Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
Abstract
A novel series of mixed-ligand complexes of the type, [M(L1)(L2)Cl]·2H2O [L1 = 2-(α-methyl salicylidene hydrazine) benzimidazole (primary ligand), L2 = 2,2′-bipyridine (bipy; secondary ligand), M = Co(ii), Ni(ii), Cu(ii) and Zn(ii)], were based on the physicoanalytical studies. The spectroscopic findings revealed tridentate nature of the Schiff base ligand (L1) and its coordination to the metal ions via azomethine nitrogen, ring nitrogen and the deprotonated phenolic oxygen atoms. Furthermore, the synthesized compounds were evaluated for antimicrobial activity against Bacillus subtilis, Escherichia coli and Salmonella typhi microorganisms. In addition, molecular docking studies were carried out against Middle East respiratory syndrome coronavirus (PDB ID: 4ZS6) and severe acute respiratory syndrome coronavirus 2 main protease (PDB ID: 6W63).
Graphical abstract
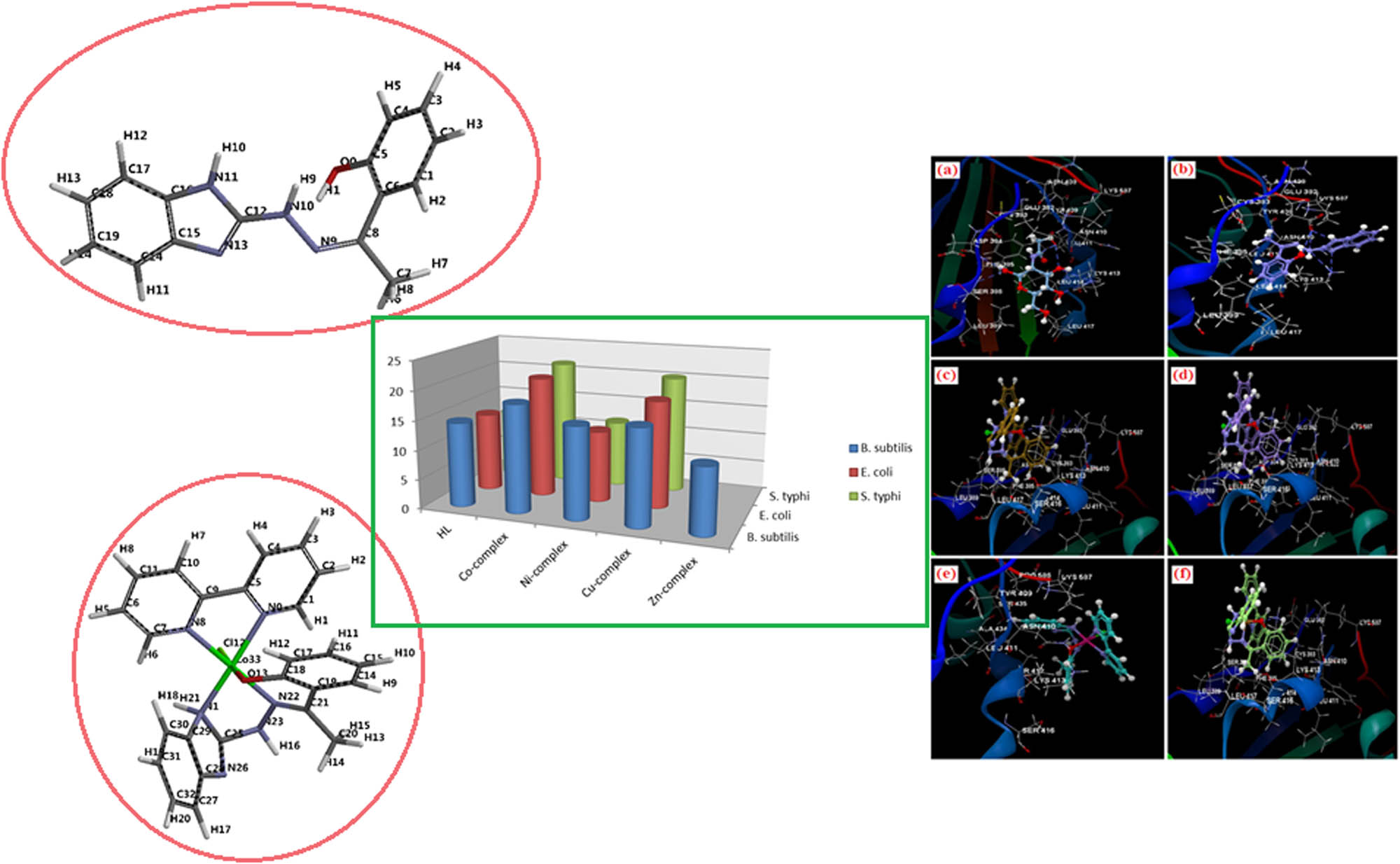
1 Introduction
Benzimidazole, an important precursor of the heterocyclic compounds, exhibits a wide range of biological applications, i.e., antiviral, antifungal, antipyretic, antidepressant and inhibitory activities in various cancers [1,2,3,4,5]. The broad biological activity of these compounds is supposed to be due to the presence of sp2 hybridized nitrogen donor atoms [6]. The ligational behavior of benzimidazole and its derivatives has been explored in coordination chemistry to form stable complexes through various modes of coordination [7]. The benzimadazole-based metal complexes exhibit broad spectrum of pharmaceutical activities, such as zinc complexes of benzimidazole find usage in anticancer activity, and reveal remarkable antimicrobial activity [8]. In addition, several benzimadazole complexes have been reported as anticancer agents [9,10]. It is also reported that the transition metal ions form stable complexes and have displayed several properties [11,12]. Over the years, bipy and its derivatives have received immense importance as binding blocks and ligands in the construction of various homo- and heteroleptic metal complexes with broad spectrum of applications in the area of both material and biological science [13,14,15,16,17].
Mixed-ligand complexes, which have two different ligands, find significant consideration in coordination chemistry because of their structural variation and diverse applications [18,19]. Besides, mixed-ligand complexes also exhibit various biological applications [20]. Recent outbreak due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has destroyed the economy and various socioeconomic sectors worldwide [21,22,23]. So far, not a single approved drug is available to treat the caused infection. Therefore, scientists are trying to find a drug to combat the COVID-19 pandemic. Several reports suggest that the progression of this pandemic can be controlled by targeting the main protease (Mpro) to develop the potential inhibitor. Molecular docking, a computational strategy to predict the binding site to assist drug repositioning for several diseases, plays a significant role in the pharmaceutical industry to bring new drugs to the market [24]. The HIV-1 protease inhibitors as the repurposed drugs for SARS-CoV-2 Mpro are discussed in literature [24,25,26]. Therefore, considering the versatile nature of mixed-ligand complexes, we are reporting four new mixed-ligand complexes of Co(ii), Ni(ii), Cu(ii) and Zn(ii) ions with benzimidazole-based Schiff base ligand (L1) as the primary ligand, and bipy as the co-ligand, (L2) in 1:1:1 molar ratio, and investigated them by various physicochemical studies. The synthesized metal complexes exhibited moderate antibacterial activity when screened against Bacillus subtilis, Escherichia coli and Salmonella typhi. In addition, we analyzed the molecular docking of the complexes of protein obtained from Middle East respiratory syndrome coronavirus (MERS-CoV; PDB ID: 4ZS6) and SARS-CoV-2 Mpro (PDB ID: 6W63).
