCCTCT-3ʹ |
| Reverse: 5ʹ-CGCGGGGAAAGAGTTTAGGT-3ʹ |
Primer sequences for RT-qPCR
| Gene | Sequence |
|---|---|
| SMAD3 (human) | Forward: 5ʹ-TGGACGCAGGTTCTCCAAAC-3ʹ |
| Reverse: 5ʹ-CCGGCTCGCAGTAGGTAAC-3ʹ | |
| ETS1 (human) | Forward: 5ʹ-GATAGTTGTGATCGCCTCACC-3ʹ |
| Reverse: 5ʹ-GTCCTCTGAGTCGAAGCTGTC-3ʹ | |
| HOXB2 (human) | Forward: 5ʹ-CGCCAGGATTCACCTTTCCTT-3ʹ |
| Reverse: 5ʹ-CCCTGTAGGCTAGGGGAGAG-3ʹ | |
| GAPDH (human) | Forward: 5ʹ-GGAGCGACATCCCTCCAAAAT-3ʹ |
| Reverse: 5ʹ-GGCTGTTGTCATACTTCTCATGG-3ʹ |
Data information of eight common malignant tumor cell lines in the SRA database
| Malignant tumor type | Number of cell lines | Reference |
|---|---|---|
| Gastric cancer | 6 | Baek Su-Jin et al. 2016 Oncotarget |
| Renal cancer | 4 | Baek Su-Jin et al. 2016 Oncotarget |
| Esophageal squamous cell carcinoma | 6 | Jiang Yuan et al. 2018 Nature communications. |
| Colorectal cancer | 24 | Jiang Yan-Yi et al. 2016 Gut |
| Bladder cancer | 3 | Cohen Andrea J et al. 2017 Nature communications |
| Breast cancer | 12 | Pattison Jillian M et al. 2016 Molecular cancer research |
| Small cell lung cancer | 14 | Franco Hector L et al. 2018 Genome research |
| Liver cancer | 2 | Huang Yu-Han et al. 2018 Genes & development |
Information and classification of SE-regulated genes in eight malignant tumor cell lines
| SE-regulated target gene type | Gene |
|---|---|
| Conservative target genes | ABALON, BCL9L, DLGAP1-AS2, LINC00963, EPHA2, MALAT1, MIR21, NEAT1, TNRC18, UBC, etc. (2154 genes in total) |
| Specific target genes | A2M, AADACL2-AS1, ABCA1, ABCA13, ABCB1, ABCB11, BCC4, ABCG1, ABHD16B, ABHD17C, etc. (35 genes in total) |
Core transcription factors in eight malignant tumor cell lines
| Malignant tumor cell line | Transcription factors with the highest score |
|---|---|
| Breast cancer 76NF2V | SMAD3, RUNX1, KLF5, TCF7L2, SOX9, MAF, ELF3, ERG, KLF4, EHF, FOXC1, MYC, FOSL2, IKZF2, TGIF1, DLX2, FOXD2 |
| Renal cancer 786-M1A | SMAD3, NR3C1, FOXL1, GLIS3, RUNX2, KLF7, NFIL3, ETV6, ETS1, HNF1B, GLI2, KLF4, RUNX1 |
| Small cell lung cancer COR-L311 | NFATC1, SOX9, GFI1B, ASCL2, POU2F3, KLF13, PRDM1, INSM1, HMX2, SOX13, MEF2D |
| Colorectal cancer HCT116 | HIF1A, NR5A2, RREB1, JUNB, BHLHE40, HES1, MEF2D, MYC, NR4A1, SMAD3 |
| Liver cancer HepG2 | TBX2, MAX, E2F6, RREB1, SMAD3, EHF, SREBF1, ELF1, HES1, FOXA1, FOXQ1, HNF1A, NR5A1, KLF16, FOXP1 |
| Esophageal squamous cell carcinoma KYSE14 | SOX2, EOMES, HOXA7 |
| Gastric cancer MKN45 | ELF3, RREB1, BHLHE40, JUNB, EHF, HES1, HNF1B, MEF2A |
| Bladder cancer T24 | SMAD3, ETS1, HOXB2 |
Reference
[1] Peng Y, Zhang Y. Enhancer and super-enhancer: positive regulators in gene transcription. Animal Model Exp Med. 2018;1(3):169–79. 10.1002/ame2.12032.Search in Google Scholar PubMed PubMed Central
[2] Suzuki HI, Young RA, Sharp PA. Super-enhancer-mediated RNA processing revealed by integrative microRNA network analysis. Cell. 2017;168(6):1000–14. 10.1016/j.cell.2017.02.015.Search in Google Scholar PubMed PubMed Central
[3] Ma Q, Yang F, Mackintosh C, Jayani RS, Oh S, Jin C, et al. Super-enhancer redistribution as a mechanism of broad gene dysregulation in repeatedly drug-treated cancer cells. Cell Rep. 2020;31(3):107532. 10.1016/j.celrep.2020.107532.Search in Google Scholar PubMed
[4] Sengupta S, George RE. Super-enhancer-driven transcriptional dependencies in cancer trends. Cancer. 2017;3(4):269–81. 10.1016/j.trecan.2017.03.006.Search in Google Scholar PubMed PubMed Central
[5] Thandapani P. Super-enhancers in cancer. Pharmacol Ther. 2019;199:129–38. 10.1016/j.pharmthera.2019.02.014.Search in Google Scholar PubMed
[6] Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320–34. 10.1016/j.cell.2013.03.036.Search in Google Scholar PubMed PubMed Central
[7] Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. 10.1038/nature08822.Search in Google Scholar PubMed PubMed Central
[8] Oldridge DA, Wood AC, Weichert-Leahey N, Crimmins I, Sussman R, Winter C, et al. Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. Nature. 2015;528(7582):418–21. 10.1038/nature15540.Search in Google Scholar PubMed PubMed Central
[9] Alam H, Tang M, Maitituoheti M, Dhar SS, Kumar M, Han CY, et al. KMT2D deficiency impairs super-enhancers to confer a glycolytic vulnerability in lung cancer. Cancer Cell. 2020;37(4):599–617. 10.1016/j.ccell.2020.03.005.Search in Google Scholar PubMed PubMed Central
[10] Ying Y, Wang Y, Huang X, Sun Y, Zhang J, Li M, et al. Oncogenic HOXB8 is driven by MYC-regulated super-enhancer and potentiates colorectal cancer invasiveness via BACH1. Oncogene. 2020;39(5):1004–17. 10.1038/s41388-019-1013-1.Search in Google Scholar PubMed
[11] Cao X, Dang L, Zheng X, Lu Y, Lu Y, Ji R, et al. Targeting super-enhancer-driven oncogenic transcription by CDK7 inhibition in anaplastic thyroid carcinoma. Thyroid. 2019;29(6):809–23. 10.1089/thy.2018.0550.Search in Google Scholar PubMed
[12] Zhang L, Xue G, Liu J, Li Q, Wang Y. Revealing transcription factor and histone modification co-localization and dynamics across cell lines by integrating ChIP-seq and RNA-seq data. BMC Genomics. 2018;19(Suppl 10):914. 10.1186/s12864-018-5278-5.Search in Google Scholar PubMed PubMed Central
[13] Wang S, Zang C, Xiao T, Fan J, Mei S, Qin Q, et al. Modeling cis-regulation with a compendium of genome-wide histone H3K27ac profiles. Genome Res. 2016;26(10):1417–29. 10.1101/gr.201574.115.Search in Google Scholar PubMed PubMed Central
[14] Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–9. 10.1093/bioinformatics/btp352.Search in Google Scholar PubMed PubMed Central
[15] Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9(9):R137. 10.1186/gb-2008-9-9-r137.Search in Google Scholar PubMed PubMed Central
[16] Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. DeepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 2014;42(Web Server Issue):W187–91. 10.1093/nar/gku365.Search in Google Scholar PubMed PubMed Central
[17] Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153(2):307–19. 10.1016/j.cell.2013.03.035.Search in Google Scholar PubMed PubMed Central
[18] Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–2. 10.1093/bioinformatics/btq033.Search in Google Scholar PubMed PubMed Central
