Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
-
Aleksandra Ciesielska
, Henryk Kołoczek
Abstract
Deionized, tap and two kinds of commercially available mineralized water, after supplementation with ammonia, were treated with low-pressure, low-temperature glow plasma (GP) of low frequency. Treating hard water with ammonia provided the removal of permanent and temporary water hardness already at room temperature. On such treatment, mineralized water supplemented with ammonia was partly demineralized. Precipitated rhombohedral deposit from hard water did not turn into scale even when maintained in suspension for 3 days at around 90°C. In such manner, the use of other chemicals for prevention from the scale formation and/or for the scale removal is entirely dispensable. The rate and yield of precipitation depended on the concentration of admixed ammonia and the GP treatment time. Ammonia served as a ligand of calcium, magnesium and ferric central atoms of corresponding salts constituting the hardness. Moreover, ammonia constituting the atmosphere of the treatment was arrested inside aqueous clathrates. So, stabilized ammonia solutions could potentially be utilized as an environmental-friendly nitrogen fertilizer. The precipitate could also be utilized for the same purpose.
1 Introduction
From a chemical point of view, water which is commonly used in everyday life cannot be considered pure. Even when either redistilled or thoroughly deionized, it is, in fact, a solution of components of the atmosphere in which these operations were carried out. The gaseous components cannot be fully evacuated from those solutions even under a deep vacuum. Such water, particularly when containing dissolved oxygen, is corrosive which is essential on its technical applications. If not either deionized or distilled, water contains dissolved mineral salts. Sulfates and hydrogen carbonates of calcium, magnesium and iron are responsible for the so-called permanent and temporary water hardness, and the other salts constitute water mineralization. Both kinds of hardness cause a scale deposition in water-heating systems and water-transmitting pipelines. Calcium and magnesium sulfates precipitate and go into scale after reaching the saturation point in a given solution. In contrast to them, magnesium and chiefly calcium bicarbonates precipitate even from their diluted solutions because of the shift in the equilibrium of the reaction
to the right side. Water-soluble hydrogen carbonate decomposes into water insoluble carbonate, water and CO2. Calcium carbonate precipitates as rhombohedral aragonite forming a mud which successively turns into developing scale rhombic calcite.
There are several unwanted consequences of the scale formation. The costs of heating water in the scale-covered heating systems producing hot water and/or steam increase. Perturbed heat transmission can result in local overheating. Gradual closing pipes require more energy for transmitting fluids across them.
In order to manage with scale, two approaches are in use. One prevents the scale formation and the other takes its removal once it forms. The most common methods involve (i) deionization of water with ion exchangers, (ii) blowing CO2 into water to hinder the decomposition of calcium/magnesium hydrogen carbonate into the corresponding carbonates, (iii) addition of polyphosphates and (iv) treatment of water with static magnetic as well as electric field. Once the precipitate of aragonite is formed, it should be removed prior to its conversion into calcite scale by either blowing with steam or air or by filtration. Moreover, (v) the removal of hardness through an accelerated precipitation of calcium carbonate may be involved. For this purpose, a blend of lime and sodium carbonate is added at 60–80°C. The selected approach depends on the destination of the water after the treatment, parameters of the installation prevented from the scale and, generally, economy of the process [1,2,3,4,5].
In former papers, the ability of low-temperature, low-pressure glow plasma (GP) of low frequency to modulate water macrostructure and, hence, its physical, physicochemical and functional properties was demonstrated. These properties depended, among others, on the time of exposure of the water to GP and the atmosphere under which the GP treatment was performed. Thus far, the effect of the air [6], oxygen-free nitrogen [7], carbon dioxide [8], methane [9] and oxygen [10] upon results of the GP treatment was published. In the course of our studies, we found that the energy of applied GP never broke valence bonds and could not initiate any chemical reaction. Thus, on the treatment of water in the air as well as under pure oxygen, neither hydrogen peroxide nor ozone was formed.
In this paper, results of the GP treatment upon either deionized or tap water under ammonia are presented. The preparation of water with potentially interesting functional properties was the driving force for this study. It has appeared that such treatment of water offered a simple removal of permanent and temporary water hardness. A fine mineral deposit which was formed had no tendency to turn into the scale. In such manner, any involvement of other chemicals appeared dispensable. Resulting water hosting ammonia in aqueous clathrates was practically odorless. Therefore, it could be either comfortably recirculated or potentially utilized as environmentally benign nitrogen fertilizer. For its composition, the precipitates could also be utilized for the same purpose.
2 Materials and methods
2.1 Water characteristics
Commercially available deionized water contained totally 20.04 mg minerals/L (0.25 mg Ca2+/L, 0.12 mg Mg2+/L, 0.08 mg Na+/L, 18.30 mg HCO3=/L, 1.28 mg SO4=/L, 0.01 mg Cl−/L).
Tap water: municipal water from the Czestochowa supply system containing totally 672.28 mg minerals/L (193.27 mg Ca2+/L, 46.21 mg Mg2+/L, 23.18 mg Na+/L, 5.27 mg K+/L, 351.90 mg HCO3=/L, 51.20 mg SO4=/L, 6.52 mg Cl−/L and 40 μg Fe/L).
Highly mineralized water: Commercially available water “Kryniczanka” highly saturated with CO2 containing totally 2246.1 mg minerals/L (368.92 mg Ca2+/L, 73.209 mg Mg2+/L, 54.71 mg Na+/L, 5.27 mg K+/L, 1721.9 mg HCO3=/L, 40.50 mg SO4=/L, 7.8 mg Cl−/L and 0.4 mg F−/L).
Weakly mineralized water: Commercially available nonsaturated with CO2 water “Kropla Beskidu” containing totally 322.27 mg minerals/L (44.9 mg Ca2+/L, 17.01 mg Mg2+/L, 11.10 mg Na+/L, 1.0 mg K+/L, 186.7 mg HCO3=/L, 43.62 mg SO4=/L and 3.19 mg Cl−/L).
2.2 Methods
2.2.1 Water treatment with GP
Either deionized, tap, high- or low-mineralized water (100 mL) was blended either with (i) saturated at room temperature aqueous ammonia puriss., anhydrous, ≥99.95% (Sigma-Aldrich, Warsaw, Poland) (1, 2.5, 5, 7.5 or 10 mL) or with (ii) aqueous 0.01 M solution of sodium hydroxide anhydrous, free-flowing pellets, ≥97% (Sigma-Aldrich) (1, 5 or 10 mL) and placed within 250 mL polyethylene bottles. The bottles were transferred into the chamber of the reactor [11,12] and exposed to plasma for either 1, 5, 15, 30, 60 or 120 min. Plasma of 38°C was generated at 5 × 10−3 mbar, 600 V, 50 mA and 280 GHz frequency. The corresponding nontreated water and the corresponding nontreated aqueous hydroxide solutions served as primary and secondary controls, respectively.
2.2.2 pH measurements
pH values were taken at ambient temperature (∼20°C) with the Metrohm 827 lab pH meter Metrohm Polska Sp. z o.o. (Opacz-Kolonia, Poland). The measurements were run in triplicates.
2.2.3 Conductivity
Conductivity measurements were performed at room temperature. Measurements were run in triplicates. An inoLab, Pol-Eko-Aparatura (Warsaw, Poland), conductometer was employed.
2.2.4 Precipitates and their analyses
2.2.4.1 Weight analysis
On the GP treatment of tap and mineralized waters, fine precipitates were separated from solutions by centrifugation at 500 rpm. They were collected either directly after separation or after their 3 days maintenance in aqueous suspensions at ∼90°C. Weights of samples were measured in triplicates with the analytical laboratory scale RADWAG AS 220.R2 (Radom, Poland) with a precision of ±0.0001 g.
2.2.4.2 Cation chromatography, estimation of Ca2+, Mg2+, Na+ ions in solutions after the removal of deposits
Estimation of cations in solutions after the removal of deposits was performed at 22°C using ionic chromatograph 940 Professional IC Vario 1 (Metrohm, Opacz-Kolonia, Poland) with conductometric detection, 919 IC automatic sampler and Metrohm Metrosep C6 (4 × 150 mm) columns. The instrument operated involving the Metrodata 2.3 computer software. 2,2’-dipirydyne-4-carboxylic acid (PDCA) (1.7 mM) with HNO3 (1.7 mM) served for elution. The eluent was stored in 1 L vessels pressurized at 8 p.s.i. using high-purity argon (BOC Gases, Guildford, Surrey, England, UK). The flow rate of 0.9 mL/min was maintained using a GP40 gradient pump (Dionex, Synnavale, California, USA). Injection volume was 100 μL.