2 Experimental
2.1 Preparation of the benzimidazole derived Schiff base ligand, L1
The precursor, 2-hydrazinobenzimidazole, was prepared following the previously reported protocol [27]. The primary ligand, L1, was prepared by the condensation reaction of 2-hydrazinobenzimidazole with o-hydroxyacetophenone in equimolar ratio in ethanol [27].
Yield 80%, color yellow; anal. calc. (%): C, 63.83; H, 4.96; N, 19.86, found (%): C, 63.76; H, 4.92; N, 19.82.
2.2 Preparation of mixed-ligand complexes
An ethanolic solution of the Schiff base ligand, L1 (0.01 mol and 20 mL), metal(ii) chloride (0.01 mol and 20 mL) and bipy, and L2 (0.01 mol and 20 mL) were mixed in 1:1:1 molar ratio and refluxed for 2.5 h at pH = 7–8 by adding catalytic amount of solid NaOH. A colored precipitate was obtained, which was separated by filtration. The precipitate was washed with methanol and dried in vacuo. The schematic representation of the synthesis of mixed-ligand complexes is given in Scheme 1.

Schematic representation of preparation of mixed-ligand complexes.
2.3 In vitro antibacterial activity
The in vitro antimicrobial activity of the studied mixed-ligand complexes was reported by Agar Well diffusion method [27] at 100 μg mL−1 concentrations against B. subtilis, E. coli and S. typhi with ciprofloxacin as the standard antibacterial drug.
2.4 Molecular docking study
In order to evaluate the biological activity, molecular docking was analyzed on CLC Drug Discovery Workbench Software to obtain accurate predictions about the structure and interactions with a protein/enzyme receptor [28]. Some protein/enzyme receptors were imported from protein data bank (http://www.rcsb.org/:PDB): MERS-CoV (PDB ID: 4ZS6 [29]) and SARS-CoV-2 Mpro (PDB ID: 6W63 [30]).
In the docking simulation, the compounds (Figure 1) were placed into a predictable binding site on the surface of a protein target. The CLC Drug Discovery Work bench utilized MMFF94 (Merck Molecular Force Field [MMFF]) force field to generate 3D structure on import. Rotation around bond generates several conformations. Thus, the ligand optimizer was realized by geometry minimization using MMFF94 force field [31], conforming the binding pocket geometry. The protein–ligand interaction was scored, and the best score-binding mode was returned for individual ligand and collected with the score. The ligand-binding mode search is effectuated inside the binding site (green sphere with a radius large enough to comprise the ligands docked to the receptor protein). After the import of the protein receptor from the PDB bank, the next step involved the setting up of the binding site and the binding pockets; binding pockets are necessary to guide the docking simulation. After the setup of the binding site and the binding pocket, the co-crystallized-natural ligand was extracted and redocked in the active binding site of the protein receptor to validate the method and the docking parameters obtained from the molecular docking studies.
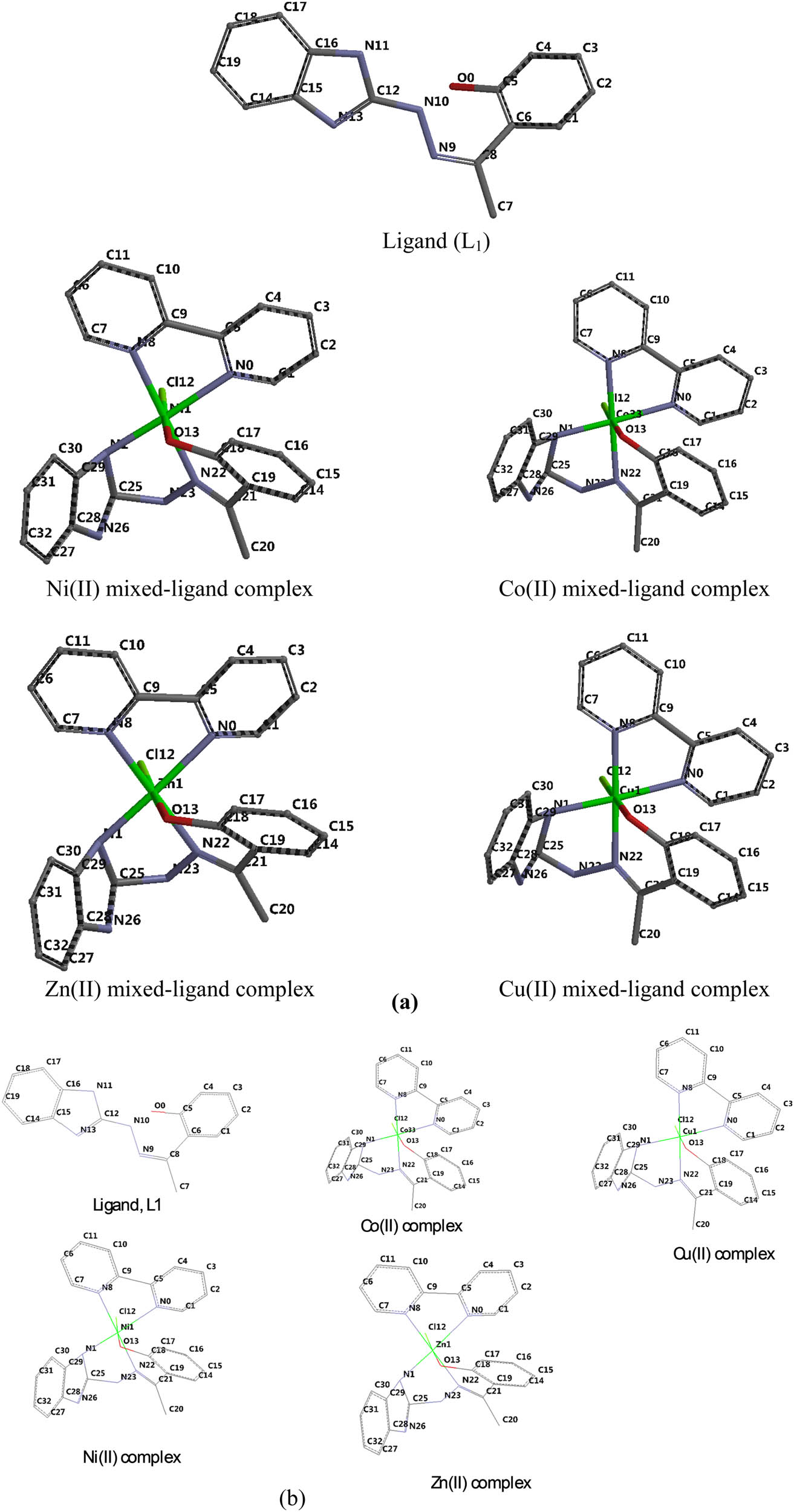
(a) Tube representation and (b) wire representation of the optimized molecular structure of ligand and mixed-ligand metal complexes (numbering of the atoms was done according to the software).
3 Results and discussion
All the synthesized mixed-ligand complexes are stable at room temperature, non-hygroscopic and soluble in dimethyl sulfoxide and N,N-dimethylformamide. The physicoanalytical data are shown in Table 1.
