Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
Abstract
By partially substituting 1,3,5-tris-(2-hydroxyethyl) cyanuric acid for glycerol as the polyol, new modified alkyd resins having a wide range of oil length and hydroxyl content were produced. FT-IR, 1H NMR, acid and iodine values, and other characterization techniques were used to confirm the chemical compositions of the modified alkyd resins. The drying qualities of the alkyd resins synthesized were significantly enhanced by adding a greater quantity of freshly prepared polyol and excess –OH, along with improvements in flexibility, gloss measurement, gloss measurement, and scratch hardness, in comparison to unaltered alkyd resin (blank). The corrosion resistance of dried coated steel panels was examined using a salt spray test. By using a specifically designed modified alkyd resin as a binder, the corrosion resistance of painted steel panels was improved. The addition of the new polyol, which contains nitrogen components, as well as the alkyd resins that provide strong adherence to the steel surface, may be responsible for such an improvement.
1 Introduction
A typical alkyd resin consists of a polyester that has been altered by adding fatty acids and other components. Alkyd resin is typically made with a source of dihydroxyl and a dicarboxylic compound or its anhydride. Alkyd resin is the reaction product of an oil or fatty acid, polyol, and a polyacid in many solvent-based paint systems (1). Glyptal was the brand name for the first alkyd resin, which was prepared from glycerol and phthalic acid. Alkyd resins have an excellent reputation due to their low cost, wide availability of raw materials, and convenient usage. Because of their oil and glycerol components, alkyd resins are more biologically degradable polymers and thus more environmentally friendly than petroleum-based polymers, which pollute and degrade the environment (2). Recently, researchers have synthesized and characterized alkyd resin from Delonix regia (Flamboyant) seeds for use in surface coating applications (3).
Alkyd resins are versatile binders that are utilized in a variety of applications, including industrial, decorative, and architectural coatings. Alkyd resins are frequently blended with different resins to achieve improved performance due to their great compatibility with other resins (4). Various alkyd resins based on newly extracted oil having a high level of unsaturated acid had been constructed as a renewable raw material. Short (I), medium (II), and long (III) alkyd resins were made with oil, glycerol, and phthalic anhydride (PA) in varying proportions. (5) Rubber seed oil, linseed oil, and their mixtures were used to make alkyd resins using the monoglyceride method. The effect of mixing on the qualities of linseed oil was then investigated for its physicochemical parameters, drying performance, and chemical resistance (6). Extensive research was conducted in order to create alkyd resins with outstanding performance for use as binders in permeable composite materials for large-size/complex-shape applications (7). The production of alkyd resins with the new sustainable raw material catalyst zirconium 2-ethyl hexanoate as a base-catalyzed alcoholysis of soybean oil catalyst has been revealed. Using zirconium octoate as a catalyst in the alcoholysis reaction prevents oil from oxidizing, resulting in monoglyceride formation, which is beneficial for monoglyceride color (8). Alkyd resins are used in various applications, including chemical resistance for wood, and mechanical stress for machine tool finishes (9). Compared to conventional petroleum-based polymers, which cause pollution and degradation in the environment, the presence of oil and glycerol in alkyd resins confers the quality of being eco-friendly; they have received considerable attention because of their inherent nontoxic nature and biodegradability (10). Seed oils, including some unsaturated oils, have been used to make polymeric resins like alkyds, depending on the kind (11). Before being utilized in primer formulations containing an effective amount of the corrosion-resistant pigment ilmenite ore, long oil alkyds were treated with naphthalene dicarboxylic acid. Significant corrosion-preventive properties were discovered (12). Preparation and characterization of a nano iron oxide impregnated alkyd coating (NIAC) with outstanding anticorrosive properties with reported. The weight-loss method was used to investigate the corrosion inhibition capability of NIAC (13). The flame-retardant (FR) properties of the modified alkyd resins and emulsion paint formula were achieved by adding hexachlorodiphosph(V) azane of kinds I–III. To assess the additives’ downsides, researchers looked at their physical, mechanical, and corrosion resistance (14). Short, medium, and long oil alkyd resins with modified reactive FR characteristics were constructed, and their diverse applications were examined (15). The synthesis, analysis, and evaluation of the performance qualities of silver nanoparticles embedded in alkyd resin as a surface coating binder were reported. The findings demonstrated that the paint films that dried quickly in the air had strong solvent resistance and light fastness and may be used as antimicrobial surface coatings (16). Using newly synthesized polyol as a modifier has created a novel alkyd resin and polyesteramide resin. The main concept is to take advantage of the new polyol resource and the increased physicochemical and anticorrosive properties. Physicochemical analyses and other spectral studies were used to describe the produced resins. The mechanical properties of polymeric films were also studied (17). A new paint formulation based on short oil alkyd resin that was blended with Co(II) and Ni(II) metal complexes by physical addition was developed and tested. The additive-modified coating improved the coated specimens’ mechanical properties (18). In the current study, we attempted to synthesize a new modified alkyd resin based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid as a source of polyol by partially replacing glycerol in order to take advantage of the aromatic ring, and nitrogen elements in cyanuric acid, which has improved physicochemical and anti-corrosive properties. Physicochemical analyses and spectral studies were used to characterize the produced resins. The physicomechanical properties of polymeric films and their corrosion resistance, were studied.