2.2.4.3 Anion chromatography, estimation of SO42−, Cl− ions in solutions after the removal of deposits
Ionic chromatograph Thermo Scientific (Dionex ICS 3000) (Dionex, Synnavale, California, USA) was used. It was equipped with gradient pump, vacuum degassing system eluent generator, automatic sampler (AS-AP), AERS™300 – 2 mm suppressor, Dionex IonPac AS20 (2 × 250 mm) column with AG20 (2 × 50 mm) precolumn for ion detection and separation, respectively, and Chromeleon 7 computer software. Aqueous KOH solution (40 mM) served as an eluent. The eluent was stored in 1 L vessels pressurized at 8 p.s.i. using high-purity argon (BOC Gases, Guildford, Surrey, England, UK). The flow rate of 0.25 mL/min was maintained using a GP40 gradient pump (Dionex, Sunnyvale, California, USA). Injection volume was 10 µL. Estimations were carried out at 30°C.
2.2.4.4 Quantitative estimation of the HCO3− anion
Samples of water (100 cm3) were titrated with 0.05 M solution of hydrochloric acid with methyl orange indicator. The HCO3− content, C, [mg/dm3] was derived from equation (1)
where V – volume of hydrochloric acid [cm3], k – titer of hydrochloric acid solution with respect to the HCO3− anions (3.05 mg HCO3−/cm3) and V0 – volume of the titrated sample [cm3]. The measurements were run in triplicates.
2.2.4.5 Quantitative nitrogen estimation
Nitrogen content in precipitates deposited from the GP-treated ammonia added tap water was performed with CHNS Elemental Analyser (©elementar Analysensysteme GmbH, Langenselbold, Germany) on 0.1 g samples. Error of the estimation was <0.2% of the estimated value. The measurements were run in triplicates.
2.2.5 Differential scanning calorimetry (DSC)
The thermal DSC-TG-DTG analysis was carried out with the NETZSCH STA-409 simultaneous thermal analyzer (Selb, Germany) calibrated with standard indium, tin, zinc and aluminum of 99.99% purity. Samples of approximately 0.020 g deionized water and deionized water containing 3% ammonia were heated in corundum crucibles with nonhermetic lids. Corundum (SINGLE *R) was the standard. The heating was performed under static conditions in the air in the range of 20–400°C with the 5 K min−1 temperature rate increase. The measurements were duplicated. They provided the ±0.5°C precision in the temperature reading.
2.2.6 Raman spectra
The spectra were taken with a Perkin-Elmer MPF44A Fluorescence Spectrophotometer (Waltham, MA, USA) equipped with a xenon lamp and 4 mL quartz cell. The spectra were recorded in the range from 350 to 650 cm−1 for deionized water, deionized water 0.1% ammonia added prior to and after exposure to plasma for 60 min.
2.2.7 X-ray powder diffractometry
X-ray powder diffractometer URD-6 (Cu-Kα radiation, step mode of scanning) (Freiberg, Germany) was used.
2.2.8 Diffractogram simulations
Diffractograms were taken following the paper by Shtender et al. [12]. Performed simulations were performed based on SHELX-97 software package [13] and Cambridge Structural Database [14,15].
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
It was shown in our recent paper [6] that in the Raman spectrum of plain water prior to its GP treatment, a sharp peak observed around 350 nm (in Raman shift) was followed by a long weak shoulder and another sharp but less intensive peak around 700 nm. On treating that water with GP, an intensive broad peak formed at around 400 nm accompanied by a broad shoulder at around 440 nm. The intensity of the band observed around 400 nm increased with time of the GP treatment, and at the same time, the shoulder around 440 nm has transformed into a separate band. It should be underlined that these bands did not form when the water was treated without contact with the air. Based on this observation and paper by Ramya and Venkanathan [16], declusterization of the water macrostructure was postulated. The declusterization was followed by the formation of aqueous clathrates hosting molecular oxygen. These bands slowly ceased within several months of storage but immediately disappeared on contact of GP-treated water with mineral acids [6].
Raman spectra of the water treated with GP in the presence of ammonia are presented in Figure 1; one could observe the spectral pattern typical for the presence of aqueous clathrates (curves 2 and 4).
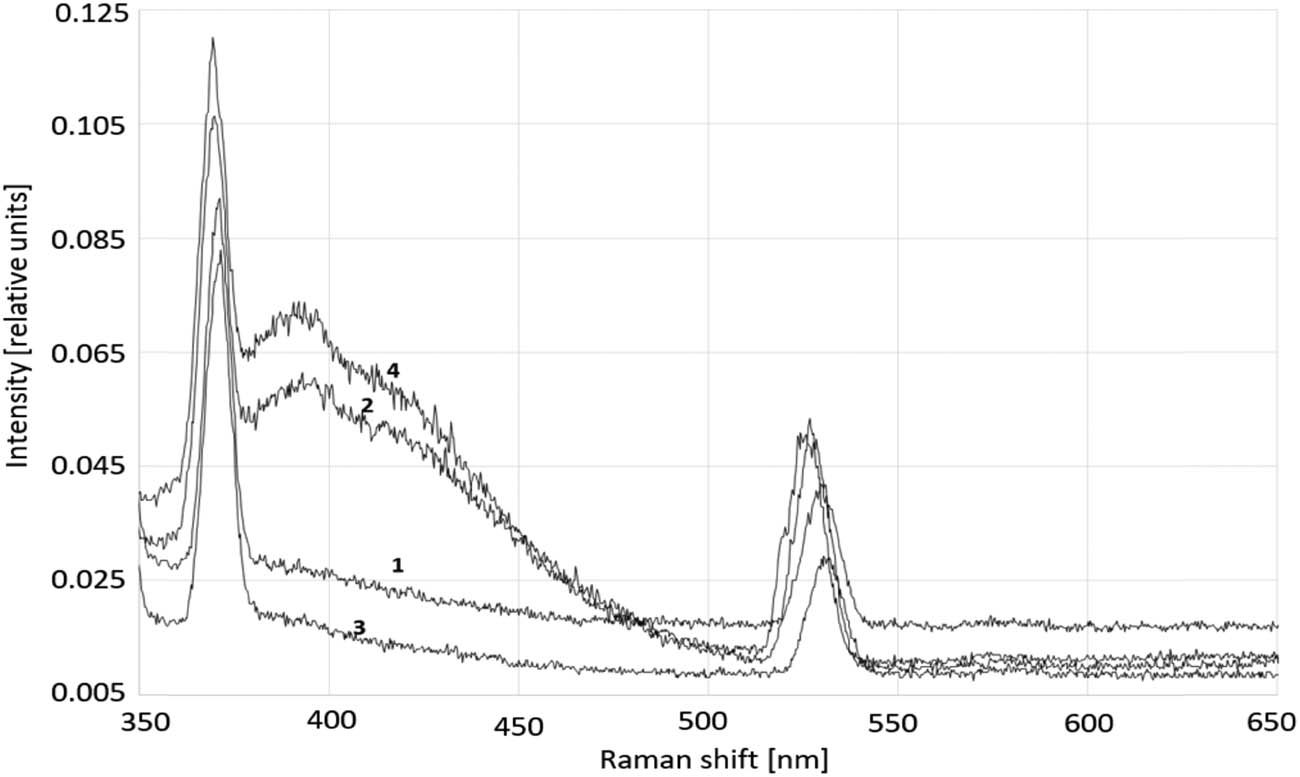
Raman spectra of deionized ammonia-free (1) and ammonia-containing water (3) prior to the GP treatment and these samples after such treatment, (2) and (4), respectively.
In terms of the particular peak intensities (Figure 1 and Table 1), ammonia facilitated declusterization. The GP treatment of plain, deionized and ammonia-saturated water developed the bands with shoulders at ∼400 and ∼430 nm, respectively. In case of plain, ammonia-free water, it was a symptom of the formation of clathrates hosting molecular oxygen. In case of water saturated with ammonia, it was an evidence of the formation of clathrates hosting ammonia. Admixture of ammonia to plain water decreased the intensity of all peaks and accompanying shoulders. Just the GP treatment of the ammonia-containing water considerably increased the intensity of all peaks.