Physicoanalytical data of the compounds
| Sl. no. | Compounds | Molecular mass | Yield (%) | Λam | C found (calcd) | H found (calcd) | N found (calcd) | M found (calcd) |
|---|---|---|---|---|---|---|---|---|
| 1 | [CoL1L2Cl]2H2O | 533.5 | 66 | 14.5 | 53.92 (53.98) | 4.08 (4.12) | 15.69 (15.74) | 11.01 (11.06) |
| 2 | [NiL1L2Cl]2H2O | 538 | 71 | 11.3 | 53.69 (53.53) | 4.02 (4.09) | 15.55 (15.61) | 11.75 (11.80) |
| 3 | [CuL1L2Cl]2H2O | 533 | 68 | 12.7 | 53.96 (54.03) | 4.07 (4.12) | 15.71 (15.76) | 10.93 (10.97) |
| 4 | [ZnL1L2Cl]2H2O | 539.5 | 65 | 9.2 | 53.32 (53.38) | 4.03 (4.07) | 15.50 (15.57) | 11.98 (12.04) |
- a
Ohm−1 cm2 mole−1.
3.1 Fourier-transform-infrared (FT-IR) spectra
The FT-IR spectra revealed the coordination of the metal ion through the deprotonated phenolic oxygen atom by the disappearance of the band at ∼3,300 cm−1 due to the phenolic –OH stretching vibration [32,33]. This was further confirmed by the hypsochromic shift of phenolic v(C–O) band at ∼1,280 cm−1 in the free ligand and L1 to ∼1,500 cm−1 in the spectra of the mixed-ligand complexes [33,34]. However, the position of the band due to vN–H (exocyclic) remains practically unaltered in the spectra, suggesting its noninvolvement in coordination [33] (Figure S1). Furthermore, noncoordination of ring nitrogen atom v(–C═N) of benzimidazole moiety in the spectra of the complexes is also ascertained by finding no change in the positions of the characteristic IR bands at ∼1,540 and ∼1,320 cm−1 due to v(C═N) (cyclic) and v(C–N) (cyclic) modes of vibration, respectively [33]. On the other hand, the position due to benzimidazole v(N–H) at ∼3,150 cm−1 reduced to lower frequency of ca. 20–25 cm−1, suggesting participation of –NH group of benzimidazole in coordination [33,35]. However, the bands due to v(C═N) and v(N–N) vibrations also show negative shift of ca. 10–20 cm−1 in the spectra of the complexes, suggesting their role in coordination [33,36]. Moreover, the vibration at ∼3,450 cm−1 is assigned to v(O–H) of the lattice water [33,34]. In addition, a sharp band at 655–680 cm−1 due to ν(C═N) of pyridine is observed in the spectra of the complexes [33,37].
TGA data for the ternary compounds
| Compounds | Temp. range of water loss (°C) | % of water loss | Decomposition temperature (°C) | % of residue | Composition of the residue | ||
|---|---|---|---|---|---|---|---|
| Found | Calc. | Found | Calc. | ||||
| [CoL1L2Cl]2H2O | 60–110 | 6.69 | 6.74 | 270 | 14.01 | 14.05 | CoO |
| [NiL1L2Cl] 2H2O | 55–110 | 6.70 | 6.75 | 260 | 13.92 | 13.98 | NiO |
| [CuL1L2Cl]2H2O | 45–95 | 6.65 | 6.69 | 255 | 14.72 | 14.77 | CuO |
| [ZnL1L2Cl]2H2O | 50–100 | 6.62 | 6.67 | 240 | 14.93 | 15.01 | ZnO |
3.2 Electronic spectra
The electronic spectral data and the magnetic moment values of the mixed-ligand complexes are listed in Table S1. The electronic spectrum of Co(ii) complex showed two main bands at ∼10,745 cm−1 (broad) and ∼22,136 cm−1 (strong) due to 4T1g(F) → 4T2g(F) (v1) and 4T1g(F) → 4T1g (P) (v3) transitions, respectively [38,39]. However, 4T1g(F) → 4A2g(F) (v2) transition was not observed because of its association with a large amount of energy cause by two electron transitions (t52g e2g → t32g e4g) [39,40]. Furthermore, the observed magnetic moment, µeff (4.72 BM), supported an octahedral geometry around Co(ii) ion [39,41]. The studied mixed-ligand complex of Ni(ii) complex showed split bands at ∼8,850 and ∼ 10,420 cm−1 assigned to 3B1g → 3Eg and 3B1g → 3B2g transitions, respectively. In addition, two characteristic bands at ∼15,350 and ∼24,575 cm−1 were assigned to 3A2g(F) → 3T1g(F) (v2) and 3A2g(F) → 3T1g(P) (v3) transitions, respectively, and suggested an octahedral geometry around Ni(ii) ion, which was confirmed by the observation of magnetic moment at 2.98 BM [39,42]. The mixed-ligand complex of Cu(ii) displayed two bands at ∼14,320 and ∼16,775 cm−1 attributed to 2B1g → 2B2g (v2) and 2B1g → 2Eg (v3) transitions, respectively, thus suggesting a distorted octahedral geometry [39,43]. However, the band due to 2B1g → 2A1g was not observed in the studied copper complex [39,44]. The µeff value for copper complex at 1.88 BM also suggested an octahedral geometry [39,45].
3.3 Thermal analysis
The thermogravimetric analysis (TGA) data for the studied mixed-ligand complexes displayed similar pattern of thermal decomposition. The lattice water degraded at temperature below 100°C in all the studied complexes. This is followed by the degradation of the anhydrous complexes in two distinct stages at temperatures 250–280°C and 360–390°C. However, the degradation of organic constituents continued until the formation of stable metal oxide as the end product. The temperature ranges of decomposition, peak temperature and the possible fragments removed are presented in (Table 2).