[19] Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38(4):576–89. 10.1016/j.molcel.2010.05.004.Search in Google Scholar PubMed PubMed Central
[20] Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. 10.1093/nar/gkn923.Search in Google Scholar PubMed PubMed Central
[21] Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. 10.1038/nprot.2008.211.Search in Google Scholar PubMed
[22] Saint-Andre V, Federation AJ, Lin CY, Abraham BJ, Reddy J, Lee TI, et al. Models of human core transcriptional regulatory circuitries. Genome Res. 2016;26(3):385–96. 10.1101/gr.197590.115.Search in Google Scholar PubMed PubMed Central
[23] Hnisz D, Abraham BJ, Lee TI, Lau A, Saint-Andre V, Sigova AA, et al. Super-enhancers in the control of cell identity and disease. Cell. 2013;155(4):934–47. 10.1016/j.Search in Google Scholar
[24] Jefferson WN, Kinyamu HK, Wang T, Miranda AX, Padilla-Banks E, Suen AA, et al. Widespread enhancer activation via ERalpha mediates estrogen response in vivo during uterine development. Nucleic Acids Res. 2018;46(11):5487–503. 10.1093/nar/gky260.Search in Google Scholar PubMed PubMed Central
[25] Deng YN, Xia Z, Zhang P, Ejaz S, Liang S. Transcription factor RREB1: from target genes towards biological functions. Int J Biol Sci. 2020;16(8):1463–73. 10.7150/ijbs.40834.Search in Google Scholar PubMed PubMed Central
[26] Niederriter AR, Varshney A, Parker SC, Martin DM. Super enhancers in cancers, complex disease, and developmental disorders. Genes (Basel). 2015;6(4):1183–200. 10.3390/genes6041183.Search in Google Scholar PubMed PubMed Central
[27] Qin Q, Fan J, Zheng R, Wan C, Mei S, Wu Q, et al. Lisa: inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP-seq data. Genome Biol. 2020;21(1):32. 10.1186/s13059-020-1934-6.Search in Google Scholar PubMed PubMed Central
[28] Ai S, Xiong H, Li CC, Luo Y, Shi Q, Liu Y, et al. Profiling chromatin states using single-cell itChIP-seq. Nat Cell Biol. 2019;21(9):1164–72. 10.1038/s41556-019-0383-5.Search in Google Scholar PubMed
[29] Light N, Adoue V, Ge B, Chen SH, Kwan T, Pastinen T. Interrogation of allelic chromatin states in human cells by high-density ChIP-genotyping. Epigenetics. 2014;9(9):1238–51. 10.4161/epi.29920.Search in Google Scholar PubMed PubMed Central
[30] Jiang Y, Jiang YY, Xie JJ, Mayakonda A, Hazawa M, Chen L, et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat Commun. 2018;9(1):3619. 10.1038/s41467-018-06081-9.Search in Google Scholar PubMed PubMed Central
[31] Liu P, Jiang W, Zhou S, Gao J, Zhang H. Combined analysis of ChIP sequencing and gene expression dataset in breast cancer. Pathol Oncol Res. 2017;23(2):361–8. 10.1007/s12253-016-0116-z.Search in Google Scholar PubMed
[32] Yevshin I, Sharipov R, Valeev T, Kel A, Kolpakov F. GTRD: a database of transcription factor binding sites identified by ChIP-seq experiments. Nucleic Acids Res. 2017;45(D1):D61–7. 10.1093/nar/gkw951.Search in Google Scholar PubMed PubMed Central
[33] Ho TH, Nateras RN, Yan H, Park JG, Jensen S, Borges C, et al. A multidisciplinary biospecimen bank of renal cell carcinomas compatible with discovery platforms at mayo clinic, Scottsdale, Arizona. PLoS One. 2015;10(7):e0132831. 10.1371/journal.pone.0132831.Search in Google Scholar PubMed PubMed Central
[34] Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell. 2019;178(2):316–29. 10.1016/j.cell.2019.06.003.Search in Google Scholar PubMed PubMed Central
[35] Anagnostou VK, Syrigos KN, Bepler G, Homer RJ, Rimm DL. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol. 2009;27(2):271–8. 10.1200/JCO.2008.17.0043.Search in Google Scholar PubMed
[36] Forloni M, Gupta R, Nagarajan A, Sun LS, Dong Y, Pirazzoli V, et al. Oncogenic EGFR represses the TET1 DNA demethylase to induce silencing of tumor suppressors in cancer cells. Cell Rep. 2016;16(2):457–71. 10.1016/j.celrep.2016.05.087.Search in Google Scholar PubMed PubMed Central
[37] Milde-Langosch K. The Fos family of transcription factors and their role in tumourigenesis. Eur J Cancer. 2005;41(16):2449–61. 10.1016/j.ejca.2005.08.008.Search in Google Scholar PubMed
[38] Tang W, Zhou W, Xiang L, Wu X, Zhang P, Wang J, et al. The p300/YY1/miR-500a-5p/HDAC2 signalling axis regulates cell proliferation in human colorectal cancer. Nat Commun. 2019;10(1):663. 10.1038/s41467-018-08225-3.Search in Google Scholar PubMed PubMed Central
© 2021 Yuan Liang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Identification of ZG16B as a prognostic biomarker in breast cancer
- Behçet’s disease with latent Mycobacterium tuberculosis infection
- Erratum
- Erratum to “Suffering from Cerebral Small Vessel Disease with and without Metabolic Syndrome”
- Research Articles
- GPR37 promotes the malignancy of lung adenocarcinoma via TGF-β/Smad pathway
- Expression and role of ABIN1 in sepsis: In vitro and in vivo studies
- Additional baricitinib loading dose improves clinical outcome in COVID-19
- The co-treatment of rosuvastatin with dapagliflozin synergistically inhibited apoptosis via activating the PI3K/AKt/mTOR signaling pathway in myocardial ischemia/reperfusion injury rats
- SLC12A8 plays a key role in bladder cancer progression and EMT
- LncRNA ATXN8OS enhances tamoxifen resistance in breast cancer
- Case Report
- Serratia marcescens as a cause of unfavorable outcome in the twin pregnancy
- Spleno-adrenal fusion mimicking an adrenal metastasis of a renal cell carcinoma: A case report and embryological background
- Research Articles
- TRIM25 contributes to the malignancy of acute myeloid leukemia and is negatively regulated by microRNA-137
- CircRNA circ_0004370 promotes cell proliferation, migration, and invasion and inhibits cell apoptosis of esophageal cancer via miR-1301-3p/COL1A1 axis
- LncRNA XIST regulates atherosclerosis progression in ox-LDL-induced HUVECs
- Potential role of IFN-γ and IL-5 in sepsis prediction of preterm neonates
- Rapid Communication
- COVID-19 vaccine: Call for employees in international transportation industries and international travelers as the first priority in global distribution
- Case Report
- Rare squamous cell carcinoma of the kidney with concurrent xanthogranulomatous pyelonephritis: A case report and review of the literature
- An infertile female delivered a baby after removal of primary renal carcinoid tumor
- Research Articles