Peak intensity in the Raman spectra of deionized water with and without ammonia prior and after GP treatment
| Type of water | Peak intensity, I,a at wavelength [cm−1] | |||
|---|---|---|---|---|
| 365 | 390 | 410b | 535 | |
| Plain, nontreated | 0.092 | 0.028 | 0.025 | 0.042 |
| Plain, GP-treated | 0.105 | 0.059 | 0.053 | 0.052 |
| Plain, ammonia added, nontreated | 0.083 | 0.018 | 0.017 | 0.029 |
| Ammonia added, GP-treated | 0.120 | 0.072 | 0.060 | 0.050 |
- a
In arbitrary units.
- b
A shoulder.
Admixed water with either NH3 or NaOH under study alkaline has been shown in Table 2.
pH of deionized, tap and mineralized water and its changes with the amount of either ammonia or hydroxide added and GP treatment time
| NH3/NaOH addeda (%) | Initial pH (water free of hydroxide) | GP treatment time [min] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 15 | 30 | 60 | 120 | ||
| Deionized watera | ||||||||
| 1 | 6.56 ± 0.05 | 11.12 ± 0.03 | 11.11 ± 0.02 | 11.08 ± 0.01 | 11.00 ± 0.03 | 11.02 ± 0.04 | 10.95 ± 0.05 | 10.99 ± 0.07 |
| 12.80 ± 0.05 | 12.10 ± 0.04 | 12.10 ± 0.03 | 11.98 ± 0.06 | 11.97 ± 0.02 | 11.94 ± 0.06 | 11.96 ± 0.05 | ||
| 2.5 | 11.75 ± 0.02 | 11.68 ± 0.06 | 11.67 ± 0.03 | 11.52 ± 0.08 | 11.43 ± 0.03 | 11.23 ± 0.03 | 11.02 ± 0.02 | |
| 5 | 11.86 ± 0.05 | 11.10 ± 0.07 | 10.52 ± 0.04 | 10.20 ± 0.02 | 10.63 ± 0.07 | 10.72 ± 0.03 | 10.34 ± 0.02 | |
| 12.06 ± 0.04 | 12.03 ± 0.02 | 11.95 ± 0.06 | 11.94 ± 0.05 | 11.99 ± 0.06 | 12.01 ± 0.07 | 11.95 ± 0.08 | ||
| 7.5 | 11.94 ± 0.04 | 10.53 ± 0.06 | 10.25 ± 0.08 | 10.45 ± 0.03 | 10.13 ± 0.08 | 10.56 ± 0.03 | 10.44 ± 0.03 | |
| 10 | 12.10 ± 0.03 | 11.85 ± 0.01 | 11.86 ± 0.03 | 12.02 ± 0.07 | 11.93 ± 0.02 | 11.83 ± 0.01 | 11.78 ± 0.03 | |
| 12.85 ± 0.02 | 12.81 ± 0.06 | 12.83 ± 0.07 | 12.87 ± 0.03 | 12.81 ± 0.01 | 12.76 ± 0.06 | 12.73 ± 0.04 | ||
| Tap watera | ||||||||
| 1 | 7.00 ± 0.04 | 11.05 ± 0.02 | 10.95 ± 0.04 | 10.94 ± 0.04 | 10.95 ± 0.01 | 10.92 ± 0.03 | 11.02 ± 0.04 | 10.92 ± 0.02 |
| 12.30 ± 0.05 | 11.70 ± 0.05 | 11.65 ± 0.04 | 11.62 ± 0.05 | 11.53 ± 0.03 | 11.55 ± 0.02 | 11.53 ± 0.04 | ||
| 2.5 | 11.46 ± 0.05 | 11.23 ± 0.07 | 11.22 ± 0.02 | 11.04 ± 0.06 | 11.06 ± 0.06 | 10.98 ± 0.06 | 10.85 ± 0.08 | |
| 5 | 11.08 ± 0.02 | 11.70 ± 0.06 | 11.30 ± 0.04 | 10.80 ± 0.03 | 10.70 ± 0.02 | 10.60 ± 0.06 | 10.70 ± 0.03 | |
| 12.90 ± 0.05 | 12.68 ± 0.03 | 12.51 ± 0.02 | 12.36 ± 0.06 | 12.28 ± 0.07 | 12.31 ± 0.06 | 12.22 ± 0.07 | ||
| 7.5 | 11.98 ± 0.04 | 11.40 ± 0.06 | 10.62 ± 0.03 | 10.42 ± 0.04 | 10.85 ± 0.07 | 10.63 ± 0.03 | 10.32 ± 0.08 | |
| 10 | 12.08 ± 0.03 | 11.56 ± 0.07 | 11.76 ± 0.03 | 11.87 ± 0.04 | 12.04 ± 0.02 | 11.93 ± 0.03 | 11.98 ± 0.03 | |
| 12.31 ± 0.04 | 12.18 ± 0.03 | 12.11 ± 0.02 | 12.08 ± 0.06 | 12.06 ± 0.04 | 12.05 ± 0.06 | 11.97 ± 0.02 | ||
| Highly mineralized watera | ||||||||
| 1 | 8.60 ± 0.02 | 10.11 ± 0.04 | 9.85 ± 0.06 | 9.82 ± 0.03 | 9.81 ± 0.06 | 8.87 ± 0.02 | 8.62 ± 0.06 | 8.31 ± 0.07 |
| 11.42 ± 0.03 | 11.40 ± 0.06 | 11.32 ± 0.03 | 11.21 ± 0.06 | 10.56 ± 0.07 | 10.41 ± 0.06 | 10.21 ± 0.04 | ||
| 2.5 | 10.44 ± 0.04 | 11.11 ± 0.06 | 11.08 ± 0.04 | 11.00 ± 0.03 | 11.02 ± 0.02 | 10.95 ± 0.02 | 10.99 ± 0.02 | |
| 5 | 10.82 ± 0.04 | 10.72 ± 0.03 | 10.76 ± 0.07 | 10.32 ± 0.03 | 10.24 ± 0.04 | 10.11 ± 0.01 | 10.21 ± 0.05 | |
| 11.03 ± 0.00 | 10.92 ± 0.06 | 10.93 ± 0.03 | 10.74 ± 0.02 | 10.42 ± 0.07 | 10.35 ± 0.05 | 10.34 ± 0.03 | ||
| 7.5 | 10.21 ± 0.07 | 9.81 ± 0.08 | 9.65 ± 0.04 | 9.62 ± 0.04 | 9.51 ± 0.03 | 9.49 ± 0.07 | 9.51 ± 0.02 | |
| 10 | 11.52 ± 0.04 | 11.37 ± 0.06 | 11.32 ± 0.07 | 11.21 ± 0.03 | 11.08 ± 0.02 | 10.52 ± 0.01 | 10.21 ± 0.05 | |
| 11.03 ± 0.05 | 10.95 ± 0.07 | 10.93 ± 0.03 | 10.82 ± 0.02 | 10.63 ± 0.06 | 10.18 ± 0.07 | 9.62 ± 0.03 | ||
| Weakly mineralized watera | ||||||||
| 1 | 6.10 ± 0.03 | 10.11 ± 0.06 | 9.84 ± 0.07 | 9.86 ± 0.04 | 9.81 ± 0.02 | 9.62 ± 0.05 | 9.34 ± 0.01 | 9.17 ± 0.06 |
| 12.01 ± 0.03 | 11.89 ± 0.03 | 11.85 ± 0.02 | 11.62 ± 0.05 | 11.34 ± 0.04 | 10.86 ± 0.02 | 10.37 ± 0.06 | ||
| 2.5 | 10.78 ± 0.04 | 11.11 ± 0.02 | 11.08 ± 0.04 | 11.00 ± 0.06 | 11.02 ± 0.03 | 10.95 ± 0.01 | 10.99 ± 0.04 | |
| 5 | 11.20 ± 0.06 | 11.01 ± 0.07 | 10.65 ± 0.04 | 10.25 ± 0.02 | 10.01 ± 0.05 | 9.65 ± 0.06 | 9.51 ± 0.03 | |
| 11.92 ± 0.04 | 11.93 ± 0.04 | 11.90 ± 0.02 | 10.91 ± 0.02 | 10.65 ± 0.04 | 10.53 ± 0.03 | 10.46 ± 0.06 | ||
| 7.5 | 11.21 ± 0.04 | 11.01 ± 0.03 | 10.52 ± 0.02 | 10.31 ± 0.06 | 10.25 ± 0.02 | 10.12 ± 0.02 | 9.84 ± 0.01 | |
| 10 | 11.43 ± 0.04 | 11.11 ± 0.04 | 11.08 ± 0.05 | 11.00 ± 0.06 | 10.02 ± 0.03 | 10.95 ± 0.04 | 10.99 ± 0.06 | |
| 11.68 ± 0.06 | 11.56 ± 0.03 | 11.60 ± 0.06 | 11.51 ± 0.07 | 11.48 ± 0.03 | 11.42 ± 0.02 | 11.36 ± 0.05 | ||
- a
Data taken in aq. NaOH solutions are given in italics.