The list of intermolecular interactions between the compounds docked with 4Z6S
| Ligand | Score* | RMSD (Å) | Group interaction | Hydrogen bond** | Bond length (Å) |
|---|---|---|---|---|---|
| Co-crystallized | −21.63 | 0.04 | LEU 417, LEU 389, LYS 413, LEU 414, LEU 411, ASN 410, TYR 409, LYS 587, ASN 408, GLU 382, CYS 383, ASP 384, SER 386, PHE 385 | N sp2 (N2)–O sp2-GLU 382 | 3.078 |
| O sp3(O1)–N sp2-PHE 385 | 3.016 | ||||
| O sp3(O1)–N sp2-SER 386 | 3.043 | ||||
| O sp3(O3)–O sp2-GLU 382 | 2.866 | ||||
| O sp3(O3)–N sp3-LYS 413 | 3.333 | ||||
| O sp3(O4)–N sp3-LYS 413 | 3.082 | ||||
| Schiff base ligand | −41.40 | 0.05 | LEU 417, LEU 389, LEU 414, LYS 413, PHE 385, LEU 411, ASN 410, TYR 409, CYS 383, GLU 382, ASN 408, LYS 587 | O sp3(O0)–O sp2-GLU 382 | 3.077 |
| N sp3(N10)–O sp2-GLU 382 | 3.018 | ||||
| N sp3(N10)–N sp3-LYS 413 | 3.155 | ||||
| N sp2(N13)–O sp2-GLU 382 | 2.696 | ||||
| N sp2(N11)–N sp3-LYS 413 | 3.084 | ||||
| N sp2(N11)–N sp2-ASN 410 | 2.849 | ||||
| [NiL1L2Cl]2H2O | −26.64 | 0.04 | LEU 417, SER 416, LYS 413, LEU 414, LEU 389, SER 386, ASN 410, LYS 587, LEU 411, TYR 409, GLU 382, PHE 385, ASP 384, CYS 383 | O sp3(O13)–N sp3-LYS 413 | 3.140 |
| [ZnL1L2Cl]2H2O | −26.10 | 0.02 | LEU 417, SER 416, LYS 413, LEU 414, LEU 389, SER 386, ASN 410, LYS 587, LEU 411, TYR 409, GLU 382, PHE 385, ASP 384, CYS 383 | O sp3(O13)–N sp3-LYS 413 | 3.156 |
| [CoL1L2Cl]2H2O | −25.85 | 0.04 | SER 416, LYS 413, THR 412, LEU 411, ASN 410, ALA 434, SER 435, TYR 409, LYS 587, PRO 586 | O sp3(O13)–N sp3-ASN 410 | 3.044 |
| [CuL1L2Cl]2H2O | 23.86 | 0.08 | LEU 417, SER 416, LYS 413, LEU 414, LEU 389, SER 386, ASN 410, LYS 587, LEU 411, TYR 409, GLU 382, PHE 385, ASP 384, CYS 383 | O sp3(O13)–N sp3-LYS 413 | 3.014 |
- *
The docking score (PLANTSPLP score) is a function described in Korb et al. [31].
- **
The numbering of the atoms was done according to the software (Figures 1 and 2).
3.3.1 Proton nuclear magnetic resonance (1H-NMR) spectra
The 1H-NMR spectra of Schiff base ligand, L1, and its mixed-ligand complex of Zn(ii) displayed a multiplet due to the aromatic protons at δ 7.3–8.1 ppm. The signals at δ 6.7 and δ 9.0 ppm were attributed to the ring NH and exocyclic NH protons, respectively (Figure S2). However, the observed de-shielding in the ring –NH proton at δ 7.0 ppm indicated its participation in coordination. Furthermore, disappearance of phenolic –OH proton in the metal complexes confirmed its role in coordination. Moreover, –CH3 signal was also observed at δ 2.4 ppm.
3.4 Antibacterial activity
The in vitro antibacterial activity was reported by Agar well diffusion method [27] against B. subtilis, E. coli and S. typhi at 100 μg mL−1 concentrations. The zone of inhibition data was studied using ciprofloxacin as the standard antibacterial drug as shown in Figure 2. The studied complexes exhibited better activity than the free ligand, which is likely due to the presence of –C═N linkage and its involvement in coordination. Furthermore, coordination through the metal ions reduces the electron density due to the partial sharing of its positive charge with the donor groups and possible π-electron delocalization [46]. In addition, solubility, dipole moment, the nature of the ligand and geometry are supposed to be the possible factor for the higher antibacterial activity displayed by the mixed-ligand complexes [47]. However, the mixed-ligand Zn(ii) complex remained inactive against E. coli and S. typhi.

In vitro antibacterial activity of the ligand and its mixed-ligand complexes.
3.4.1 Docking evaluation against MERS-CoV
Docking studies were performed to obtain accurate predictions on the optimized conformations for both the ligands and protein target to form a stable complex. All the compounds were docked on the crystal structure of MERS CoV (PDB ID: 4Z6S). The docking pose of the co-crystallized N-acetyl-d-glucosamine (NAG) interacting with the residues of amino acid of active site are listed in Figure 3. The co-crystallized NAG displayed the occurrence of six hydrogen bonds: two with GLU 382 (3.078 and 2.866 Å), two with LYS 413 (3.333 and 3.082 Å), PHE 385 (3.016 Å) and SER 386 (3.043 Å). The co-crystallized NAG was considered as the reference ligand to compare the docking results of the studied compounds. The docking studies revealed that the docking score of all the metal complexes are greater than the co-crystallized NAG (docking score: −21.63; root-mean-square deviation [RMSD]: 0.04 Å) but smaller than L1 (docking score: −41.40; RMSD: 0.05 Å; Table 4). The L1 showed the presence of six hydrogen bonds: three with GLU 382 (3.077, 3.018 and 2.696 Å), two with LYS 413 (3.155 and 3.084 Å) and one with ASN 410 (2.849 Å). The Ni(ii) complex with the best docking score (−26.64; RMSD: 0.04) displayed one hydrogen bond with LYS 413 (3.140 Å). With LYS 413 amino acid, Zn(ii) complex (3.156 Å) and Cu(ii) complex (3.014 Å) realized one more hydrogen bond. However, Co(ii) complex realized one hydrogen bond with ASN 410 (3.044 Å). The docking pose of the compounds interacting with amino acid residues is presented in Figure 3. The amino acid residues that formed the interacting group of each compound are listed in Table 3. After analyzing the data, it was noticed that all the studied compounds were placed in the same binding site (represented by a green sphere) of 4ZS6 as the co-crystallized one and have the same orientation as the co-crystallized NAG (Figure 4).
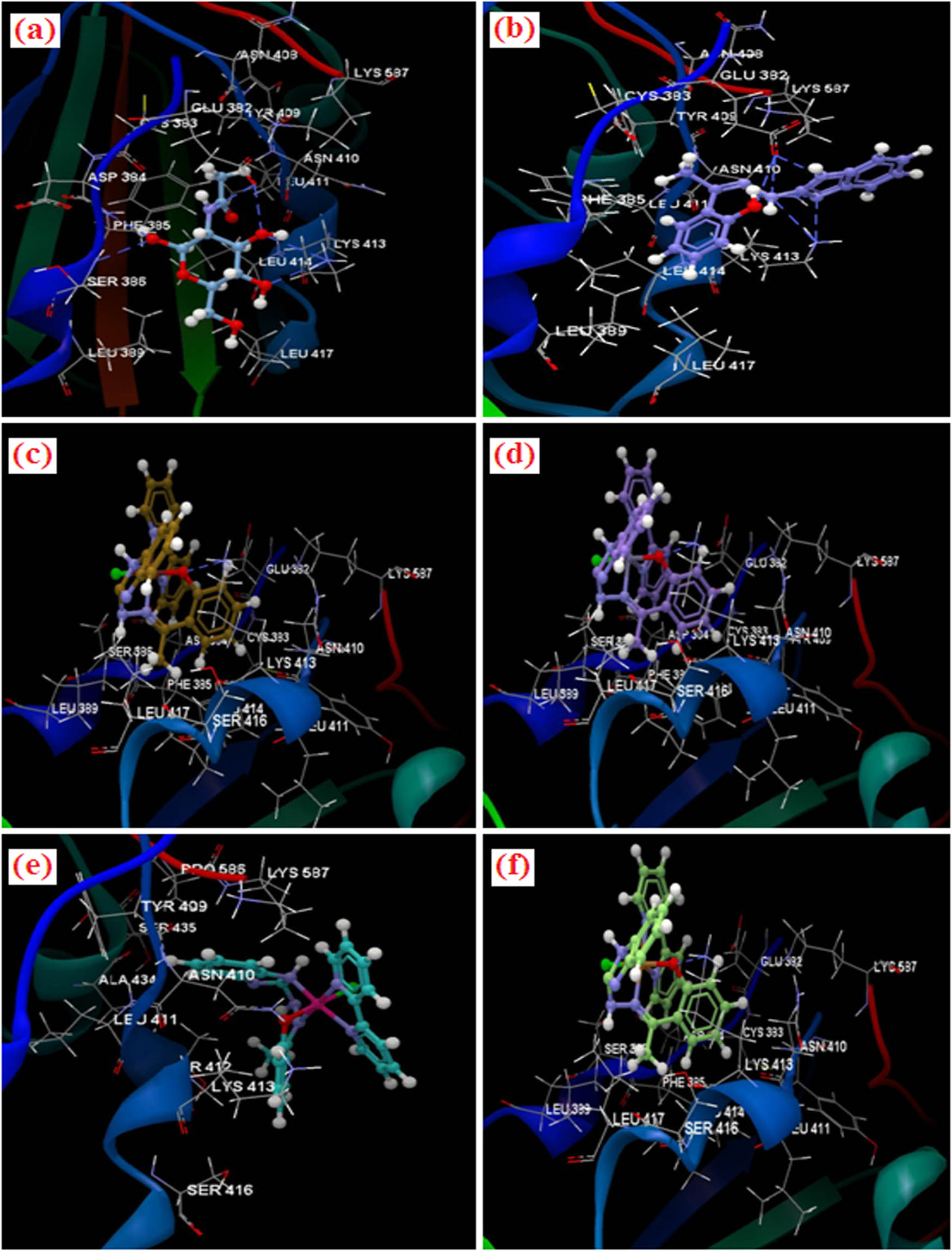
Docking pose of the compounds interacting with the residues of amino acid of binding site of 4Z6S; (a) co-crystallized NAG, (b) Schiff base ligand, (c) Ni(ii) complex, (d) Zn(ii) complex, (e) Co(ii) complex and (f) Cu(ii) complex.