- Hypertension, BMI, and cardiovascular and cerebrovascular diseases
- Case Report
- Coexistence of bilateral macular edema and pale optic disc in the patient with Cohen syndrome
- Research Articles
- Correlation between kinematic sagittal parameters of the cervical lordosis or head posture and disc degeneration in patients with posterior neck pain
- Review Articles
- Hepatoid adenocarcinoma of the lung: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database
- Research Articles
- Thermography in the diagnosis of carpal tunnel syndrome
- Pemetrexed-based first-line chemotherapy had particularly prominent objective response rate for advanced NSCLC: A network meta-analysis
- Comparison of single and double autologous stem cell transplantation in multiple myeloma patients
- The influence of smoking in minimally invasive spinal fusion surgery
- Impact of body mass index on left atrial dimension in HOCM patients
- Expression and clinical significance of CMTM1 in hepatocellular carcinoma
- miR-142-5p promotes cervical cancer progression by targeting LMX1A through Wnt/β-catenin pathway
- Comparison of multiple flatfoot indicators in 5–8-year-old children
- Early MRI imaging and follow-up study in cerebral amyloid angiopathy
- Intestinal fatty acid-binding protein as a biomarker for the diagnosis of strangulated intestinal obstruction: A meta-analysis
- miR-128-3p inhibits apoptosis and inflammation in LPS-induced sepsis by targeting TGFBR2
- Dynamic perfusion CT – A promising tool to diagnose pancreatic ductal adenocarcinoma
- Biomechanical evaluation of self-cinching stitch techniques in rotator cuff repair: The single-loop and double-loop knot stitches
- Review Articles
- The ambiguous role of mannose-binding lectin (MBL) in human immunity
- Case Report
- Membranous nephropathy with pulmonary cryptococcosis with improved 1-year follow-up results: A case report
- Fertility problems in males carrying an inversion of chromosome 10
- Acute myeloid leukemia with leukemic pleural effusion and high levels of pleural adenosine deaminase: A case report and review of literature
- Metastatic renal Ewing’s sarcoma in adult woman: Case report and review of the literature
- Burkitt-like lymphoma with 11q aberration in a patient with AIDS and a patient without AIDS: Two cases reports and literature review
- Skull hemophilia pseudotumor: A case report
- Judicious use of low-dosage corticosteroids for non-severe COVID-19: A case report
- Adult-onset citrullinaemia type II with liver cirrhosis: A rare cause of hyperammonaemia
- Clinicopathologic features of Good’s syndrome: Two cases and literature review
- Fatal immune-related hepatitis with intrahepatic cholestasis and pneumonia associated with camrelizumab: A case report and literature review
- Research Articles
- Effects of hydroxyethyl starch and gelatin on the risk of acute kidney injury following orthotopic liver transplantation: A multicenter retrospective comparative clinical study
- Significance of nucleic acid positive anal swab in COVID-19 patients
- circAPLP2 promotes colorectal cancer progression by upregulating HELLS by targeting miR-335-5p
- Ratios between circulating myeloid cells and lymphocytes are associated with mortality in severe COVID-19 patients
- Risk factors of left atrial appendage thrombus in patients with non-valvular atrial fibrillation
- Clinical features of hypertensive patients with COVID-19 compared with a normotensive group: Single-center experience in China
- Surgical myocardial revascularization outcomes in Kawasaki disease: systematic review and meta-analysis
- Decreased chromobox homologue 7 expression is associated with epithelial–mesenchymal transition and poor prognosis in cervical cancer
- FGF16 regulated by miR-520b enhances the cell proliferation of lung cancer
- Platelet-rich fibrin: Basics of biological actions and protocol modifications
- Accurate diagnosis of prostate cancer using logistic regression
- miR-377 inhibition enhances the survival of trophoblast cells via upregulation of FNDC5 in gestational diabetes mellitus
- Prognostic significance of TRIM28 expression in patients with breast carcinoma
- Integrative bioinformatics analysis of KPNA2 in six major human cancers
- Exosomal-mediated transfer of OIP5-AS1 enhanced cell chemoresistance to trastuzumab in breast cancer via up-regulating HMGB3 by sponging miR-381-3p
- A four-lncRNA signature for predicting prognosis of recurrence patients with gastric cancer
- Knockdown of circ_0003204 alleviates oxidative low-density lipoprotein-induced human umbilical vein endothelial cells injury: Circulating RNAs could explain atherosclerosis disease progression
- Propofol postpones colorectal cancer development through circ_0026344/miR-645/Akt/mTOR signal pathway
- Knockdown of lncRNA TapSAKI alleviates LPS-induced injury in HK-2 cells through the miR-205/IRF3 pathway
- COVID-19 severity in relation to sociodemographics and vitamin D use
- Clinical analysis of 11 cases of nocardiosis
- Cis-regulatory elements in conserved non-coding sequences of nuclear receptor genes indicate for crosstalk between endocrine systems
- Four long noncoding RNAs act as biomarkers in lung adenocarcinoma
- Real-world evidence of cytomegalovirus reactivation in non-Hodgkin lymphomas treated with bendamustine-containing regimens
- Relation between IL-8 level and obstructive sleep apnea syndrome
- circAGFG1 sponges miR-28-5p to promote non-small-cell lung cancer progression through modulating HIF-1α level
- Nomogram prediction model for renal anaemia in IgA nephropathy patients
- Effect of antibiotic use on the efficacy of nivolumab in the treatment of advanced/metastatic non-small cell lung cancer: A meta-analysis
- NDRG2 inhibition facilitates angiogenesis of hepatocellular carcinoma
- A nomogram for predicting metabolic steatohepatitis: The combination of NAMPT, RALGDS, GADD45B, FOSL2, RTP3, and RASD1
- Clinical and prognostic features of MMP-2 and VEGF in AEG patients
- The value of miR-510 in the prognosis and development of colon cancer
- Functional implications of PABPC1 in the development of ovarian cancer
- Prognostic value of preoperative inflammation-based predictors in patients with bladder carcinoma after radical cystectomy