2 Materials and methods
2.1 Materials
All of the chemicals used in the studies reported here were of pure grade. Linseed oil fatty acid (LOFA) was a product of Sample Steal Bruxelles, Belgium (99.9%). Phthalic acid “PA” and glycerol were products of El-Nasr Pharmaceutical Chemical Company, Egypt. PA (99.9%), glycerol (99.8%), xylene (99.7%), methanol (99.6%), potassium hydroxide (100%), and phenolphthalein (99.3%) were supplied by El-goumhouria Co. (Cairo, Egypt). Zr octoate and mineral spirit were supplied by a trade Co. 1,3,5-Tris-(2-hydroxyethyl)cyanuric acid (100%) was a product of Merck, Germany.
2.2 Methods: synthesis of the long, medium, and short oil alkyd resins
A condensation polymerization reaction and a solvent technique were used to prepare alkyd resins in a single step. Thus, a mixture of calculated amounts of LOFA, PA as the source of the dibasic acid ingredient, and anhydrous glycerol, which was partially replaced with 1,3,5-tris-(2-hydroxyethyl)cyanuric acid as the source of the polyol ingredient, was refluxed in the presence of 10% xylene as a solvent in a 250 mL round-bottomed flask fitted with a Dean and Stark trap. During the reaction, the theoretical amount of water liberated was observed to help determine the route of esterification. The resins were designed to work with a wide range of oil length and hydroxyl content (long, medium, and short). It should be emphasized that the total number of acid and hydroxyl equivalent for the various runs was kept consistent within each set of formulations (19). Alkyd resin calculations can be used to predict the final formulation, resin properties, reaction water liberation, and gelation risk. Calculation of water was developed as a tool for tracking the progress of the esterification reaction and calculating the theoretical yield, as shown in Tables 1 and 2. The formulation of a modified alkyd vehicle based on the novel modifier (HECA) and driers Zr and Co octoate is shown in Table 2. Scheme 1 shows the equations for synthesizing monoglyceride and alkyd resin.
Resin constants for HECA – modified alkyd resins
| Resin No. | Excess –OH% | Ingredients | E | F | e 0 | e A | e B | R | H2O off (mL) |
|---|---|---|---|---|---|---|---|---|---|
| I a–d | 0 | G | 30.7 | 3 | 0.520 | 0.260 | 0.260 | 1.00 | 3.34 |
| HECA | 87 | 2 | |||||||
| LOFA | 280 | 1 | |||||||
| PA | 74.1 | 2 | |||||||
| II a–d | 10 | G | 30.7 | 3 | 0.573 | 0.273 | 0.300 | 1.10 | 3.36 |
| HECA | 87 | 2 | |||||||
| LOFA | 280 | 1 | |||||||
| PA | 74.1 | 2 | |||||||
| III a–d | 20 | G | 30.7 | 3 | 0.656 | 0.298 | 0.358 | 1.20 | 3.42 |
| HECA | 87 | 2 | |||||||
| LOFA | 280 | 1 | |||||||
| PA | 74.1 | 2 | |||||||
| IV a–d | 30 | G | 30.7 | 3 | 0.778 | 0.339 | 0.439 | 1.30 | 3.55 |
| HECA | 87 | 2 | |||||||
| LOFA | 280 | 1 | |||||||
| PA | 74.1 | 2 |
LOFA – linseed oil fatty acid, PA – phthalic anhydride, HECA – 1,3,5-tris-(2-hydroxyethyl) cyanuric acid, G – glycerol, E – equivalent weight, e A – number of acid equivalent, e B – number of hydroxyl equivalent, e 0 – total equivalent present at the start of the reaction, m 0 – total moles present at the start of the reaction, F – functionality, K – alkyl constant (m 0/e A), R – ratio of total –OH groups to total –COOH groups (e B/e A), W – weight (W gm = e 0 E).
Formulation of modified alkyd vehecle based on HECA as new polyol
| Ingredient (wt%) | Formulation no. |
|---|---|
| Alkyd resin | 82 |
| Xylene | 16 |
| Benton | 1 |
| Methanol | 0.2 |
| Zr-octoate | 0.4 |
| Co-octoate | 0.2 |
| Anti-skin | 0.2 |

Prepared alkyd resin based on 1,3,5-tris-(2-hydroxyethyl) cyanuric acid. (a) Stage 1: Formation of monoglyceride. (b) Stage 2: Esterification and formation of alkyd.