The list of intermolecular interactions between the compounds docked with 6W63
| Ligand | Score* | RMSD (Å) | Group interaction | Hydrogen bond** | Bond length (Å) |
|---|---|---|---|---|---|
| Co-crystallized | −56.57 | 1.53 | THR 190, GLN189, VAL 186, ARG 188, MET 49, TYR 54, ASP 187, PRO 168, LEU 167, GLU 166, MET 165,HIS 164, HIS 172, HIS 163, CYS 145, ASN 142, GLY 143, SER 144, LEU 141, PHE 140, GLY 143, LEU 127, THR 26, THR 25, VAL 42, HIS 41, CYS 44 | O sp2 (O01)–N sp2-GLY 143 | 3.202 |
| O sp2 (O13)–N sp2-GLU 166 | 2.721 | ||||
| Schiff base ligand | −49.91 | 0.87 | ALA 191, VAL 186, GLN 192, THR 190, GLN 189, ARG 188, ASP 187, HIS 41, LEU 50, PRO 52, ASP 48, CYS 44, TYR 54, MET 49, PRO 168, LEU 167, GLU 166, MET 165 | O sp3(O0)–N sp2-GLN 192 | 3.028 |
| O sp3(O0)–O sp2-THR 190 | 2.558 | ||||
| O sp3(O0)–N sp2-THR 190 | 2.924 | ||||
| O sp3(O0)–O sp2-ARG 188 | 3.025 | ||||
| [CoL1L2Cl]2H2O | −52.52 | 0.002 | ALA 191, GLN 192, THR 190, GLN 189, ARG 188, ASP 187, MET 49, THR 169, PRO 168, LEU 167, GLY 170, GLU 166, MET 165, HIS 164, HIS 163, CYS 145, ASN 142 | N sp3(N23)–N sp2-GLN 189 | 3.143 |
| [NiL1L2Cl]2H2O | −52.35 | 0.006 | ALA 191, GLN 192, THR 190, GLN 189, ARG 188, ASP 187, MET 49, THR 169, PRO 168, LEU 167, GLY 170, GLU 166, MET 165, HIS 164, HIS 163, CYS 145, ASN 142 | N sp3(N23)–N sp2-GLN 189 | 3.145 |
| [ZnL1L2Cl]2H2O | −52.23 | 0.01 | ALA 191, GLN 192, THR 190, GLN 189, ARG 188, ASP 187, MET 49, THR 169, PRO 168, LEU 167, GLY 170, GLU 166, MET 165, HIS 164, HIS 163, CYS 145, ASN 142 | N sp3(N23)–N sp2-GLN 189 | 3.136 |
| [CuL1L2Cl]2H2O | −51.97 | 0.01 | ALA 191, GLN 192, THR 190, GLN 189, ARG 188, ASP 187, MET 49, HIS 41, VAL 186, THR 169, PRO 168, LEU 167, GLY 170, GLU 166, MET 165, HIS 164, HIS 163, CYS 145, ASN 142 | N sp3(N23)–N sp2-GLN 189 | 3.134 |
- *
The docking score (PLANTSPLP score) is a function described in Korb et al. [31].
- **
The numbering of the atoms was done according to the software (Figures 1 and 2).

(a) Docking pose of the co-crystallized NAG and all compounds in the binding site of 4Z6S and (b) docking pose of the co-crystallized X77 and all compounds in the binding site of 6W63.
3.4.2 Docking evaluation against SARS-CoV-2 Mpro
All the compounds were docked on the crystal structure of SARS-CoV-2 Mpro (PDB ID: 6W63). The docking pose of the co-crystallized X77 interacting with amino acid residues of the active site and the hydrogen bonds created with GLU 166 (2.721 Å) and GLY 143(3.202 Å) is shown in Figure 5. The co-crystallized X77 (N-(4-tert-butylphenyl)-N-[(1R)-2-(cyclohexylamino)-2-oxo-1-(pyridin-3-yl)ethyl]-1H-imidazole-4-carboxamide) was considered as a standard ligand to compare the docking results of the studied compounds. The docking studies revealed that the docking score of all the metal complexes are close to that of the co-crystallized X77 (docking score: −56.57; RMSD: 1.53 Å) and greater than the Schiff base ligand, L1 (docking score: −49.91; RMSD: 0.87 Å; Table 5). The Schiff base ligand, L1, showed the occurrence of four hydrogen bonds: one with GLN 192 (3.028 Å), two with THR 190 (2.558 and 2.924 Å) and one with ARG 188 (3.025 Å). The Co(ii) complex with the best docking score (−52.52; RMSD: 0.002) displayed one hydrogen bond with GLN 189 (3.143 Å). With the GLN189 amino acid, Ni(ii) complex (3.145 Å), Zn(ii) complex (3.145 Å) and Cu(ii) complex (3.134 Å) realized one more hydrogen bond. The docking pose of the compounds interacting with amino acid residues is presented in Figure 5. The amino acid residues that formed the interacting group of each compound are listed in Table 4. After analyzing the data, it was noticed that the all studied compounds were placed in the same binding site (represented by a green sphere) of 6W63 as the co-crystallized one and have the same orientation as the co-crystallized X77 (Figure 5).

Docking pose of the compounds interacting with the amino acid residues of binding site of 4Z6S; (a) co-crystallized X77, (b) Schiff base ligand, HL, (c) Co(ii) complex, (d) Ni(ii) complex, (e) Zn(ii) complex and (f) Cu(ii) complex.