- Sublingual immunotherapy increases Treg/Th17 ratio in allergic rhinitis
- Prediction of improvement after anterior cruciate ligament reconstruction
- Effluent Osteopontin levels reflect the peritoneal solute transport rate
- circ_0038467 promotes PM2.5-induced bronchial epithelial cell dysfunction
- Significance of miR-141 and miR-340 in cervical squamous cell carcinoma
- Association between hair cortisol concentration and metabolic syndrome
- Microvessel density as a prognostic indicator of prostate cancer: A systematic review and meta-analysis
- Characteristics of BCR–ABL gene variants in patients of chronic myeloid leukemia
- Knee alterations in rheumatoid arthritis: Comparison of US and MRI
- Long non-coding RNA TUG1 aggravates cerebral ischemia and reperfusion injury by sponging miR-493-3p/miR-410-3p
- lncRNA MALAT1 regulated ATAD2 to facilitate retinoblastoma progression via miR-655-3p
- Development and validation of a nomogram for predicting severity in patients with hemorrhagic fever with renal syndrome: A retrospective study
- Analysis of COVID-19 outbreak origin in China in 2019 using differentiation method for unusual epidemiological events
- Laparoscopic versus open major liver resection for hepatocellular carcinoma: A case-matched analysis of short- and long-term outcomes
- Travelers’ vaccines and their adverse events in Nara, Japan
- Association between Tfh and PGA in children with Henoch–Schönlein purpura
- Can exchange transfusion be replaced by double-LED phototherapy?
- circ_0005962 functions as an oncogene to aggravate NSCLC progression
- Circular RNA VANGL1 knockdown suppressed viability, promoted apoptosis, and increased doxorubicin sensitivity through targeting miR-145-5p to regulate SOX4 in bladder cancer cells
- Serum intact fibroblast growth factor 23 in healthy paediatric population
- Algorithm of rational approach to reconstruction in Fournier’s disease
- A meta-analysis of exosome in the treatment of spinal cord injury
- Src-1 and SP2 promote the proliferation and epithelial–mesenchymal transition of nasopharyngeal carcinoma
- Dexmedetomidine may decrease the bupivacaine toxicity to heart
- Hypoxia stimulates the migration and invasion of osteosarcoma via up-regulating the NUSAP1 expression
- Long noncoding RNA XIST knockdown relieves the injury of microglia cells after spinal cord injury by sponging miR-219-5p
- External fixation via the anterior inferior iliac spine for proximal femoral fractures in young patients
- miR-128-3p reduced acute lung injury induced by sepsis via targeting PEL12
- HAGLR promotes neuron differentiation through the miR-130a-3p-MeCP2 axis
- Phosphoglycerate mutase 2 is elevated in serum of patients with heart failure and correlates with the disease severity and patient’s prognosis
- Cell population data in identifying active tuberculosis and community-acquired pneumonia
- Prognostic value of microRNA-4521 in non-small cell lung cancer and its regulatory effect on tumor progression
- Mean platelet volume and red blood cell distribution width is associated with prognosis in premature neonates with sepsis
- 3D-printed porous scaffold promotes osteogenic differentiation of hADMSCs
- Association of gene polymorphisms with women urinary incontinence
- Influence of COVID-19 pandemic on stress levels of urologic patients
- miR-496 inhibits proliferation via LYN and AKT pathway in gastric cancer
- miR-519d downregulates LEP expression to inhibit preeclampsia development
- Comparison of single- and triple-port VATS for lung cancer: A meta-analysis
- Fluorescent light energy modulates healing in skin grafted mouse model
- Silencing CDK6-AS1 inhibits LPS-induced inflammatory damage in HK-2 cells
- Predictive effect of DCE-MRI and DWI in brain metastases from NSCLC
- Severe postoperative hyperbilirubinemia in congenital heart disease
- Baicalin improves podocyte injury in rats with diabetic nephropathy by inhibiting PI3K/Akt/mTOR signaling pathway
- Clinical factors predicting ureteral stent failure in patients with external ureteral compression
- Novel H2S donor proglumide-ADT-OH protects HUVECs from ox-LDL-induced injury through NF-κB and JAK/SATA pathway
- Triple-Endobutton and clavicular hook: A propensity score matching analysis
- Long noncoding RNA MIAT inhibits the progression of diabetic nephropathy and the activation of NF-κB pathway in high glucose-treated renal tubular epithelial cells by the miR-182-5p/GPRC5A axis
- Serum exosomal miR-122-5p, GAS, and PGR in the non-invasive diagnosis of CAG
- miR-513b-5p inhibits the proliferation and promotes apoptosis of retinoblastoma cells by targeting TRIB1
- Fer exacerbates renal fibrosis and can be targeted by miR-29c-3p
- The diagnostic and prognostic value of miR-92a in gastric cancer: A systematic review and meta-analysis
- Prognostic value of α2δ1 in hypopharyngeal carcinoma: A retrospective study
- No significant benefit of moderate-dose vitamin C on severe COVID-19 cases
- circ_0000467 promotes the proliferation, metastasis, and angiogenesis in colorectal cancer cells through regulating KLF12 expression by sponging miR-4766-5p
- Downregulation of RAB7 and Caveolin-1 increases MMP-2 activity in renal tubular epithelial cells under hypoxic conditions
- Educational program for orthopedic surgeons’ influences for osteoporosis
- Expression and function analysis of CRABP2 and FABP5, and their ratio in esophageal squamous cell carcinoma
- GJA1 promotes hepatocellular carcinoma progression by mediating TGF-β-induced activation and the epithelial–mesenchymal transition of hepatic stellate cells
- lncRNA-ZFAS1 promotes the progression of endometrial carcinoma by targeting miR-34b to regulate VEGFA expression
- Anticoagulation is the answer in treating noncritical COVID-19 patients
- Effect of late-onset hemorrhagic cystitis on PFS after haplo-PBSCT
- Comparison of Dako HercepTest and Ventana PATHWAY anti-HER2 (4B5) tests and their correlation with silver in situ hybridization in lung adenocarcinoma
- VSTM1 regulates monocyte/macrophage function via the NF-κB signaling pathway
- Comparison of vaginal birth outcomes in midwifery-led versus physician-led setting: A propensity score-matched analysis
- Treatment of osteoporosis with teriparatide: The Slovenian experience