Regardless of the added concentration of either NH3 or NaOH, the pH of the nontreated deionized as well as the tap water only slightly decreased with the GP treatment time. That effect was slightly more remarkable in case of tap water. Also, that decrease in water with NH3 was slightly more remarkable than in water alkalized with NaOH. It could suggest some evolution of ammonia as the treatment time progressed. However, the pH of the water-containing NaOH also decreased on the GP treatment. Thus, evidently, the GP treatment influenced the dissociation of the hydroxides added. Since that effect was stronger in case of ammonia, an intervention of the GP treatment into the equilibrium of the reaction
and hydration conditions of the reaction participants could be taken under consideration. The pattern of the Raman spectra of the GP-treated water-containing ammonia (a maximum and its shoulder between 390 and 450 cm−1) (Figure 1) suggests the formation of clathrates [6,16]. Nonlinear changes of pH against the GP treatment time support this assumption. The same behavior observed in case of the water treated in the air was interpreted as dependent on the treatment time changes of the structure of clathrates and the content of their interior.
The observed decrease in pH was accompanied by changes of the conductivity (Table 3). At a given concentration of the base added, these changes were irregular against the treatment time. Changes of the clathrate structure and composition on the treatment [6,16] could rationalize these irregularities.
Conductivity of deionized, tap and mineralized water and its changes with the amount of hydroxide added and the GP treatment time
| NH3/NaOH addeda [%] | Water | GP treatment time [min] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 15 | 30 | 60 | 120 | ||
| Deionized water | ||||||||
| 1 | 0.001 ± 0.001 | 0.326 ± 0.004 | 0.354 ± 0.002 | 0.364 ± 0.002 | 0.353 ± 0.001 | 0.342 ± 0.005 | 0.352 ± 0.005 | 0.322 ± 0.002 |
| 0.392 ± 0.005 | 0.393 ± 0.004 | 0.395 ± 0.002 | 0.396 ± 0.002 | 0.397 ± 0.002 | 0.394 ± 0.001 | 0.392 ± 0.002 | ||
| 2.5 | 0.455 ± 0.006 | 0.353 ± 0.003 | 0.315 ± 0.002 | 0.327 ± 0.002 | 0.328 ± 0.005 | 0.294 ± 0.006 | 0.313 ± 0.003 | |
| 5 | 0.355 ± 0.003 | 0.367 ± 0.005 | 0.349 ± 0.002 | 0.310 ± 0.004 | 0.305 ± 0.006 | 0.323 ± 0.003 | 0.302 ± 0.005 | |
| 0.415 ± 0.001 | 0.407 ± 0.006 | 0.409 ± 0.005 | 0.397 ± 0.001 | 0.403 ± 0.002 | 0.391 ± 0.003 | 0.394 ± 0.002 | ||
| 7.5 | 0.352 ± 0.007 | 0.315 ± 0.005 | 0.328 ± 0.004 | 0.303 ± 0.007 | 0.297 ± 0.004 | 0.315 ± 0.002 | 0.323 ± 0.005 | |
| 10 | 0.006 ± 0.002 | 0.912 ± 0.003 | 0.862 ± 0.002 | 0.916 ± 0.003 | 0.954 ± 0.002 | 0.967 ± 0.003 | 0.995 ± 0.003 | 1.013 ± 0.002 |
| 0.455 ± 0.003 | 0.444 ± 0.003 | 0.446 ± 0.001 | 0.439 ± 0.006 | 0.436 ± 0.002 | 0.435 ± 0.005 | 0.424 ± 0.004 | ||
| Tap water | ||||||||
| 1 | 0.844 ± 0.002 | 0.959 ± 0.004 | 0.756 ± 0.009 | 0.794 ± 0.005 | 0.813 ± 0.002 | 0.622 ± 0.004 | 0.564 ± 0.002 | 0.388 ± 0.007 |
| 0.953 ± 0.004 | 0.904 ± 0.005 | 0.874 ± 0.003 | 0.866 ± 0.002 | 0.866 ± 0.005 | 0.815 ± 0.001 | 0.792 ± 0.003 | ||
| 2.5 | 1.107 ± 0.003 | 0.820 ± 0.006 | 0.625 ± 0.002 | 0.513 ± 0.005 | 0.347 ± 0.006 | 0.285 ± 0.002 | 0.025 ± 0.001 | |
| 5 | 1.127 ± 0.006 | 0.953 ± 0.002 | 0.842 ± 0.003 | 0.637 ± 0.005 | 0.529 ± 0.007 | 0.356 ± 0.003 | 0.205 ± 0.001 | |
| 1.132 ± 0.005 | 1.124 ± 0.002 | 1.116 ± 0.002 | 0.993 ± 0.003 | 0.902 ± 0.004 | 0.697 ± 0.001 | 0.563 ± 0.002 | ||
| 7.5 | 1.237 ± 0.003 | 1.028 ± 0.006 | 0.814 ± 0.002 | 0.653 ± 0.002 | 0.356 ± 0.006 | 0.267 ± 0.004 | 0.118 ± 0.006 | |
| 10 | 1.184 ± 0.008 | 0.425 ± 0.002 | 0.316 ± 0.004 | 0.307 ± 0.003 | 0.319 ± 0.002 | 0.304 ± 0.002 | 0.302 ± 0.001 | |
| 1.224 ± 0.002 | 1.193 ± 0.008 | 1.195 ± 0.006 | 1.027 ± 0.003 | 0.938 ± 0.004 | 0.815 ± 0.003 | 0.635 ± 0.004 | ||
| Highly mineralized water | ||||||||
| 1 | 0.115 ± 0.004 | 0.213 ± 0.005 | 0.235 ± 0.003 | 0.249 ± 0.06 | 0.247 ± 0.003 | 0.238 ± 0.006 | 0.176 ± 0.007 | 0.164 ± 0.004 |
| 0.426 ± 0.003 | 0.418 ± 0.004 | 0.364 ± 0.001 | 0.352 ± 0.002 | 0.324 ± 0.005 | 0.306 ± 0.002 | 0.268 ± 0.003 | ||
| 2.5 | 0.325 ± 0.006 | 0.317 ± 0.004 | 0.300 ± 0.002 | 0.294 ± 0.002 | 0.273 ± 0.005 | 0.263 ± 0.003 | 0.253 ± 0.002 | |
| 5 | 0.414 ± 0.003 | 0.357 ± 0.003 | 0.315 ± 0.004 | 0.244 ± 0.003 | 0.239 ± 0.001 | 0.185 ± 0.002 | 0.155 ± 0.003 | |
| 0.414 ± 0.009 | 0.383 ± 0.009 | 0.393 ± 0.003 | 0.373 ± 0.004 | 0.367 ± 0.002 | 0.295 ± 0.003 | 0.263 ± 0.002 | ||
| 7.5 | 0.328 ± 0.006 | 0.353 ± 0.003 | 0.363 ± 0.005 | 0.353 ± 0.006 | 0.348 ± 0.003 | 0.354 ± 0.002 | 0.324 ± 0.003 | |
| 10 | 0.985 ± 0.003 | 0.428 ± 0.007 | 0.419 ± 0.004 | 0.403 ± 0.006 | 0.362 ± 0.003 | 0.352 ± 0.002 | 0.322 ± 0.001 | |
| 0.526 ± 0.002 | 0.498 ± 0.005 | 0.489 ± 0.001 | 0.464 ± 0.004 | 0.413 ± 0.002 | 0.366 ± 0.002 | 0.316 ± 0.005 | ||
| Weakly mineralized water | ||||||||
| 1 | 0.054 ± 0.002 | 0.387 ± 0.004 | 0.329 ± 0.003 | 0.284 ± 0.002 | 0.283 ± 0.005 | 0.241 ± 0.001 | 0.198 ± 0.005 | 0.182 ± 0.002 |
| 0.353 ± 0.002 | 0.355 ± 0.003 | 0.336 ± 0.003 | 0.308 ± 0.005 | 0.269 ± 0.002 | 0.214 ± 0.004 | 0.193 ± 0.002 | ||
| 2.5 | 0.254 ± 0.006 | 0.236 ± 0.007 | 0.210 ± 0.003 | 0.179 ± 0.003 | 0.163 ± 0.002 | 0.159 ± 0.003 | 0.152 ± 0.003 | |
| 5 | 0.397 ± 0.006 | 0.350 ± 0.002 | 0.310 ± 0.003 | 0.286 ± 0.001 | 0.243 ± 0.002 | 0.201 ± 0.005 | 0.165 ± 0.002 | |
| 0.397 ± 0.002 | 0.399 ± 0.003 | 0.370 ± 0.003 | 0.363 ± 0.003 | 0.326 ± 0.002 | 0.296 ± 0.005 | 0.236 ± 0.004 | ||
| 7.5 | 0.484 ± 0.005 | 0.432 ± 0.007 | 0.372 ± 0.002 | 0.326 ± 0.002 | 0.279 ± 0.003 | 0.213 ± 0.001 | 0.182 ± 0.002 | |
| 10 | 0.536 ± 0.008 | 0.418 ± 0.003 | 0.400 ± 0.006 | 0.364 ± 0.003 | 0.322 ± 0.002 | 0.282 ± 0.007 | 0.236 ± 0.004 | |
| 0.417 ± 0.002 | 0.419 ± 0.003 | 0.395 ± 0.003 | 0.384 ± 0.004 | 0.354 ± 0/005 | 0.347 ± 0.002 | 0.315 ± 0.002 | ||
- a
Data taken in aq. NaOH solutions are given in italics.