2.3 Characterization of the prepared polyol
2.3.1 Spectroscopic analysis
FTIR spectra were obtained using a JASCO FTIR 6100 in the range of 4,000–400 cm−1, using KBr pellets. 1H NMR spectra were recorded on a Varian Mercury 400 1H NMR spectrometer, using DMSO as the solvent.
2.3.2 Physiochemical properties of the alkyd samples
Color (physical observation) (ASTM Paint and Coatings Testing Handbook ISBN 0-8031-2060-5), acid value (22) (ASTM-D1980-1987 R98 Edition), and viscosity using Gardner Standard Bubble Viscometers (ASTM D1545, D1545, D1725) were assessed.
2.3.3 Determination of iodine value
The iodine value was used to determine the amount of unsaturation present in the alkyd samples and was reported (20) according to ASTM-D5554-2015 R21 Edition-Current.
2.3.4 Film casting and testing
Coating films were prepared following the surface preparation (ASTM method D609-17). The dry film thickness was assessed following ASTM D1005-13. A specular gloss was measured according to ASTM method D523-18. The scratch hardness was assessed following ASTM method D3363-11. The adhesion of the coating films to the test panels was measured following ASTM method D3359-17. The flexibility of the coating films was assessed following ASTM Method D522-17.
2.3.5 Chemical resistance of the alkyd resin’s film to different media
After a week, the coated glass and steel plates were subjected to chemical resistance testing according to ASTM D1647(1996), D870(1997), and D1308(1998).
2.3.6 Scanning electron microscope (SEM) analysis
An SEM (Joel Jsm 6360LA, Japan) operating at an accelerated voltage of 10 kV was employed to analyze the morphology of the surface of the coating films. The fracture surfaces were vacuum-coated with gold for SEM analyses.
2.3.7 Evaluation of the modified alkyd resin as a corrosion inhibitor
Five paint formulations rely on the % of surplus –OH for the created modified alkyd and another formulation including unmodified alkyd was constructed to examine the valuable effects of the modified alkyd resin as an anticorrosive binder. To compare the results, all of the formulations were tested without any corrosion inhibitor, as indicated in Table 6. Corrosion inhibitors of this type act by reducing the coating’s permeability to water, oxygen, and hostile ions. According to ASTM B117, the paint formulations were tested on steel panels in a salt spray cabinet for 500 h.
3 Results and discussion
3.1 Spectral analysis of HECA-modified alkyd resins
3.1.1 FT-IR spectra
Figure 1 shows the FT-IR spectra of the alkyd resins concerned. These spectra show the existence of the key ester group linkages, olefinic double bonds, and other distinguishing peaks. Table 3 shows the characteristic peaks found in the FT-IR spectra of alkyd resins. In the case of the synthetic resin, the C═O peak emerges at 1,735 cm−1. The presence of the hydroxyl group is indicated by the peak at 3,472 cm−1. C═C stretching for unsaturated fatty acids and aromatics is indicated by the peak at 1,590 cm−1 (8).

IR spectra of the modified alkyd resin.
IR spectral of new HECA modified alkyd showed the following data
| Functional groups | IR peak (cm−1) |
|---|---|
| O‒H stretching vibration | 3,472.55 |
| Olefinic C‒H stretching vibration | 3,008.8 |
| C‒H aliphatic stretching vibration | 2,925.65 |
| 2,854.38 | |
| C═O stretching frequency of ester | 1,735.1 |
| C═C stretching frequency of alkene an aromatic band | 1,599.45 |
| 1,580.28 | |
| Symmetric and asymmetric bending of methyl groups | 1,489.18 |
| 1,377.7 | |
| C‒O‒C stretching vibrations attached with aliphatic and aromatic moiety | 1,280.77 |
| 1,041.64 | |
| Out of plane aromatic C–H bending vibration | 742.65 |
| 705.98 |
3.1.2 1H NMR spectra of the prepared modified alkyd resins
1H NMR spectra (Figure 2) were measured in DMSO-d6 and showed signals at δ: 1.2–1.6 ppm for CH2 of fatty acid chains; at δ: 2.1–2.4 ppm for DMSO; at δ: 4.1–4.2 ppm for glycerol CH2, –CH; at 5.4 ppm for 2H, 2OH humps; and at 7–8 ppm for m, 3H, Ar-H (21).

The H1 NMR spectra of the prepared modified alkyd resin.