According to Lipinski’s rule of five, the calculated parameters (Table S2) may predict the property of a molecule to turn into an active drug [48] on the basis of the number of violations made. It is observed that all metal complexes have two violations of Lipinski’s rule of five (Lipinski violation is 2), namely, molecular weight >500 Da and octanol–water partition coefficient (log P) > 5.
3.5 Electrostatic potential (ESP) surface analysis
The ESP surface of Schiff base ligand, L1, was analyzed by using Argus Lab 4.0.1 software as shown in Figure 6. The surface contains a number of possible sites for electrophilic attack. The ESP surface displayed a specific data about the charge distribution. The negative regions were mainly over phenolic oxygen, azomethine nitrogen and benzimidazole ring nitrogen atoms as indicated in red and were involved in coordination. However, the other nitrogen atom was not involved in coordination likely due to the steric effect.
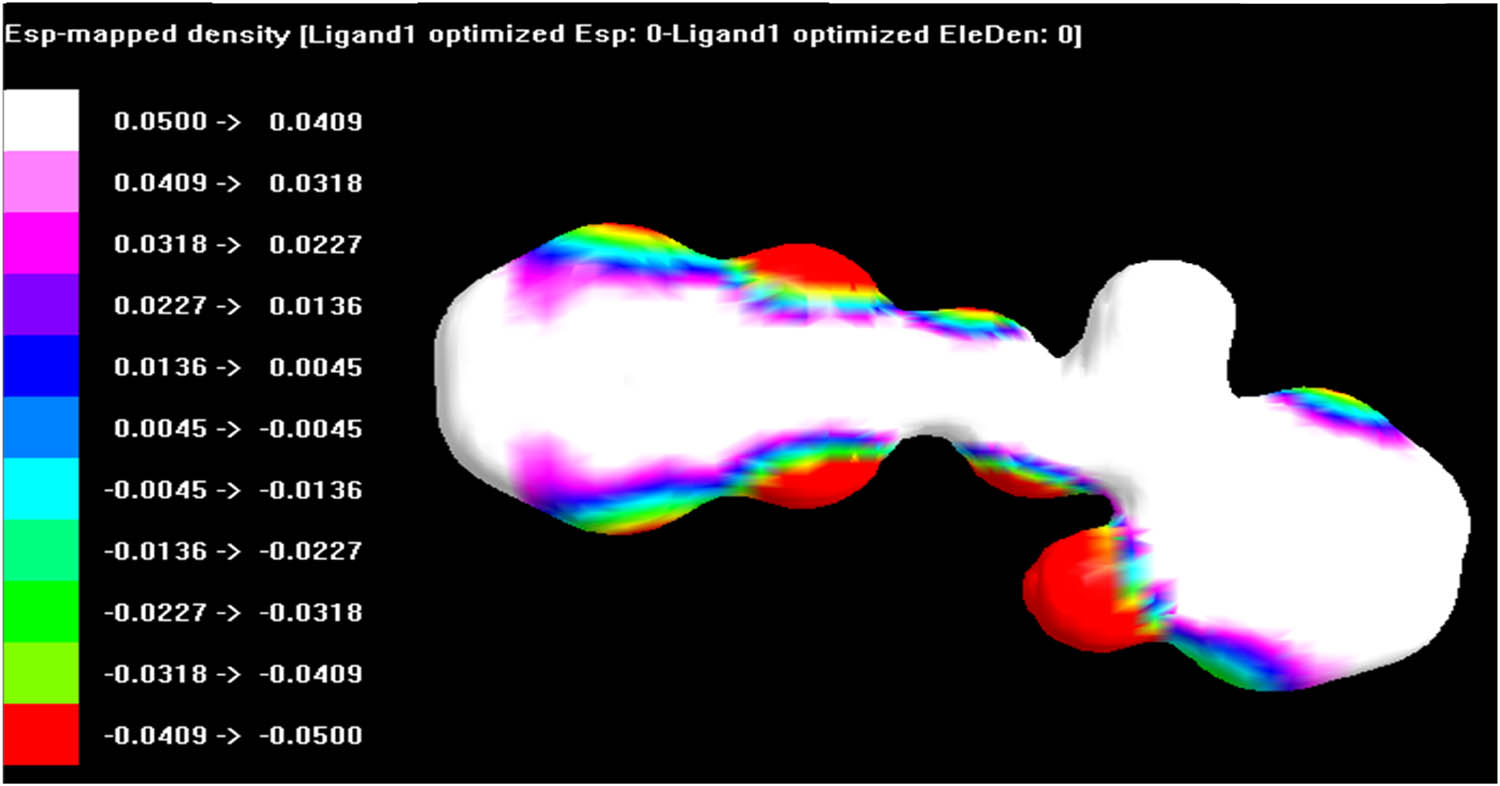
The ESP surface of the Schiff base ligand, L1.
4 Conclusion
A series of mixed-ligand complexes with Cu(ii), Co(ii), Ni(ii) and Zn(ii) ions were investigated. The antibacterial activity suggested the moderate antibacterial activity for all the complexes in comparison to the Schiff base ligand. However, the Zn(ii) complex was inactive against E. coli and S. typhi. Moreover, molecular docking studies recorded against MERS-CoV (PDB ID: 4ZS6) and SARS-CoV-2 Mpro (PDB ID: 6W63) revealed that all the studied metal complexes demonstrated two violations of all the parameters involved in the Lipinski’s rule of five. The Ni(ii) complex displayed the best docking score (−26.64; RMSD: 0.04) against MERS-CoV whereas the Co(ii) complex displayed the best docking score (−52.52; RMSD: 0.002) against SARS-CoV-2 Mpro. This study may be helpful for the researchers in designing new potent drugs. In addition, the ESP surface of the Schiff base ligand, L1, was analyzed to understand the possible sites for electrophilic attack.
Acknowledgments
The authors acknowledge the financial support through Researchers Supporting Project number (RSP-2020/147), King Saud University, Riyadh, Saudi Arabia. The authors are highly thankful to Dr. Lucia Pintilie for her valuable support in the docking analyses.
Research funding: The authors acknowledge the financial support through Researchers Supporting Project number (RSP-2020/147), King Saud University, Riyadh, Saudi Arabia.
Author contribution: Ranjan K. Mohapatra designed and supervised the study. V. P. Saikishore performed the study. Susanta K. Biswal supervised and was in charge of the initial draft. Mohammad Azam was involved in final editing and also revised the manuscript.
Conflict of interest: There are no conflicts to declare.
Data availability statement: All data presented in the study are included in the article and supplementary material.
Ethics approval: The conducted research is not related to either human or animal use.