Conductivity of the deionized water also irregularly decreased with the treatment time (Table 3). It was almost negligible in case of deionized water and remarkable in case of water-containing mineral solutes, particularly tap water. The time-depending decrease in the conductivity noted for a given kind of water was stronger in case of solutions of ammonium hydroxide than in case of solutions with sodium hydroxide. Moreover, it was slightly nonlinear against the treatment time suggesting intervention of the declusterization of the water macrostructure followed by building clusters of varying structures [6].
GP treatment of tap and highly and weakly mineralized water alkalized with either ammonia or sodium hydroxide resulted in the precipitation of a deposit. The effects became more remarkable with an increase in the content of either ammonia or sodium hydroxide added (Table 4). Ammonia provided more efficient precipitation.
Amount [mg] of precipitation after 120 min GP treatment
| Type of water | Additive [%] | |||||
|---|---|---|---|---|---|---|
| NaOH | NH3 | |||||
| 1 | 5 | 10 | 1 | 5 | 10 | |
| Deionized | 0 | 0 | 0 | 0 | 0 | 0 |
| Tape | 98 | 112 | 118 | 136 | 152 | 153 |
| Highly mineralized | 112 | 123 | 148 | 158 | 173 | 182 |
| Weakly mineralized | 46 | 42 | 43 | 79 | 75 | 76 |
On the treatment of deionized water with either ammonia or sodium hydroxide, no precipitates were formed (Table 4). Thus, apart from obvious hydrates, a possibility of the formation of aqueous clathrates including ammonia and ions, respectively, could be taken into account. DSC studies (Figure 2) carried out for deionized water containing ammonia supported such assumption.
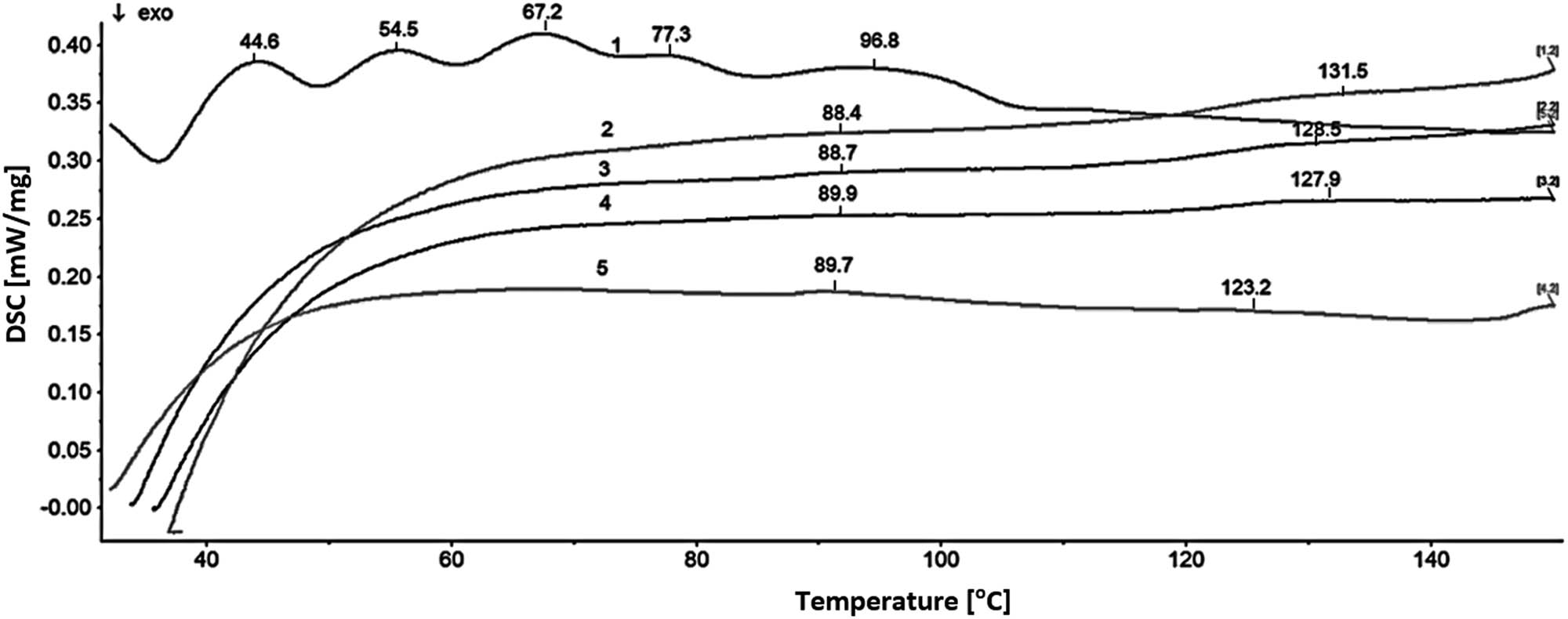
Differential scanning calorimetry (DSC) diagrams for nontreated deionized water (1), deionized nontreated water with 3% ammonia (2), deionized water with 5% ammonia treated for 1 (3), 5 (4) and 30 min (5).
The diagram for nontreated deionized, ammonia-free water contained several endothermic peaks associated with the rearrangements of the water macrostructure. These effects were exhaustively discussed by Prouzet et al. [17]. The pattern of the curve for deionized, nontreated water containing 3% ammonia was entirely different. Initially, it strongly rose as a result of an endothermic process. Then, around 60°C, the curve became almost planar. Two slightly endothermic effects at 88.4 and 131.5°C could be recognized on that segment which relatively slightly elevated up to 150°C. The course of that segment pointed to an overall endothermic character of the changes occurring in the sample macrostructure. After the 1- and 5-min treatment, the pattern of the curve remained identical; however, the overall process turned into less endothermic. The decrease in the endothermic character of the overall process (line 1 in Figure 2) followed an increase in the treatment time of the samples. Likely, the endothermic process was associated with declusterizing of the water macrostructure on the GP treatment. That process was evidently facilitated by ammonia.
In the sample of the ammonia-containing water treated for 30 min, the deal of the declusterization process was significantly reduced. In contrast to former curves, the semi-planar section of that line declined up to about 145°C in order to increase again above that temperature. The endothermic effect formerly observed between 88 and 90°C appeared at 89.7°C. It was essentially stronger, whereas the effect formerly observed between 131.5 and 127.9°C appeared at 123.2°C and was hardly recognizable. These differences evoked by the treatment pointed to a possibility of the formation of aqueous ammonia clathrates.
Data in Table 5 demonstrate efficiency of the removal of ions responsible for the water hardness increasing with the concentration of ammonia added and the GP treatment time. Accompanying demineralization of water in terms of the removal of sodium and chloride ions was negligible, and a higher concentration of ammonia added obstructed, to a certain extent, the removal of those ions.