3.2 Evaluation of the physical and mechanical properties of the modified alkyd resin varnish based on HECA
3.2.1 Degree of polymerization (DP)
The acid value, which is an industrial metric for conversion and can be obtained by titration with an ethanolic solution of potassium hydroxide, was used to indicate the DP of the resins (KOH). The acid value of the modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid was less than 8 mg KOH per gram resins. These values indicate that the amount of residual free carboxylic acid groups in the resins is low, implying that the majority of diacid monomers and fatty acids have been involved in the polycondensation. Also, iodine values ranged from 170 to 177, which confirmed that the unsaturated bonds in the structure of linseed fatty acids were not affected (Table 4) (22).
Varnishes characteristics data of HECA modified alkyd resins
| Resin No. | Excess –OH% | Replacement % of HECA | Reaction time (h) | Viscosity (Gardner stander) | Color (Gardner) | Acid value (mg KOH‧g−1) | Air drying time (h) | Iodin value | Stoving dry at 110 after 1 h | Stoving dry at 120 after 1 h |
|---|---|---|---|---|---|---|---|---|---|---|
| Ia | 0 | 0 | 6.00 | H | Brown | 12 | 6 | 170 | ST | HD |
| b | 10 | 6.20 | H–I | Dark brown | 10 | 6 | 175 | ST | HD | |
| c | 20 | 6.35 | L–M | Dark brown | 10 | 5.50 | 175 | VST | HD | |
| d | 30 | 6.45 | N | Dark brown | 8 | 5.50 | 177 | VST | HD | |
| IIa | 10 | 0 | 6.00 | H | Dark brown | 12 | 6.0 | 175 | ST | HD |
| b | 10 | 6.30 | I | Dark brown | 10 | 5.40 | 175 | ST | HD | |
| c | 20 | 5.40 | M | Dark brown | 8 | 5.30 | 177 | VST | HD | |
| d | 30 | 6.55 | N–O | Dark brown | 7 | 5.20 | 175 | VST | HD | |
| IIIa | 20 | 0 | 6.10 | H | Dark brown | 11 | 6.0 | 176 | ST | HD |
| b | 10 | 5.25 | L | Dark brown | 9 | 5.35 | 175 | ST | HD | |
| c | 20 | 6.40 | L–M | Dark brown | 8 | 5.20 | 175 | VST | HD | |
| d | 30 | 6.58 | O | Dark brown | 6 | 5.00 | 177 | HD | HD | |
| IVa | 30 | 0 | 6.10 | H–I | Dark brown | 10 | 5.0 | 174 | ST | HD |
| b | 10 | 5.28 | L | Dark brown | 7 | 4.40 | 175 | HD | HD | |
| c | 20 | 6.45 | O | Dark brown | 6 | 4.20 | 176 | HD | HD | |
| d | 30 | 7.00 | P | Dark brown | 6 | 4.00 | 177 | HD | HD |
3.2.2 Color parameters by the Gardner color scale
The results displayed in Table 4 also show that the color of the alkyd resins is dark brown, and this could be attributed to the conditions of alkyd production and also due to the nature of the fatty acid used in the alkyd resin preparation; the fatty acid was LOFA, which tends to yellow more than other fatty acids. The other reason for the dark brown color may be attributed to the presence of some elements such as nitrogen in the chemical structure of the new polyol, which is used in the preparation of alkyd resins (Figure 3) (23), according to ASTM Paint and Coatings Testing Manual ISBN 0-8031-2060-5.

Color of the prepared alkyd resins.
3.2.3 Viscosity by Gardner standard bubble viscometers ASTM D1545
As shown in Table 4, the viscosity increased with increasing percentage replacement of the modifier polyol and oil content in excess –OH. These increases may be attributed to the increased molecular weight of the modified alkyd resins based on the new modifier. Also, attributed to the nature of the used fatty acids in the polymerization LOFA, 30% excess –OH alkyd resins were observed to possess the highest viscosity due to all of the previously mentioned reasons (6).
3.2.4 Drying time for the prepared modified resins
Table 4 shows the time taken by the modified resins to dry, where the first is set-to-touch; these characteristics are critical in determining the rate and extent of the coating’s drying when applied to a surface. The time taken by the resin to harden was the tack-free time. The drying schedule for the various alkyd samples was either a hard dry-through in air or a stove drying plan. By auto-oxidation, the alkyd resin sheet dries. Mechanistic studies of the autoxidation drying process of alkyd resin coatings have focused on methylene-interrupted fatty acids as a result of oxygen absorption from the atmosphere, taking place during air drying of the samples that dried in air within 4–6 h and were hard-dry at 120°C within 1 h only, which enhanced the cross-linked network of the coated films. Finally, we observed that all sample films based on 30% excess –OH alkyd resins dried faster because of the oil content, and this excess –OH alkyd represented a short oil alkyd that is characterized as a fast dry alkyd resin. All these interesting mechanisms speed up the network formation and result in shorter drying times.