- New targets of morphine postconditioning protection of the myocardium in ischemia/reperfusion injury: Involvement of HSP90/Akt and C5a/NF-κB
- Superenhancer–transcription factor regulatory network in malignant tumors
- β-Cell function is associated with osteosarcopenia in middle-aged and older nonobese patients with type 2 diabetes: A cross-sectional study
- Clinical features of atypical tuberculosis mimicking bacterial pneumonia
- Proteoglycan-depleted regions of annular injury promote nerve ingrowth in a rabbit disc degeneration model
- Effect of electromagnetic field on abortion: A systematic review and meta-analysis
- miR-150-5p affects AS plaque with ASMC proliferation and migration by STAT1
- MALAT1 promotes malignant pleural mesothelioma by sponging miR-141-3p
- Effects of remifentanil and propofol on distant organ lung injury in an ischemia–reperfusion model
- miR-654-5p promotes gastric cancer progression via the GPRIN1/NF-κB pathway
- Identification of LIG1 and LIG3 as prognostic biomarkers in breast cancer
- MitoQ inhibits hepatic stellate cell activation and liver fibrosis by enhancing PINK1/parkin-mediated mitophagy
- Dissecting role of founder mutation p.V727M in GNE in Indian HIBM cohort
- circATP2A2 promotes osteosarcoma progression by upregulating MYH9
- Prognostic role of oxytocin receptor in colon adenocarcinoma
- Review Articles
- The function of non-coding RNAs in idiopathic pulmonary fibrosis
- Efficacy and safety of therapeutic plasma exchange in stiff person syndrome
- Role of cesarean section in the development of neonatal gut microbiota: A systematic review
- Small cell lung cancer transformation during antitumor therapies: A systematic review
- Research progress of gut microbiota and frailty syndrome
- Recommendations for outpatient activity in COVID-19 pandemic
- Rapid Communication
- Disparity in clinical characteristics between 2019 novel coronavirus pneumonia and leptospirosis
- Use of microspheres in embolization for unruptured renal angiomyolipomas
- COVID-19 cases with delayed absorption of lung lesion
- A triple combination of treatments on moderate COVID-19
- Social networks and eating disorders during the Covid-19 pandemic
- Letter
- COVID-19, WHO guidelines, pedagogy, and respite
- Inflammatory factors in alveolar lavage fluid from severe COVID-19 pneumonia: PCT and IL-6 in epithelial lining fluid
- COVID-19: Lessons from Norway tragedy must be considered in vaccine rollout planning in least developed/developing countries
- What is the role of plasma cell in the lamina propria of terminal ileum in Good’s syndrome patient?
- Case Report
- Rivaroxaban triggered multifocal intratumoral hemorrhage of the cabozantinib-treated diffuse brain metastases: A case report and review of literature
- CTU findings of duplex kidney in kidney: A rare duplicated renal malformation
- Synchronous primary malignancy of colon cancer and mantle cell lymphoma: A case report
- Sonazoid-enhanced ultrasonography and pathologic characters of CD68 positive cell in primary hepatic perivascular epithelioid cell tumors: A case report and literature review
- Persistent SARS-CoV-2-positive over 4 months in a COVID-19 patient with CHB
- Pulmonary parenchymal involvement caused by Tropheryma whipplei
- Mediastinal mixed germ cell tumor: A case report and literature review
- Ovarian female adnexal tumor of probable Wolffian origin – Case report
- Rare paratesticular aggressive angiomyxoma mimicking an epididymal tumor in an 82-year-old man: Case report
- Perimenopausal giant hydatidiform mole complicated with preeclampsia and hyperthyroidism: A case report and literature review
- Primary orbital ganglioneuroblastoma: A case report
- Primary aortic intimal sarcoma masquerading as intramural hematoma
- Sustained false-positive results for hepatitis A virus immunoglobulin M: A case report and literature review
- Peritoneal loose body presenting as a hepatic mass: A case report and review of the literature
- Chondroblastoma of mandibular condyle: Case report and literature review
- Trauma-induced complete pacemaker lead fracture 8 months prior to hospitalization: A case report
- Primary intradural extramedullary extraosseous Ewing’s sarcoma/peripheral primitive neuroectodermal tumor (PIEES/PNET) of the thoracolumbar spine: A case report and literature review
- Computer-assisted preoperative planning of reduction of and osteosynthesis of scapular fracture: A case report
- High quality of 58-month life in lung cancer patient with brain metastases sequentially treated with gefitinib and osimertinib
- Rapid response of locally advanced oral squamous cell carcinoma to apatinib: A case report
- Retrieval of intrarenal coiled and ruptured guidewire by retrograde intrarenal surgery: A case report and literature review
- Usage of intermingled skin allografts and autografts in a senior patient with major burn injury
- Retraction
- Retraction on “Dihydromyricetin attenuates inflammation through TLR4/NF-kappa B pathway”
- Special Issue Computational Intelligence Methodologies Meets Recurrent Cancers - Part I
- An artificial immune system with bootstrap sampling for the diagnosis of recurrent endometrial cancers
- Breast cancer recurrence prediction with ensemble methods and cost-sensitive learning
Articles in the same Issue
- Research Articles
- Identification of ZG16B as a prognostic biomarker in breast cancer
- Behçet’s disease with latent Mycobacterium tuberculosis infection
- Erratum
- Erratum to “Suffering from Cerebral Small Vessel Disease with and without Metabolic Syndrome”
- Research Articles
- GPR37 promotes the malignancy of lung adenocarcinoma via TGF-β/Smad pathway
- Expression and role of ABIN1 in sepsis: In vitro and in vivo studies
- Additional baricitinib loading dose improves clinical outcome in COVID-19
- The co-treatment of rosuvastatin with dapagliflozin synergistically inhibited apoptosis via activating the PI3K/AKt/mTOR signaling pathway in myocardial ischemia/reperfusion injury rats
- SLC12A8 plays a key role in bladder cancer progression and EMT
- LncRNA ATXN8OS enhances tamoxifen resistance in breast cancer
- Case Report
- Serratia marcescens as a cause of unfavorable outcome in the twin pregnancy