Amount of ions [mg/L] remaining in the treated water containing 0.5 and 10% ammonia after separation of precipitate
| Type of water | GP treatment duration [min] | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 15 | 30 | 60 | 120 | |
| Ion content [mg/L] | ||||||
| Ca2+ | ||||||
| Deionized | 0.25 | 0.1 | 0.05 | 0.03 | 0.03 | 0.02 |
| Tapea | 193.27 | 185.2 | 180.3 | 176.3 | 175.5 | 174.3 |
| 73.2 | 20.4 | 12.3 | 10.2 | 6.5 | ||
| Highly mineralized | 368.92 | 125.8 | 53.84 | 12.32 | 10.85 | 8.21 |
| Weakly mineralized | 44.9 | 11.5 | 10.2 | 9.52 | 5.23 | 4.25 |
| Mg2+ | ||||||
| Deionized | 0.12 | 0.11 | 0.05 | 0.04 | 0.04 | 0.03 |
| Tapea | 46.21 | 45.8 | 43.2 | 40.9 | 39.8 | 38.9 |
| 38.4 | 32.14 | 25.63 | 12.39 | 5.32 | ||
| Highly mineralized | 73.209 | 68.72 | 65.23 | 53.28 | 31.02 | 17.21 |
| Weakly mineralized | 17.01 | 15.07 | 10.25 | 6.52 | 4.25 | 2.18 |
| Na+ | ||||||
| Deionized | 0.08 | 0.07 | 0.08 | 0.08 | 0.06 | 0.07 |
| Tapea | 23.18 | 22.1 | 21.9 | 21.7 | 21.6 | 21.5 |
| 22.8 | 22.5 | 22.4 | 22.38 | 22.18 | ||
| Highly mineralized | 54.71 | 53.8 | 53.4 | 53.5 | 53.2 | 53.2 |
| Weakly mineralized | 1 | 0.95 | 0.95 | 0.94 | 0.94 | 0.93 |
| HCO3− | ||||||
| Deionized | 18.3 | 13.5 | 8.98 | 7.65 | 6.21 | 5.14 |
| Tapea | 351.9 | 346.8 | 345.8 | 344.7 | 344.6 | 344.2 |
| 128.7 | 83.5 | 35.8 | 34.8 | 33.2 | ||
| Highly mineralized | 1721.9 | 438.2 | 211.5 | 193.4 | 128.3 | 67.2 |
| Weakly mineralized | 186.7 | 56.8 | 36.8 | 23.5 | 14.9 | 12.3 |
| SO4= | ||||||
| Deionized | 1.28 | 0.98 | 0.87 | 0.69 | 0.62 | 0.43 |
| Tapea | 51.2 | 50.9 | 50.8 | 50.1 | 49.9 | 49.8 |
| 34.8 | 16.8 | 12.8 | 8.7 | 6.2 | ||
| Highly mineralized | 40.5 | 30.1 | 23.1 | 18.9 | 14.3 | 9.1 |
| Weakly mineralized | 43.62 | 29.5 | 25.3 | 20.2 | 14 | 10.8 |
| Cl− | ||||||
| Deionized | 0.01 | 0.01 | 0.01 | 0 | 0.01 | 0.01 |
| Tapea | 6.52 | 6.48 | 6.42 | 6.39 | 6.38 | 6.38 |
| 6.32 | 6.25 | 6.08 | 6.05 | 6.07 | ||
| Highly mineralized | 7.8 | 7.68 | 7.65 | 7.52 | 7.52 | 7.46 |
| Weakly mineralized | 3.19 | 3.15 | 3.11 | 3.11 | 3.09 | 3.02 |
- a
The upper values are for the treatment of 0.5% ammonia added and the lower values in italics are for the treatment water with 10% ammonia.
It was interesting that precipitates formed on the treatment from each water-containing mineral did not exhibit any tendency to turn into a scale. Such behavior could result from ligation of the Ca and Mg atoms with the NH3 ligands.
Alkaline earth atoms readily coordinate O-ligands and that ability can be controlled by their counterions [18]. These central atoms can also coordinate N-ligands. For instance, there are known numerous coordination compounds of magnesium and calcium with pyridine ligands [19]. These central atoms coordinate four and six NH3 molecules. The smaller Mg atom is a stronger electron donor than the Ca atom. The structure of the first solvation shell is sharper for Mg, which has a larger charge and smaller radius than Ca. Weak solvation leads to rapid dynamics in terms of the diffusion coefficients of the NH3 molecules [20,21]. Thus, likely, Mg and Ca carbonates and sulfates could coordinate NH3 ligands.
Indeed, the formula of the deposition immediately after its precipitation, Ca0.85Mg0.10Fe0.05[CO3](NH3)2, and after being suspended for 3 days in the solution, turned into Ca0.83Mg0.12Fe0.05[CO3](NH3)2, pointing to a gradual passing of calcium into the solution. It might suggest that, for instance, ionic interactions could contribute to the formation of precipitates.
The rhombohedral crystals of the precipitate belonged to the
Network constants and atom coordinates of the atoms of freshly precipitated deposit
| Network constants | a = 0.4985 nm, c = 1.6994 nm, V = 0.3646 nm3 a | ||||
|---|---|---|---|---|---|
| Atom coordinates | Elements | Crystallographic axis | x | y | z |
| O | 18e | 0.2577 | 0 | 1/4 | |
| 0.85Ca + 0.10Mg + 0.05Fe | 6b | 0 | 0 | 0 | |
| C | 6a | 0 | 0 | 1/4 | |
- a
The volume of the crystallographic cell.
Experimental and numerically simulated diffractograms of freshly deposited precipitates are presented in Figures 3 and 4, respectively. Their patterns and localization of particular reflexes satisfactorily fitted one another.
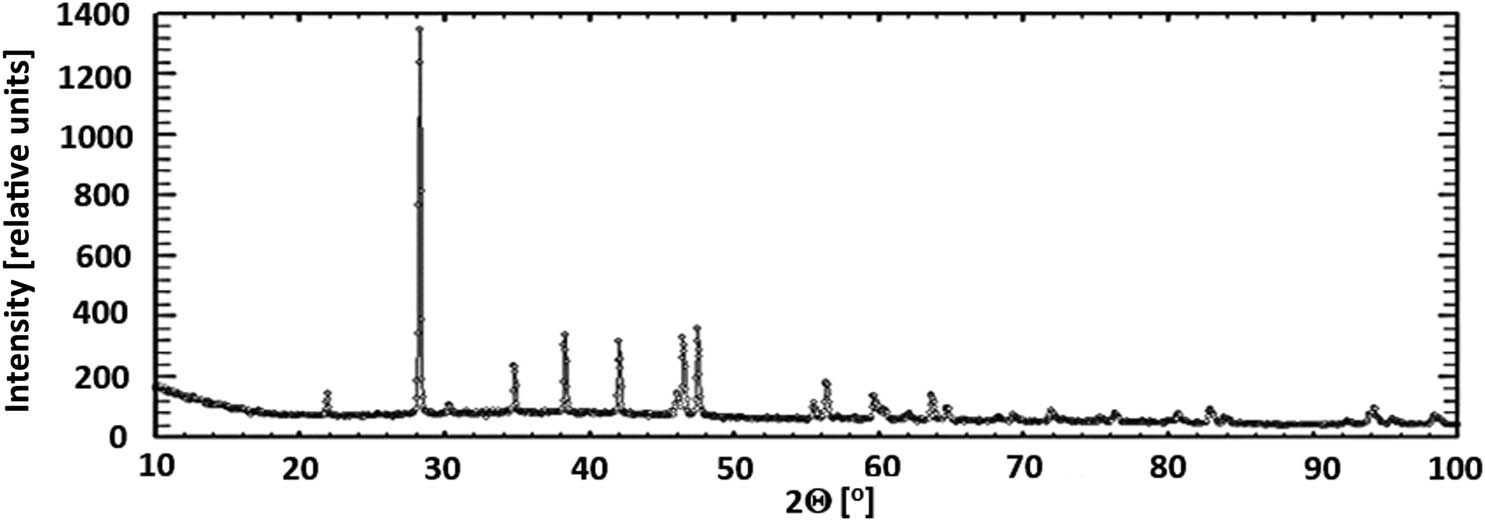
Experimental diffractogram of freshly deposited precipitate.