3.3 Mechanical properties of various coating films based on HECA-modified alkyd resins
3.3.1 Gloss
The observed gloss values are consistent with this type of vehicle, which has a high gloss value if no pigment or fillers are present. Gloss values were also improved by increasing the modified polyol in the alkyd resin structure, with values ranging from 90 to 98 at 20°. This is because alkyd resins contain an aromatic moiety. All of the resins have good gloss characteristics.
3.3.2 Scratch hardness
The scratch hardness was observed to be from <1.2 kg to >2.0 kg as shown in Table 5. The obtained results of the different coated samples indicated that the coating resistance to scratching increases with an increase in the percentage content of the modifier polyol which increases the nitrogen content resulting from the increase of its percentage in the alkyd resin content. Also, the presence of the rigid aromatic moiety (21) may be attributed to ester repeat units (COOR) in the polymeric chain of the oil-based alkyd resin that enhances the hardness of the coating, causing the application to be easier and drying to be faster.
Mechanical characteristics of various modified alkyd resins
| Resin no. | Scratch hardness (kg) | Gloss at 20° | Adhesion | Flexibility | Acid resistance | Alkali resistance | Water resistance | Solvent resistance |
|---|---|---|---|---|---|---|---|---|
| Ia | <1.2 | 90 | 5B | Pass | Pass | Poor | Pass | Pass |
| b | <1.5 | 92 | 5B | Pass | Pass | Poor | Pass | Pass |
| c | >1.5 | 95 | 5B | Pass | Pass | Poor | Pass | Pass |
| d | >1.8 | 95 | 5B | Pass | Pass | Pass | Pass | Pass |
| IIa | <1.5 | 93 | 5B | Pass | Pass | Poor | Pass | Pass |
| b | <1.6 | 94 | 5B | Pass | Pass | Poor | Pass | Pass |
| c | >1.9 | 96 | 5B | Pass | Pass | Pass | Pass | Pass |
| d | >2.0 | 95 | 5B | Pass | Pass | Pass | Pass | Pass |
| IIIa | <1.5 | 95 | 5B | Pass | Pass | Poor | Pass | Pass |
| b | <1.8 | 96 | 5B | Pass | Pass | Poor | Pass | Pass |
| c | >2.0 | 98 | 5B | Pass | Pass | Pass | Pass | Pass |
| d | >2.0 | 98 | 5B | Pass | Pass | Pass | Pass | Pass |
| IVa | <1.6 | 96 | 5B | Pass | Pass | Poor | Pass | Pass |
| b | <1.8 | 97 | 5B | Pass | Pass | Poor | Pass | Pass |
| c | >2.0 | 98 | 5B | Pass | Pass | Pass | Pass | Pass |
| d | >2.0 | 98 | 5B | Pass | Pass | Pass | Pass | Pass |
3.3.3 Crosshatch adhesion
As can be seen from Table 4, all coated films had good crosshatch adhesion. As a result, it was determined that the alkyd resins prepared had no negative impact on the varnish system’s adhesion qualities, which also can be attributed to the formation of a highly cross-linked network. Coated films showed good adhesion strength (100%) as per the crosshatch tape (24).
3.3.4 Flexibility test
Adhesion of film may be due to the higher valence forces and interlocking action of polar groups of the resin on the metal surface. Oil-based coatings are well known for good flexibility (24). The varnishes were deemed to have satisfactory flexibility if, after the bending operation, no crack mark or dislodging was noted. It was found that all of the coated films, as shown in Table 5, showed good adherent properties that can be attributed to the presence of polar ester bonds (21), which may be also due to that the final resin is composed of a polyester backbone, with its own highly branched polymer and dangling fatty acids. All of the mechanical characteristics of various modified alkyd resins are presented in Table 5.
3.4 Chemical resistance of the new modified alkyd resin
The chemical resistance properties of all of the samples of resins were comparable to those of the reference sample (unmodified alkyd). Alkyd films of all modified resins are highly resistant to acid, water, and solvent. However, greater resistance to alkali than unmodified alkyd (blank sample) may be due to the presence of a large amount of the rigid aromatic moiety of PA. As 1,3,5-tris-(2-hydroxyethyl)cyanuric acid content increases, the alkali resistance decreases, which may be attributed to the factors such as higher molar mass because of the high percentage of 1,3,5-tris-(2-hydroxyethyl)cyanuric acid in alkyd resin structure and the high cross-linked density (21,25). The results obtained are tabulated in Table 5.