References
[1] Devinder MRJ, Michael BR, Sean MK. Synthesis and evaluation of anticancer benzoxazoles and benzimidazoles related to UK-1. Bioorg Med Chem. 2002;10:3997–4004.10.1016/S0968-0896(02)00327-9Suche in Google Scholar
[2] Hong SY, Chung KH, You JH, Choi IH, Chae MJ, Han JY, et al. Synthesis and biological evaluation of benzimidazole-4,7-diones that inhibit vascular smooth muscle cell proliferation. Bioorg Med Chem Lett. 2004;14:3563–6.10.1016/j.bmcl.2004.04.051Suche in Google Scholar
[3] Lopez VF, Medina-Franco JL, Hernandez-Campos A, Rodriguez-Morales S, Yepez L, et al. Molecular modeling of some 1H-benzimidazole derivatives with biological activity against Entamoebahistolytica: a comparative molecular field analysis study. Bioorg Med Chem. 2007;15:1117–26.10.1016/j.bmc.2006.10.019Suche in Google Scholar
[4] Chkirate K, Karrouchi K, Dege N, Sebbar NK, Ejjoummany A, Radi S, et al. Co(ii) and Zn(ii) pyrazolyl-benzimidazole complexes with remarkable antibacterial activity. New J Chem. 2020;44:2210–21.10.1039/C9NJ05913JSuche in Google Scholar
[5] White AW, Curtin NJ, Eastman BW, Golding BT, Hostomsky Z, Kyle S, et al. Potentiation of cytotoxic drug activity in human tumour cell lines, by amine substituted 2-arylbenzimidazole-4-carboxamide PARP-1 inhibitors. Bioorg Med Chem Lett. 2004;14:2433–7.10.1016/j.bmcl.2004.03.017Suche in Google Scholar
[6] Driessen WL, Wiesmeijer WGR, Schipper-Zablotskaja M, De Graaff RAG, Reedijk J. Transition metal coordination compounds of two pyrazole-substituted ammonia ligands. X-ray structure of [bis(1-pyrazolylmethyl)aminecobalt(ii)] bis(nitrate). Inorg Chim Acta. 1989;162:233–8.10.1016/S0020-1693(00)83153-9Suche in Google Scholar
[7] Boca M, Jameson RF, Linert W. Fascinating variability in the chemistry and properties of 2,6-bis-(benzimidazol-2-yl)-pyridine and 2,6-bis-(benzthiazol-2-yl)-pyridine and their complexes. Coord Chem Rev. 2011;255:290–317.10.1016/j.ccr.2010.09.010Suche in Google Scholar
[8] Bouchouit M, Said ME, Kara Ali M, Bouacida S, Merazig H, KacemChaouche H, et al. Synthesis, X-ray structure, theoretical investigation, corrosion inhibition and antimicrobial activity of benzimidazole thioether and theirs metal complexes. Polyhedron. 2016;119:248–59.10.1016/j.poly.2016.08.045Suche in Google Scholar
[9] Gocke M, Utku S, Gur S, Ozkul A, Gumus F. Synthesis, in vitro cytotoxic and antiviral activity of cis-[Pt(R(−) and S(+)-2-α-hydroxybenzylbenzimidazole)Cl] complexes. Eur J Med Chem. 2005;40:135–41.10.1016/j.ejmech.2004.09.017Suche in Google Scholar PubMed
[10] Saczewski F, Dziemidowicz-Borys E, Bednarski PJ, Grünert R, Gdaniec M, Tabin P. Synthesis, crystal structure and biological activities of copper(ii) complexes with chelating bidentate 2-substituted benzimidazole ligands. J Inorg Biochem. 2006;100:1389–98.10.1016/j.jinorgbio.2006.04.002Suche in Google Scholar PubMed
[11] Khan SA, Noor A, Kempe R, Subhan H, Shah A, Khan E. Syntheses, molecular structure, and electrochemical investigations of cobalt(ii), copper(ii), palladium(ii), and zinc(ii) complexes with 3-methylpyrazole. J Coord Chem. 2014;67:2425–34.10.1080/00958972.2014.938066Suche in Google Scholar
[12] Khan E, Khan SA, Zahoor M, Tahir MN, Noor A, Altaf AA. Cu(ii) coordination polymers stabilized by pyridine-2,6-dicarboxylate anion and pyrazole derivatives through ligand hydrolysis. J Coord Chem. 2018;71:2658–73.10.1080/00958972.2018.1501562Suche in Google Scholar
[13] Alvareza N, Mendesb LFS, Kramerc MG, Torrea MH, Costa-Filhob AJ, Ellenad J, et al. Development of copper(ii)-diimine-iminodiacetate mixed ligand complexes as potential antitumor agents. Inorg Chim Acta. 2018;483:61–70.10.1016/j.ica.2018.07.052Suche in Google Scholar
[14] Albobaledia Z, Esfahania MH, Behzada M, Abbasi A. Mixed ligand Cu(II) complexes of an unsymmetrical Schiff base ligand and N-donor heterocyclic co-ligands: Investigation of the effect of co-ligand on the antibacterial properties. Inorg Chim Acta. 2020;499:119185.10.1016/j.ica.2019.119185Suche in Google Scholar
[15] Eremina JA, Lidera EV, Samsonenko DG, Sheludyakova LA, Berezina AS, Klyushova LS, et al. Mixed-ligand copper(ii) complexes with tetrazole derivatives and 2,2′-bipyridine, 1,10-phenanthroline: Synthesis, structure and cytotoxic activity. Inorg Chim Acta. 2019;487:138–44.10.1016/j.ica.2018.12.011Suche in Google Scholar
[16] Bianchia A, Delgado-Pinar E, García-España E, Giorgia C, Pina F. Highlights of metal ion-based photochemical switches. Coord Chem Rev. 2014;260:156–215.10.1016/j.ccr.2013.09.023Suche in Google Scholar
[17] Costa RD, Orti E, Bolink HJ, Monti F, Accorsi G, Armaroli N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew Chem Int Ed. 2012;51:8178–211.10.1002/anie.201201471Suche in Google Scholar PubMed
[18] Katari M, Carmichael D, Jacquemin D, Frison G. Structure of electronically reduced N-donor bidentate ligands and their heteroleptic four-coordinate zinc complexes: a survey of density functional theory results. Inorg Chem. 2019;58:7169–79.10.1021/acs.inorgchem.8b03549Suche in Google Scholar PubMed
[19] Lu J, Guo J, Sang W, Guo H. Mixed-ligand oxovanadium complexes incorporating Schiff base ligands: synthesis, DNA interactions, and cytotoxicities. Trans Met Chem. 2013;38:481–8.10.1007/s11243-013-9714-8Suche in Google Scholar
[20] Sigel H, Sigel A. Metal ions in biological systems. Vols. 12 and 13. New York: Marcel Dekker; 2005.10.1201/9780849346071Suche in Google Scholar
[21] Mohapatra RK, Pintilie L, Kandi V, Sarangi AK, Das D, Sahu R, Perekhoda L. The recent challenges of highly contagious COVID-19causing respiratory infections: symptoms, diagnosis, transmission, possible vaccines, animal models and immunotherapy. Chem Biol Drug Des. 2020;96:1187–120.10.1111/cbdd.13761Suche in Google Scholar PubMed PubMed Central
[22] Mohapatra RK, Rahman M. Is it possible to control the outbreak of COVID-19 in Dharavi, Asia’s largest slum situated in Mumbai? Anti-Infective Agents. 2020. 10.2174/2211352518999200831142851.Suche in Google Scholar
[23] Mohapatra RK, Das PK, Kandi V. Challenges in controlling COVID-19 in migrants in Odisha, India, diabetes & metabolic syndrome. Clin Res Rev. 2020;14:1593–4.Suche in Google Scholar