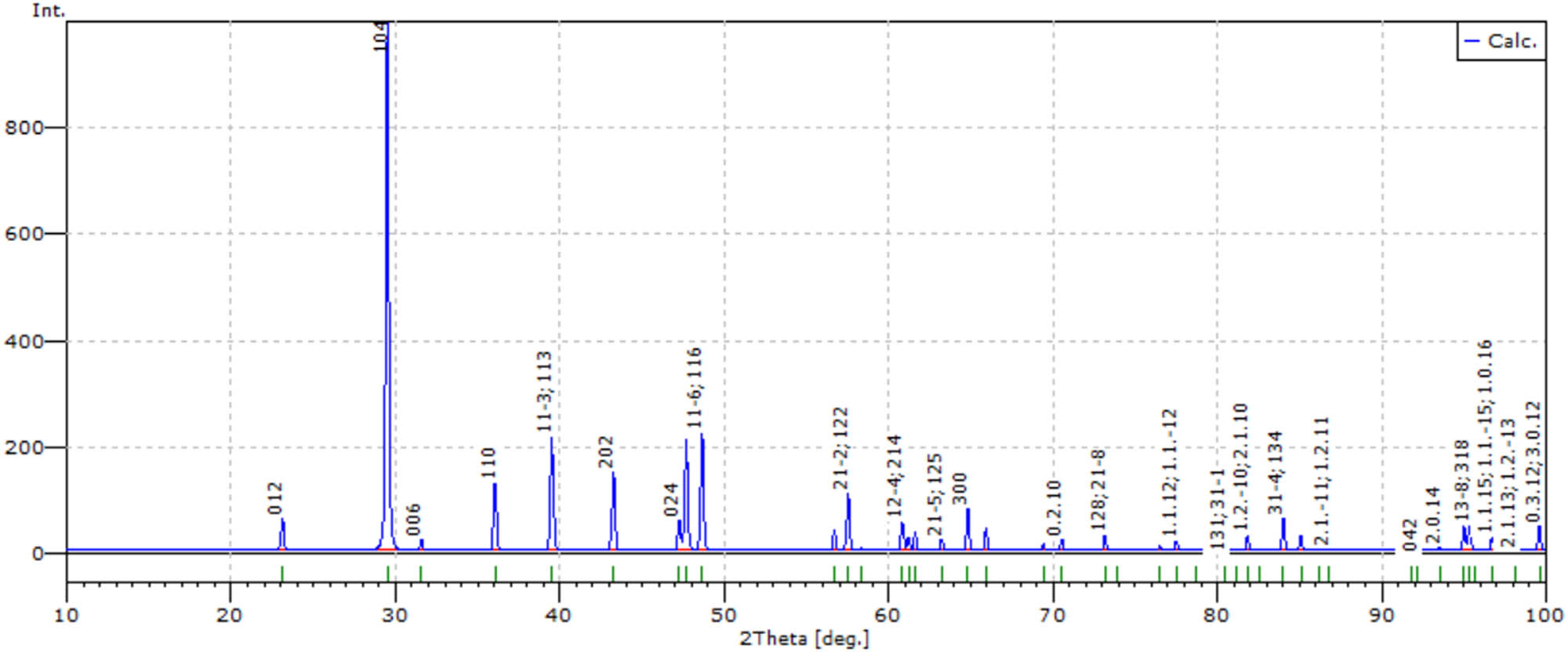
Numerically simulated diffractogram of freshly deposited precipitate.
After three days in suspension at around 90°C, the crystallographic structure of the precipitate slightly changed (Table 7 and Figures 5, 6). The volume of the crystallographic cell remained unchanged. The shift of the diffraction reflexes toward higher 2θ angles (Figure 7) informed on decrease in the network constants due to decrease in the calcium content and, simultaneously, an increase in the magnesium content.
Network constants and atom coordinates of the atoms of precipitated deposit taken after 3 days
| Network constants | a = 0.4979 nm, c = 1.6989 nm, V = 0.3646 nm3 a | ||||
|---|---|---|---|---|---|
| Atom coordinates | Elements | Crystallographic axis | x | y | z |
| O | 18e | 0.2577 | 0 | 1/4 | |
| 0.83Ca + 0.12Mg + 0.05Fe | 6b | 0 | 0 | 0 | |
| C | 6a | 0 | 0 | 1/4 | |
- a
The volume of the crystallographic cell.
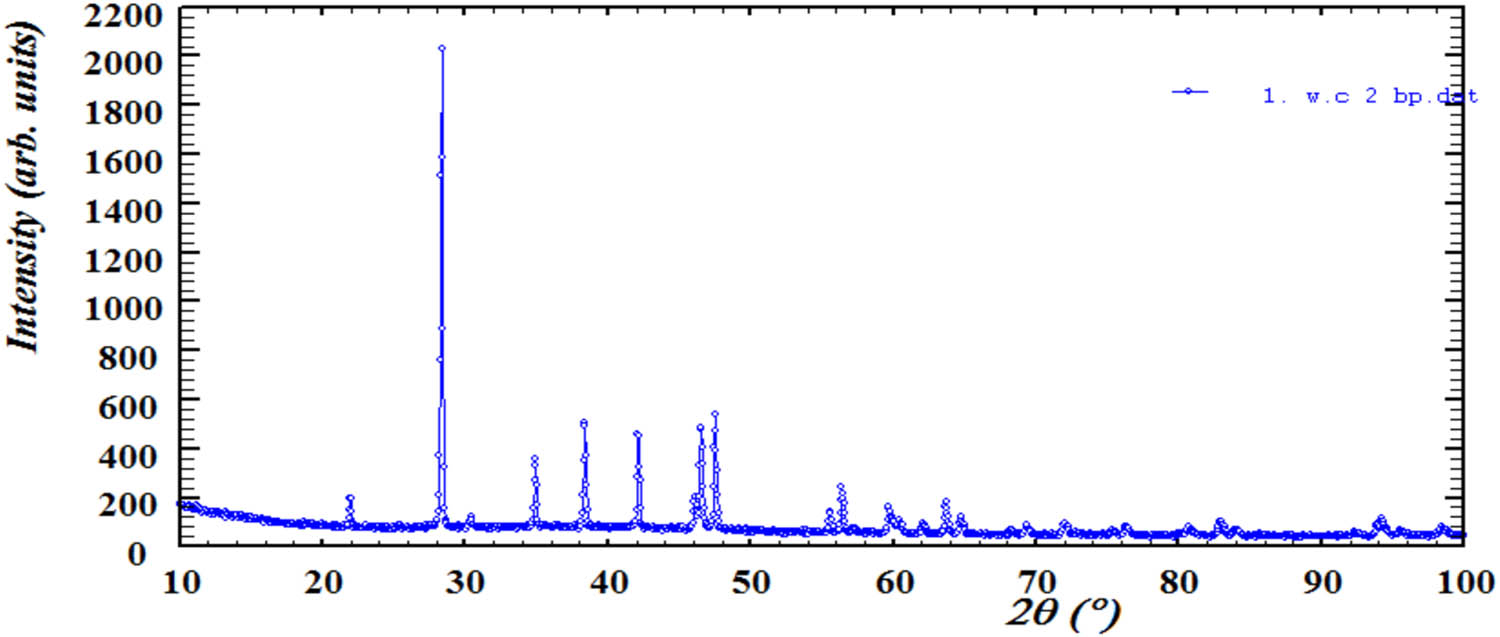
Experimental diffractogram of deposited precipitate stored for 3 days.