3.5 Evaluation of the corrosion resistance of coated steel panels
Four coating formulations based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid-modified alkyd resin were prepared and are illustrated in Table 6. The prepared coatings were applied to carbon steel panels. These panels were treated before coating application to ensure that they were free from any surface contamination. The treatments employed in this study involved sanding using sanding paper and brushing using a mechanical wire brush. The dried coated panels were subjected to anticorrosion tests in a salt spray corrosion cabinet. The tested panels were then inspected for any corrosion-related damage. The results of corrosion resistance represented in Table 7 and Figures 4–6 indicated that the corrosion resistance increases with increasing excess –OH in the low oil content and also the percentage of the new polyol (HECA). The polyol (HECA) is rich in nitrogen element, which enhances corrosion resistance. Thus, the improvement in corrosion resistance is attributed to the introduction of the nitrogen element and the numerous free lone pairs of electrons in the modified alkyd compared with the unmodified alkyd resin (blank sample), in addition to many valence bonds (double and triple bonds) in the type of fatty acid used (linseed fatty acid), and all forms of aromatic rings may be responsible for the improved corrosion resistance outcomes. To determine the magnitude of blistering, photographic reference criteria were employed to indicate whether the coating film was becoming more corrosion resistant. The percentage of the corroded area was determined by visually comparing the surface of the tested panels to photographic reference standards, as shown in Figure 6, which shows the results of salt spray tests after 500 h of exposure (26).
Percent composition of the prepared anticorrosive primer formulations
| Composition | Formulation no. | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Type of alkyd | Unmodified | 10% HECA | 20% HECA | 30% HECA |
| Alkyd resin | 50 | 50 | 50 | 50 |
| TiO2 | 15 | 15 | 15 | 15 |
| CaCO3 | 15 | 15 | 15 | 15 |
| Talc | 8 | 8 | 8 | 8 |
| Solvent | 10 | 10 | 10 | 10 |
| Additives | 2 | 2 | 2 | 2 |
Evaluation of corrosion resistance of the dry painted films
| Formula No. | Modification | Degree of rusting | Blistering | Scribe failure rating | |
|---|---|---|---|---|---|
| Size | Frequency | ||||
| 1 | Unmodified alkyd | 5 | 4 | MD | 5 |
| 2 | 10% Replacement | 6 | 4 | M | 7 |
| 3 | 20% Replacement | 7 | 6 | M | 8 |
| 4 | 30% Replacement | 9 | 8 | F | 9 |

Degree of resting for painted samples based on modified and unmodified alkyd primer formulation.

Scribe failure resistance of modified alkyd primer formulation.

The photographic of painted steel samples exposed to salt spray based on modification by using 1,3,5-tris-(2-hydroxyethyl) cyanuric acid and for unmodified alkyd resin.
3.6 SEM analysis of coating films based on modified and unmodified alkyd resins on steel panels after corrosion tests
It can be observed from Figure 7 that prior to corrosion testing, the surface of the control steel panel showed evidence of abrading scratches. Close examination of the SEM electron micrographs indicates that the surface of the control sample has been damaged due to an aggressive attack by the seawater medium. However, in the presence of the modified alkyd resin, the steel is observed to have a smooth surface with only a relatively small number of small notches, especially for the coatings based on 0% and 10% excess –OH. However, for paint-based 20% and 30% excess –OH, such results imply that both varnishes and paint formulations based on the modified alkyd resin were smooth and there was no creaking or damage. It is clear that the modified alkyd resin based on the high percentage of 1,3,5-tris-(2-hydroxyethyl)cyanuric acid as polyol reduces the corrosion rate and therefore affords better protection against corrosion.
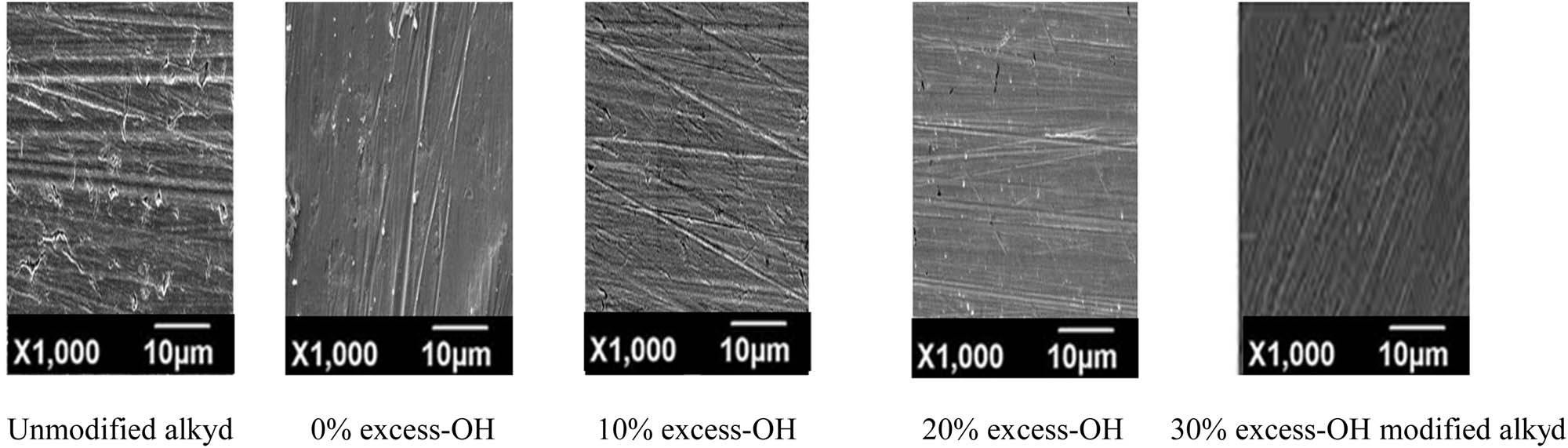
SEM images of the steel surface after corrosion for unmodified and 0%, 10%, 20%, and 30% excess-OH of modified alkyd resins.