- Spleno-adrenal fusion mimicking an adrenal metastasis of a renal cell carcinoma: A case report and embryological background
- Research Articles
- TRIM25 contributes to the malignancy of acute myeloid leukemia and is negatively regulated by microRNA-137
- CircRNA circ_0004370 promotes cell proliferation, migration, and invasion and inhibits cell apoptosis of esophageal cancer via miR-1301-3p/COL1A1 axis
- LncRNA XIST regulates atherosclerosis progression in ox-LDL-induced HUVECs
- Potential role of IFN-γ and IL-5 in sepsis prediction of preterm neonates
- Rapid Communication
- COVID-19 vaccine: Call for employees in international transportation industries and international travelers as the first priority in global distribution
- Case Report
- Rare squamous cell carcinoma of the kidney with concurrent xanthogranulomatous pyelonephritis: A case report and review of the literature
- An infertile female delivered a baby after removal of primary renal carcinoid tumor
- Research Articles
- Hypertension, BMI, and cardiovascular and cerebrovascular diseases
- Case Report
- Coexistence of bilateral macular edema and pale optic disc in the patient with Cohen syndrome
- Research Articles
- Correlation between kinematic sagittal parameters of the cervical lordosis or head posture and disc degeneration in patients with posterior neck pain
- Review Articles
- Hepatoid adenocarcinoma of the lung: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database
- Research Articles
- Thermography in the diagnosis of carpal tunnel syndrome
- Pemetrexed-based first-line chemotherapy had particularly prominent objective response rate for advanced NSCLC: A network meta-analysis
- Comparison of single and double autologous stem cell transplantation in multiple myeloma patients
- The influence of smoking in minimally invasive spinal fusion surgery
- Impact of body mass index on left atrial dimension in HOCM patients
- Expression and clinical significance of CMTM1 in hepatocellular carcinoma
- miR-142-5p promotes cervical cancer progression by targeting LMX1A through Wnt/β-catenin pathway
- Comparison of multiple flatfoot indicators in 5–8-year-old children
- Early MRI imaging and follow-up study in cerebral amyloid angiopathy
- Intestinal fatty acid-binding protein as a biomarker for the diagnosis of strangulated intestinal obstruction: A meta-analysis
- miR-128-3p inhibits apoptosis and inflammation in LPS-induced sepsis by targeting TGFBR2
- Dynamic perfusion CT – A promising tool to diagnose pancreatic ductal adenocarcinoma
- Biomechanical evaluation of self-cinching stitch techniques in rotator cuff repair: The single-loop and double-loop knot stitches
- Review Articles
- The ambiguous role of mannose-binding lectin (MBL) in human immunity
- Case Report
- Membranous nephropathy with pulmonary cryptococcosis with improved 1-year follow-up results: A case report
- Fertility problems in males carrying an inversion of chromosome 10
- Acute myeloid leukemia with leukemic pleural effusion and high levels of pleural adenosine deaminase: A case report and review of literature
- Metastatic renal Ewing’s sarcoma in adult woman: Case report and review of the literature
- Burkitt-like lymphoma with 11q aberration in a patient with AIDS and a patient without AIDS: Two cases reports and literature review
- Skull hemophilia pseudotumor: A case report
- Judicious use of low-dosage corticosteroids for non-severe COVID-19: A case report
- Adult-onset citrullinaemia type II with liver cirrhosis: A rare cause of hyperammonaemia
- Clinicopathologic features of Good’s syndrome: Two cases and literature review
- Fatal immune-related hepatitis with intrahepatic cholestasis and pneumonia associated with camrelizumab: A case report and literature review
- Research Articles
- Effects of hydroxyethyl starch and gelatin on the risk of acute kidney injury following orthotopic liver transplantation: A multicenter retrospective comparative clinical study
- Significance of nucleic acid positive anal swab in COVID-19 patients
- circAPLP2 promotes colorectal cancer progression by upregulating HELLS by targeting miR-335-5p
- Ratios between circulating myeloid cells and lymphocytes are associated with mortality in severe COVID-19 patients
- Risk factors of left atrial appendage thrombus in patients with non-valvular atrial fibrillation
- Clinical features of hypertensive patients with COVID-19 compared with a normotensive group: Single-center experience in China
- Surgical myocardial revascularization outcomes in Kawasaki disease: systematic review and meta-analysis
- Decreased chromobox homologue 7 expression is associated with epithelial–mesenchymal transition and poor prognosis in cervical cancer
- FGF16 regulated by miR-520b enhances the cell proliferation of lung cancer
- Platelet-rich fibrin: Basics of biological actions and protocol modifications
- Accurate diagnosis of prostate cancer using logistic regression
- miR-377 inhibition enhances the survival of trophoblast cells via upregulation of FNDC5 in gestational diabetes mellitus
- Prognostic significance of TRIM28 expression in patients with breast carcinoma
- Integrative bioinformatics analysis of KPNA2 in six major human cancers
- Exosomal-mediated transfer of OIP5-AS1 enhanced cell chemoresistance to trastuzumab in breast cancer via up-regulating HMGB3 by sponging miR-381-3p
- A four-lncRNA signature for predicting prognosis of recurrence patients with gastric cancer
- Knockdown of circ_0003204 alleviates oxidative low-density lipoprotein-induced human umbilical vein endothelial cells injury: Circulating RNAs could explain atherosclerosis disease progression
- Propofol postpones colorectal cancer development through circ_0026344/miR-645/Akt/mTOR signal pathway
- Knockdown of lncRNA TapSAKI alleviates LPS-induced injury in HK-2 cells through the miR-205/IRF3 pathway
- COVID-19 severity in relation to sociodemographics and vitamin D use
- Clinical analysis of 11 cases of nocardiosis
- Cis-regulatory elements in conserved non-coding sequences of nuclear receptor genes indicate for crosstalk between endocrine systems
- Four long noncoding RNAs act as biomarkers in lung adenocarcinoma
- Real-world evidence of cytomegalovirus reactivation in non-Hodgkin lymphomas treated with bendamustine-containing regimens