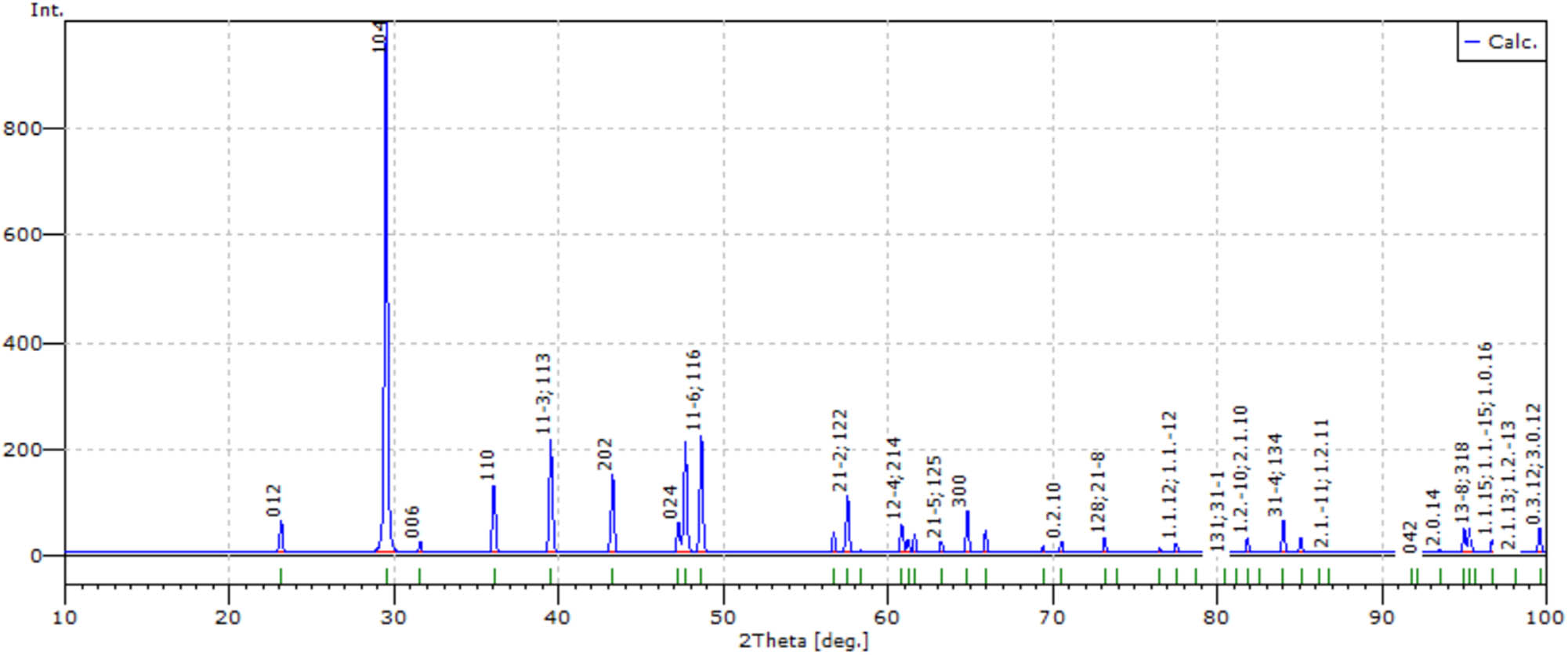
Numerically simulated diffractogram of deposited precipitate stored for 3 days.

The shift of reflexes in the diffractograms of precipitates due to decrease in the calcium content and simultaneously increase in the magnesium content; (1) and (2) diffractograms of freshly deposited and collected after 3-days precipitates, respectively.
The presented study proposes the removal of permanent and temporary water hardness at room temperature and without any necessity of the removal of deposit which was formed. For this purpose, water should be blended with ammonia followed by the treatment with GP. Admixture of ammonia makes water noncorrosive. The stabilization of the rhombohedral precipitate against scaling is afforded by coordination of ammonia ligands to the Mg and Ca atoms.
Thus, the treated water was suitable for protecting water-heating systems and water-transmitting pipelines against the scale and corrosion, eliminating other chemical, physical and physicochemical methods used for that purpose.
Additionally, on the GP treatment of ammonia-containing water, the formation of aqueous clathrates of ammonia was postulated. Water with ammonia within clathrates may serve as an alternative, environmentally benign nitrogen fertilizer. Resulting precipitates can also be utilized for the same purpose.
4 Conclusions
Permanent and temporary water hardness-removed water at room temperature to which ammonia was admixed is subjected to treatment with low-pressure, low-temperature GP of low frequency.
Fine deposit which is formed does not turn into scale even within 3-day maintenance at about 90°C.
Water treated under ammonia with low-pressure, low-temperature GP of low frequency protects water-heating systems and water-transmitting pipelines against scale and corrosion providing elimination of other chemical, physical and physicochemical methods used for that purpose.
Water treated under ammonia with low-pressure, low-temperature GP of low frequency as well as precipitates can serve as an alternative, environmentally benign nitrogen fertilizer.
Conflict of interest: The authors declare no conflict of interest.
References
[1] Chaplin M, Water www.1.lsbu.ac.uk/water/water_descaling.html [cited Oct. 22nd 2018].Suche in Google Scholar
[2] Crabtree M, Eslinger D, Fletcher P, Johnson A, King G. Fighting scale-removal and prevention. Oilfield Reviev. 1999;Autumn:30–48.Suche in Google Scholar
[3] Nitsch C, Heitland H-J, Marsen M, Schluessler H-J. Cleansing agents. Ullman’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley–VCH; 2005.Suche in Google Scholar
[4] Weingaertner H. Water. In: Ullman’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley–VCH; 2006.10.1002/14356007.a28_001Suche in Google Scholar
[5] Kamal MS, Hussein I, Mahmoud M, Sultan AS, Saad MAS. Oilfield scale formation and chemical removal: a review. J Petr Sci Eng. 2018;171:127–39.10.1016/j.petrol.2018.07.037Suche in Google Scholar
[6] Bialopiotrowicz T, Ciesielski W, Domanski J, Doskocz M, Fiedorowicz M, Graz K, et al. Structure and physicochemical properties of water treated with low-temperature low-frequency glow plasma. Curr Phys Chem. 2016;6:312–20.10.2174/1877946806666161118152613Suche in Google Scholar
[7] Chwastowski J, Ciesielska K, Ciesielski W, Khachatryan K, Kołoczek H, Kulawik D, et al. Structure and physicochemical properties of water treated under nitrogen with low-temperature glow plasma. Water. 2020;12:1314. 103390/w12051314.Suche in Google Scholar
[8] Ciesielska A, Ciesielski W, Khachatryan K, Koloczek H, Kulawik D, Oszczeda Z, et al. Structure and physicochemical properties of water treated under methane with low-temperature glow plasma of low frequency. Water. 2020;12:1638. 10.3390/w12061638.Suche in Google Scholar
[9] Ciesielska A, Ciesielski W, Khachatryan K, Koloczek H, Kulawik D, Oszczeda Z, et al. Structure and physicochemical properties of water treated under carbon dioxide with low-temperature low-pressure glow plasma of low frequency. Water. 2020;12:1920. 10.3390/w12071920.Suche in Google Scholar
[10] Chwastowski J, Ciesielski W, Khachatryan K, Kołoczek H, Kulawik D, Oszczęda Z, et al. Water of increased content of molecular oxygen. Water. 2020;12:2488. 10.3390/w12092488.Suche in Google Scholar
[11] Reszke E, Yelkin I, Oszczęda Z. 2017. Plasming lamp with power supply. Polish Patent 2017, PL 227530 B1.Suche in Google Scholar
[12] Shtender VV, Pavlyuk VV, Zelinska OYa, Nitek W, Paul-Boncour V, Dmytriv GS, et al. The Y–Mg–Co ternary system: alloys synthesis, phase diagram at 500°C and crystal structure of the new compounds. J Alloys Compd. 2019;812:152072. 10.1016/j.jallcom.2019.152072.Suche in Google Scholar
[13] Sheldrick GM. SHELXS-97and SHELXL-97 – WinGX Version. Release 97–2. Germany: University of Göttingen; 1997.Suche in Google Scholar
[14] Rodriguez-Carvajal J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys B. 1993;192:55–69.10.1016/0921-4526(93)90108-ISuche in Google Scholar
[15] Roisnel T, Rodriguez-Carvajal J. WinPLOTR, a windows tool for powder diffraction pattern analysis. Mater Sci Forum. 2001;378–381:118–23.10.4028/www.scientific.net/MSF.378-381.118Suche in Google Scholar
[16] Ramya KR, Venkathnathan A. Density functional theory study of oxygen clathrate hydrates. Indian J Chem. 2013;52A:1063–5.Suche in Google Scholar
[17] Prouzet E, Brubach J-B, Roy P. Differential scanning calorimetry study of the structure of water confined within AOT lamellar mesophases. J Phys Chem B. 2010;114:8081–8.10.1021/jp101176vSuche in Google Scholar PubMed
[18] Chaban VV, Prezhdo OV. Electron solvation in liquid ammonia: lithium, sodium, magnesium, and calcium as electron sources. J Phys Chem B. 2016;120:2500–6.10.1021/acs.jpcb.6b00412Suche in Google Scholar PubMed
[19] Chaban VV, Andreeva NA, Vorontsov-Velyaminova PN. A weakly coordinating anion substantially enhances carbon dioxide fixation by calcium and barium salts. Energy Fuels. 2017;31:9668–74.10.1021/acs.energyfuels.7b01024Suche in Google Scholar
[20] Tomasik P, Ratajewicz Z. Pyridine–metal complexes. In: Newkome GR, Strekowski L, editors. Interscience. Vol. 1, ch. 2, New York: J. Wiley; 1985.Suche in Google Scholar
[21] Kaufman Katz A, Glusker JS, Beebe SA, Bock CW. Calcium ion coordination: a comparison with that of beryllium, magnesium, and zinc. J Am Chem Soc. 1996;118:5752–63.10.1021/ja953943iSuche in Google Scholar
© 2020 Aleksandra Ciesielska et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