4 Conclusion
As mentioned above, modified alkyd resins containing 1,3,5-tris-(2-hydroxyethyl)cyanuric acid as a polyol source have been effectively prepared. It was discovered that the amount of 1,3,5-tris-(2-hydroxyethyl)cyanuric acid in the polymer structure had a significant impact on the resins’ properties. The resins acquire desirable film qualities in terms of drying time, gloss, hardness, adhesion, bending, and chemical resistance, among others, as well as corrosion resistance, making them beneficial for the protection of steel surfaces and as a composite binder, for example. The performance of the alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid was found to be comparable to that of unmodified resin. According to the findings, it is concluded that 1,3,5-tris-(2-hydroxyethyl)cyanuric acid-based alkyd resins can be used as a source of effective anticorrosive vehicles for surface coating applications.
Acknowledgments
We are grateful to the Faculty of Science, King Khalid University, Saudi Arabia, for their support to this study. We also acknowledge the Department of Color Science, School of Chemistry, University of Leeds, UK, and the Department of Chemistry, Faculty of Science, Al-Azhar University, Cairo, Egypt, for their unwavering assistance and support throughout this work. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Small Groups (project under grant number RGP.1/252/43).
-
Funding information: The funding for this work from the Deanship of Scientific Research at King Khalid University for through Small Groups (project under grant number RGP.1/252/43).
-
Author contributions: Fatimah Ali M. Zahrani: writing – original draft, writing – methodology, data curation, formal analysis. review and editing, Long Lin: writing – original draft, writing – review and editing; H. Abd El-Wahab: conceptualization, methodology, data curation, formal analysis, writing – original draft, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
(1) Ezugwu MU, Obidiegwu MU, Ezugwu CH, Dasofunjo K, Berinyuy EB. Synthesis and characterization of alkyd resin from Delonix Regia (Flamboyant) seeds. J Polym Sci Appl 3. 2019;1:2.Search in Google Scholar
(2) Mukhtar A, Habib U, Mukhtar H. Fatty acid composition of tobacco seed oil and synthesis of alkyd resin. Chin J Chem Eng. 2007;87:705–8.10.1002/cjoc.200790132Search in Google Scholar
(3) Ezugwu MU, Obidiegwu MU, Ezugwu CH, Dasofunjo K, Berinyuy EB. Synthesis and characterization of alkyd resin from Delonix Regia (Flamboyant) seeds. J Polym Sci Appl. 2019;3:1.Search in Google Scholar
(4) Assanvo EF, Gogoi P, Dolui SK, Baruah SD. Synthesis, characterization and performance characteristics of alkyd resins based on Ricinodendron heudelotii oil and their blending with epoxy resins. Ind Crop Products. 2015;65:293–302.10.1016/j.indcrop.2014.11.049Search in Google Scholar
(5) REHİM EM, Ashery RE. Synthesis and characterization of alkyd resin based on soybean oil and glycerin using zirconium octoate as catalyst. Int J Chem Technol. 2018;2(1):34–43.10.32571/ijct.347670Search in Google Scholar
(6) Otabor GO, Ifijen IH, Mohammed FU, Aigbodion AI, Ikhuoria EU. Alkyd resin from rubber seed oil/linseed oil blend: A comparative study of the physiochemical properties. Heliyon. 2019;5:e01621.10.1016/j.heliyon.2019.e01621Search in Google Scholar PubMed PubMed Central
(7) Gutowski WV, Błędzki AK. Fast-Setting permeable alkyd/polyester composites: moulding sands. Polymers. 2021;13:4386. 10.3390/polym13244386.Search in Google Scholar PubMed PubMed Central
(8) REHİM EM, Ashery RE. Synthesis and characterization of alkyd resin based on soybean oil and glycerin using zirconium octoate as catalyst. Int J Chem Technol. 2018;2(1):34–43.10.32571/ijct.347670Search in Google Scholar
(9) Uzoh CF, Obodo NJ, Onukwuli OD. Exploring the effect of styrene and anhydride ratio on the coating properties of non-drying vegetable oil-based alkyd resin. J King Saud University – Engineering Sci. 2018;30:12–21.10.1016/j.jksues.2015.12.004Search in Google Scholar