- Relation between IL-8 level and obstructive sleep apnea syndrome
- circAGFG1 sponges miR-28-5p to promote non-small-cell lung cancer progression through modulating HIF-1α level
- Nomogram prediction model for renal anaemia in IgA nephropathy patients
- Effect of antibiotic use on the efficacy of nivolumab in the treatment of advanced/metastatic non-small cell lung cancer: A meta-analysis
- NDRG2 inhibition facilitates angiogenesis of hepatocellular carcinoma
- A nomogram for predicting metabolic steatohepatitis: The combination of NAMPT, RALGDS, GADD45B, FOSL2, RTP3, and RASD1
- Clinical and prognostic features of MMP-2 and VEGF in AEG patients
- The value of miR-510 in the prognosis and development of colon cancer
- Functional implications of PABPC1 in the development of ovarian cancer
- Prognostic value of preoperative inflammation-based predictors in patients with bladder carcinoma after radical cystectomy
- Sublingual immunotherapy increases Treg/Th17 ratio in allergic rhinitis
- Prediction of improvement after anterior cruciate ligament reconstruction
- Effluent Osteopontin levels reflect the peritoneal solute transport rate
- circ_0038467 promotes PM2.5-induced bronchial epithelial cell dysfunction
- Significance of miR-141 and miR-340 in cervical squamous cell carcinoma
- Association between hair cortisol concentration and metabolic syndrome
- Microvessel density as a prognostic indicator of prostate cancer: A systematic review and meta-analysis
- Characteristics of BCR–ABL gene variants in patients of chronic myeloid leukemia
- Knee alterations in rheumatoid arthritis: Comparison of US and MRI
- Long non-coding RNA TUG1 aggravates cerebral ischemia and reperfusion injury by sponging miR-493-3p/miR-410-3p
- lncRNA MALAT1 regulated ATAD2 to facilitate retinoblastoma progression via miR-655-3p
- Development and validation of a nomogram for predicting severity in patients with hemorrhagic fever with renal syndrome: A retrospective study
- Analysis of COVID-19 outbreak origin in China in 2019 using differentiation method for unusual epidemiological events
- Laparoscopic versus open major liver resection for hepatocellular carcinoma: A case-matched analysis of short- and long-term outcomes
- Travelers’ vaccines and their adverse events in Nara, Japan
- Association between Tfh and PGA in children with Henoch–Schönlein purpura
- Can exchange transfusion be replaced by double-LED phototherapy?
- circ_0005962 functions as an oncogene to aggravate NSCLC progression
- Circular RNA VANGL1 knockdown suppressed viability, promoted apoptosis, and increased doxorubicin sensitivity through targeting miR-145-5p to regulate SOX4 in bladder cancer cells
- Serum intact fibroblast growth factor 23 in healthy paediatric population
- Algorithm of rational approach to reconstruction in Fournier’s disease
- A meta-analysis of exosome in the treatment of spinal cord injury
- Src-1 and SP2 promote the proliferation and epithelial–mesenchymal transition of nasopharyngeal carcinoma
- Dexmedetomidine may decrease the bupivacaine toxicity to heart
- Hypoxia stimulates the migration and invasion of osteosarcoma via up-regulating the NUSAP1 expression
- Long noncoding RNA XIST knockdown relieves the injury of microglia cells after spinal cord injury by sponging miR-219-5p
- External fixation via the anterior inferior iliac spine for proximal femoral fractures in young patients
- miR-128-3p reduced acute lung injury induced by sepsis via targeting PEL12
- HAGLR promotes neuron differentiation through the miR-130a-3p-MeCP2 axis
- Phosphoglycerate mutase 2 is elevated in serum of patients with heart failure and correlates with the disease severity and patient’s prognosis
- Cell population data in identifying active tuberculosis and community-acquired pneumonia
- Prognostic value of microRNA-4521 in non-small cell lung cancer and its regulatory effect on tumor progression
- Mean platelet volume and red blood cell distribution width is associated with prognosis in premature neonates with sepsis
- 3D-printed porous scaffold promotes osteogenic differentiation of hADMSCs
- Association of gene polymorphisms with women urinary incontinence
- Influence of COVID-19 pandemic on stress levels of urologic patients
- miR-496 inhibits proliferation via LYN and AKT pathway in gastric cancer
- miR-519d downregulates LEP expression to inhibit preeclampsia development
- Comparison of single- and triple-port VATS for lung cancer: A meta-analysis
- Fluorescent light energy modulates healing in skin grafted mouse model
- Silencing CDK6-AS1 inhibits LPS-induced inflammatory damage in HK-2 cells
- Predictive effect of DCE-MRI and DWI in brain metastases from NSCLC
- Severe postoperative hyperbilirubinemia in congenital heart disease
- Baicalin improves podocyte injury in rats with diabetic nephropathy by inhibiting PI3K/Akt/mTOR signaling pathway
- Clinical factors predicting ureteral stent failure in patients with external ureteral compression
- Novel H2S donor proglumide-ADT-OH protects HUVECs from ox-LDL-induced injury through NF-κB and JAK/SATA pathway
- Triple-Endobutton and clavicular hook: A propensity score matching analysis
- Long noncoding RNA MIAT inhibits the progression of diabetic nephropathy and the activation of NF-κB pathway in high glucose-treated renal tubular epithelial cells by the miR-182-5p/GPRC5A axis
- Serum exosomal miR-122-5p, GAS, and PGR in the non-invasive diagnosis of CAG
- miR-513b-5p inhibits the proliferation and promotes apoptosis of retinoblastoma cells by targeting TRIB1
- Fer exacerbates renal fibrosis and can be targeted by miR-29c-3p
- The diagnostic and prognostic value of miR-92a in gastric cancer: A systematic review and meta-analysis
- Prognostic value of α2δ1 in hypopharyngeal carcinoma: A retrospective study
- No significant benefit of moderate-dose vitamin C on severe COVID-19 cases
- circ_0000467 promotes the proliferation, metastasis, and angiogenesis in colorectal cancer cells through regulating KLF12 expression by sponging miR-4766-5p
- Downregulation of RAB7 and Caveolin-1 increases MMP-2 activity in renal tubular epithelial cells under hypoxic conditions
- Educational program for orthopedic surgeons’ influences for osteoporosis