[24] Ancy I, Sivanandam M, Kumaradhas P. Possibility of HIV-1 protease inhibitors-clinical trial drugs as repurposed drugs for SARS-CoV-2 main protease: a molecular docking, molecular dynamics and binding free energy simulation study. J Biomol Struct Dyn. 2020. 10.1080/07391102.2020.1786459Suche in Google Scholar PubMed PubMed Central
[25] Sang P, Tian S-H, Meng Z-H, Yang L-Q. Anti-HIV drug repurposing against SARS-CoV-2. RSC Adv. 2020;10:15775–83.10.1039/D0RA01899FSuche in Google Scholar PubMed PubMed Central
[26] Cardoso WB, Mendanha SA. Molecular dynamics simulation of docking structures of SARS-CoV-2 main protease and HIV protease inhibitors. J Mol Struct. 2021;1225:129143.10.1016/j.molstruc.2020.129143Suche in Google Scholar PubMed PubMed Central
[27] Mohapatra RK, Das PK, El-ajaily MM, Mishra U, Dash DC. Synthesis, spectral, thermal, kinetic and antibacterial studies of transition metal complexes with benzimidazolyl-2-hydrazones of o-hydroxyacetophenone, o-hydroxybenzophenone and o-vanillin. Bull Chem Soc Ethiop. 2018;32(3):437–50.10.4314/bcse.v32i3.3Suche in Google Scholar
[28] CLC Drug Discovery Workbench, available from http://www.clcbio.com.Suche in Google Scholar
[29] Yu X, Zhang S, Jiang L, Cui Y, Li D, Wang D, et al. Structural basis for the neutralization of MERS-CoV by a human monoclonal antibody MERS-27. Sci Rep. 2015;5:13133. 10.1038/srep13133Suche in Google Scholar PubMed PubMed Central
[30] Mesecar AD. A taxonomically-driven approach to development of potent, broad-spectrum inhibitors of coronavirus main protease including SARS-CoV-2 (COVID-19); 2020. https://www.rcsb.org/structure/6W63.Suche in Google Scholar
[31] Korb O, Stützle T, Exner TE. Empirical scoring functions for advanced protein-ligand docking with plants. J Chem Inf Model. 2009;49:84–96.10.1021/ci800298zSuche in Google Scholar PubMed
[32] Azam M, AlResayes SI. Phenoxy-bridged binuclear Zn(ii) complex holding salen ligand: synthesis and structural characterization. Mol Structure. 2016;1107:77–81.10.1016/j.molstruc.2015.11.017Suche in Google Scholar
[33] Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. New York: Wiley; 1986.Suche in Google Scholar
[34] Azam M, Dwivedi S, AlResayesSI, Adil SF, Islam MS, Kruszynska AT, Kruszynski R, Lee DU. Cu(ii) salen complex with propylene linkage: An efficient catalyst in the formation of C–X bonds (X = N, O, S) and biological investigations. J Mol Struct. 2017;1130:122–7.10.1016/j.molstruc.2016.10.021Suche in Google Scholar
[35] Azam M, Khan AA, AlResayes SI, Islam MS, Saxena AK, Dwivedi S, Musarrat J, Kruszynska AT, Kruszynski R. Synthesis and characterization of 2-substituted benzimidazoles and their evaluation as anticancer agent. Spectrochim Acta Part A. 2015;142:286–91.10.1016/j.saa.2015.01.106Suche in Google Scholar PubMed
[36] Azam M, AlResayes SI, Kruszynska AT, Kruszynski R, Verma A, Pati UK. Chiral anionic binuclear zinc complexes based on diaminocyclohexane ligand and their in vitro antiproliferative studies. Inorg Chem Commun. 2014;46:73–80.10.1016/j.inoche.2014.05.029Suche in Google Scholar
[37] Abd El-halim HF, Omar MM, Mohamed GG. Synthesis, structural, thermal studies and biological activity of a tridentate Schiff base ligand and their transition metal complexes. Spectrochim Acta Part A. 2011;78:36–44.10.1016/j.saa.2010.06.003Suche in Google Scholar PubMed
[38] Firdaus F, Fatma K, Azam M, Khan SN, Khan AU, Shakir M. Template synthesis and physico-chemical studies of 14-membered hexamacrocyclic complexes with Co(II), Ni(II), Cu(II) and Zn(II): a comparative spectroscopic approach on DNA binding with Cu(II) and Ni(II) complexes. Trans Met Chem. 2008;33:467–73.10.1007/s11243-008-9066-ySuche in Google Scholar
[39] Lever ABP. Inorganic electronic spectroscopy. 2rd edn. Amsterdam: Elsevier; 1984.Suche in Google Scholar
[40] Firdaus F, Fatma K, Azam M, Khan SN, Khan AU, Shakir M. Template synthesis and physic-chemical characterization of 14-membered tetraiminemacrocyclic complexes, [MLX2] [M = Co(II), Ni(II), Cu(II) and Zn(II)]: DNA binding study on [CoLCl2] complex. Spectrochim Acta Part A. 2009;72:591–6.10.1016/j.saa.2008.10.054Suche in Google Scholar PubMed
[41] Shakir M, Azam M, Naseem S, Khan AU. Template synthesis and physic-chemical studies of 14-membered functionalized Pendant arm Schiff base macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II): DNA binding studies on Cu(II). Synth React Inorg-Metal Org Nano-Met Chem. 2011;41:1056–62.10.1080/15533174.2011.591345Suche in Google Scholar
[42] Shakir M, Azam M, Parveen S, Khan AU, Firdaus F. Synthesis and spectroscopic studies on complexes of N,N-bis(-(2-pyridenecarboxaldimine)-1,8-diaminonaphthalene (L): DNA binding studies on Cu(II) complex. Spectrochim Acta Part A. 2009;71:1851–6.10.1016/j.saa.2008.07.002Suche in Google Scholar PubMed
[43] Shakir M, Azam M, Azim Y, Parveen S, Khan AU. Synthesis and physico-chemical studies on complexes of 1,2-diaminophenyl-N,N′-bis(2-pyridenecarboxaldimine) (L): a spectroscopic approach on DNA binding studies on Cu(II) complex. Polyhedron. 2007;26:5513–5518.10.1016/j.poly.2007.08.032Suche in Google Scholar
[44] Cotton FA, Wilkinson G. Advanced inorganic chemistry. 5th edn. New York: Wiley; 1988.Suche in Google Scholar
[45] Shakir M, Abbasi A, Azam M, Khan AU. Synthesis, spectroscopic studies and crystal structure of the Schiff base ligand L derived from condensation of 2-thiophenecarboxaldehyde and 3,3′ diaminobenzi-dine and complexes with Co(II), Ni(II), Cu(II), Cd(II) and Hg(II). Comparative DNA binding studies of L and its Co(II), Ni(II) and Cu(II) complexes. Spectrochim Acta Part A. 2011;79:1866–75.10.1016/j.saa.2011.05.077Suche in Google Scholar PubMed
[46] Balhausen CJ. An introduction to ligand field. New York: McGraw Hill; 1962.Suche in Google Scholar
[47] El-Gamel NEA. Metal chelates of ampicillin versus amoxicillin: synthesis, structural investigation, and biological studies. J Coord Chem. 2010;63:534–43.10.1080/00958970903494157Suche in Google Scholar
[48] Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev. 2001;46:3–26.10.1016/S0169-409X(00)00129-0Suche in Google Scholar
© 2020 Ranjan K. Mohapatra et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