(10) Liang D, Zhang Q, Zhang W, Liu L, Liang H, Quirino RL. Tunable thermophysical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J Clean Prod. 2019;210:1207–15.10.1016/j.jclepro.2018.11.047Search in Google Scholar
(11) Zhang C, Garrison TF, Madbouly SA, Kessler MR. Recent advances in vegetable oil-based polymers and their composites. Prog Polym Sci. 2017;71:91–143.10.1016/j.progpolymsci.2016.12.009Search in Google Scholar
(12) Abd El-Wahab H, Abd EL-Fattah M, Abdoub MI, Abd El-Hai F. New anticorrosive coating compositions incorporated ilmenite ore. Prog Org Coat. 2009;66:242–7.10.1016/j.porgcoat.2009.07.010Search in Google Scholar
(13) Jeyasubramanian K, Benitha VS, Parkavi V. Nano iron oxide dispersed alkyd coating as an efficient anticorrosive coating for industrial structures. Prog Org Coat. 2019;132:76–85.10.1016/j.porgcoat.2019.03.023Search in Google Scholar
(14) Abd El-Wahab H, Abd El-Fattah M, Gabr MY. Preparation and characterization of flame-retardant solvent-based and emulsion paints. Prog Org Coat. 2010;69:272–7.10.1016/j.porgcoat.2010.06.005Search in Google Scholar
(15) Abd El-Wahab H, Abd El-Fattah M, Abd El-Khalik N, Kazlauciunas A. Synthesis and performance of new modified reactive flame-retardant alkyd resin based on tetrabromophthalic anhydride as varnish for surface coatings. J Coat Technol Res. 2015;12(1):97–105.10.1007/s11998-014-9615-6Search in Google Scholar
(16) Idumah CI, Obele CM, Ezeani EO. Understanding interfacial dispersions in ecobenign polymer nano-biocomposites. Polym-Plast Technol Mater. 2021 Feb 11;60(3):233–52.10.1080/25740881.2020.1811312Search in Google Scholar
(17) Mohamed A, Mustafa A, Elgaby MSA, Abed SA, Kazlauciunas A, Abd El-Wahab H. New modified poly (ester amide) resins and their uses as a binder for surface coating with different applications. Pigment & Resin Technol. 2021;50(2):146–56.10.1108/PRT-06-2020-0060Search in Google Scholar
(18) Younis AA, Faheim AA, Elsawy MM, El-Wahab H. Novel flame retardant paint based on Co (II) and Ni (II) metal complexes as new additives for surface coating applications. Appl Organomet Chem. 2021;35(1):e6070.10.1002/aoc.6070Search in Google Scholar
(19) Patton TC. Alkyd resin technology, formulating techniques and allied calculations. Interscience manuals. 8, New York: Wiley; 1962.Search in Google Scholar
(20) Saad B, Ling CW, Jab MS, Lim BP, Abdussalam SMA. Determination of free fatty acids in palm oil samples using non-aqueous flow injection titrimetric method. Food Chem. 2007;102:1407–14.10.1016/j.foodchem.2006.05.051Search in Google Scholar
(21) Abd El-Wahab H, Abd El-Fattah M, Abdou MI, Abd El-Hai F. New anticorrosive coating compositions incorporated ilmenite ore. Prog Org Coat. 2009;66:242–7.10.1016/j.porgcoat.2009.07.010Search in Google Scholar
(22) Hulsbosch J, Claes L, Jonckheere D, Mestach D, De Vos DE. Synthesis and characterization of alkyd resins with glutamic acid-based monomers. RSC Adv. 2018;8:8220–7.10.1039/C8RA00060CSearch in Google Scholar PubMed PubMed Central
(23) Hunterlab. The Gardner scale: Modern applications for color measurement of transparent liquids. Retrieved; 2020-09-4.Search in Google Scholar
(24) Ahmad S, Ashraf SM, Naqvi F, Yadav S, Hasnat A. A polyesterimide from annona squamous oil for anticorrosive coating. J Polym Mater. 2001;18:53.Search in Google Scholar
(25) Ahmad S, Ashraf SM, Zafar F. Development of linseed oil based polyesteramide without organic solvent at lower temperature. J Appl Polym Sci. 2007;104:1143–8.10.1002/app.25774Search in Google Scholar
(26) Saleh NM, Elsawy MM, Abd El-Wahab H, Salem SS, Abd El-Sattar NE. New coating formulation based on synthesized benzodiazepine derivatives as double function additives for industrial application. Pigment Resin Technol. 2021 Oct 14. (online) 10.1108/PRT-06-2021-0061.Search in Google Scholar
© 2022 Fatimah Ali M. Al-Zahrani et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