- Expression and function analysis of CRABP2 and FABP5, and their ratio in esophageal squamous cell carcinoma
- GJA1 promotes hepatocellular carcinoma progression by mediating TGF-β-induced activation and the epithelial–mesenchymal transition of hepatic stellate cells
- lncRNA-ZFAS1 promotes the progression of endometrial carcinoma by targeting miR-34b to regulate VEGFA expression
- Anticoagulation is the answer in treating noncritical COVID-19 patients
- Effect of late-onset hemorrhagic cystitis on PFS after haplo-PBSCT
- Comparison of Dako HercepTest and Ventana PATHWAY anti-HER2 (4B5) tests and their correlation with silver in situ hybridization in lung adenocarcinoma
- VSTM1 regulates monocyte/macrophage function via the NF-κB signaling pathway
- Comparison of vaginal birth outcomes in midwifery-led versus physician-led setting: A propensity score-matched analysis
- Treatment of osteoporosis with teriparatide: The Slovenian experience
- New targets of morphine postconditioning protection of the myocardium in ischemia/reperfusion injury: Involvement of HSP90/Akt and C5a/NF-κB
- Superenhancer–transcription factor regulatory network in malignant tumors
- β-Cell function is associated with osteosarcopenia in middle-aged and older nonobese patients with type 2 diabetes: A cross-sectional study
- Clinical features of atypical tuberculosis mimicking bacterial pneumonia
- Proteoglycan-depleted regions of annular injury promote nerve ingrowth in a rabbit disc degeneration model
- Effect of electromagnetic field on abortion: A systematic review and meta-analysis
- miR-150-5p affects AS plaque with ASMC proliferation and migration by STAT1
- MALAT1 promotes malignant pleural mesothelioma by sponging miR-141-3p
- Effects of remifentanil and propofol on distant organ lung injury in an ischemia–reperfusion model
- miR-654-5p promotes gastric cancer progression via the GPRIN1/NF-κB pathway
- Identification of LIG1 and LIG3 as prognostic biomarkers in breast cancer
- MitoQ inhibits hepatic stellate cell activation and liver fibrosis by enhancing PINK1/parkin-mediated mitophagy
- Dissecting role of founder mutation p.V727M in GNE in Indian HIBM cohort
- circATP2A2 promotes osteosarcoma progression by upregulating MYH9
- Prognostic role of oxytocin receptor in colon adenocarcinoma
- Review Articles
- The function of non-coding RNAs in idiopathic pulmonary fibrosis
- Efficacy and safety of therapeutic plasma exchange in stiff person syndrome
- Role of cesarean section in the development of neonatal gut microbiota: A systematic review
- Small cell lung cancer transformation during antitumor therapies: A systematic review
- Research progress of gut microbiota and frailty syndrome
- Recommendations for outpatient activity in COVID-19 pandemic
- Rapid Communication
- Disparity in clinical characteristics between 2019 novel coronavirus pneumonia and leptospirosis
- Use of microspheres in embolization for unruptured renal angiomyolipomas
- COVID-19 cases with delayed absorption of lung lesion
- A triple combination of treatments on moderate COVID-19
- Social networks and eating disorders during the Covid-19 pandemic
- Letter
- COVID-19, WHO guidelines, pedagogy, and respite
- Inflammatory factors in alveolar lavage fluid from severe COVID-19 pneumonia: PCT and IL-6 in epithelial lining fluid
- COVID-19: Lessons from Norway tragedy must be considered in vaccine rollout planning in least developed/developing countries
- What is the role of plasma cell in the lamina propria of terminal ileum in Good’s syndrome patient?
- Case Report
- Rivaroxaban triggered multifocal intratumoral hemorrhage of the cabozantinib-treated diffuse brain metastases: A case report and review of literature
- CTU findings of duplex kidney in kidney: A rare duplicated renal malformation
- Synchronous primary malignancy of colon cancer and mantle cell lymphoma: A case report
- Sonazoid-enhanced ultrasonography and pathologic characters of CD68 positive cell in primary hepatic perivascular epithelioid cell tumors: A case report and literature review
- Persistent SARS-CoV-2-positive over 4 months in a COVID-19 patient with CHB
- Pulmonary parenchymal involvement caused by Tropheryma whipplei
- Mediastinal mixed germ cell tumor: A case report and literature review
- Ovarian female adnexal tumor of probable Wolffian origin – Case report
- Rare paratesticular aggressive angiomyxoma mimicking an epididymal tumor in an 82-year-old man: Case report
- Perimenopausal giant hydatidiform mole complicated with preeclampsia and hyperthyroidism: A case report and literature review
- Primary orbital ganglioneuroblastoma: A case report
- Primary aortic intimal sarcoma masquerading as intramural hematoma
- Sustained false-positive results for hepatitis A virus immunoglobulin M: A case report and literature review
- Peritoneal loose body presenting as a hepatic mass: A case report and review of the literature
- Chondroblastoma of mandibular condyle: Case report and literature review
- Trauma-induced complete pacemaker lead fracture 8 months prior to hospitalization: A case report
- Primary intradural extramedullary extraosseous Ewing’s sarcoma/peripheral primitive neuroectodermal tumor (PIEES/PNET) of the thoracolumbar spine: A case report and literature review
- Computer-assisted preoperative planning of reduction of and osteosynthesis of scapular fracture: A case report
- High quality of 58-month life in lung cancer patient with brain metastases sequentially treated with gefitinib and osimertinib
- Rapid response of locally advanced oral squamous cell carcinoma to apatinib: A case report
- Retrieval of intrarenal coiled and ruptured guidewire by retrograde intrarenal surgery: A case report and literature review
- Usage of intermingled skin allografts and autografts in a senior patient with major burn injury
- Retraction
- Retraction on “Dihydromyricetin attenuates inflammation through TLR4/NF-kappa B pathway”
- Special Issue Computational Intelligence Methodologies Meets Recurrent Cancers - Part I
- An artificial immune system with bootstrap sampling for the diagnosis of recurrent endometrial cancers
- Breast cancer recurrence prediction with ensemble methods and cost-sensitive learning

