Abstract
An electrochemical sensor based on guanine-, polythionine-, and nitrogen-doped graphene modified glassy carbon electrode (G/PTH/NG/GCE) was fabricated and applied for antioxidant capacity evaluation of natural compounds and complexes in electrochemical method since natural sources of active compounds exhibited various antioxidant activities. When the antioxidants existed in the system, the generated hydroxyl radicals were scavenged and the damage to guanine immobilized on the electrode was reduced less resulting in the oxidation peak current increased in square wave voltammetry. After the modifications of polythionine- and nitrogen-doped graphene, the oxidation peak current was improved. The effects of pH, incubation time, and concentrations of guanine and Fe2+ ions on the performances of the electrode were investigated and optimized. The G/PTH/NG/GCE showed good linearity, reproducibility, and storage stability for antioxidant capacity evaluation of ascorbic acid at the optimum conditions. The antioxidant capacities of three flavonoids and three plant extracts were measured using the G/PTH/NG/GCE and DPPH methods. Myricetin showed the highest antioxidant capacity in both electrochemical and DPPH methods. The proposed G/PTH/NG/GCE exhibited easy fabrication procedure, rapid detection time, and low cost for the detection of antioxidant activity for various kinds of samples.
1 Introduction
Reactive oxygen species (ROS) were common in natural biological processes. However, when there was an accumulation of ROS induced by environment and chemicals, the oxidative stress would occur in the body and cause damage of proteins, lipids, and even cells [1]. This process could lead to various chronic diseases such as cancer, muscular dystrophy, heart disease, and atherosclerosis [2]. Antioxidant compounds existing in various fruits, vegetables, and beverages could react with ROS by scavenging free radicals, transition metal chelation, and singlet oxygen quenching [3]. Flavonoids and polyphenols compounds were the major class of antioxidants that came from diet possessing many activities in anti-inflammation and anti-virus [4]. Accordingly, efficient detection of antioxidant capacity for various samples could promote the discovery of new antioxidants and related researches. Different methods were proposed as tools for the analysis of antioxidant capacity for compounds or complex mixtures such as absorption spectroscopy and high-performance liquid chromatography [5]. However, the main problem of spectroscopic methods was the color of samples that would interfere with the reaction system and affect the precision of detection [6]. As an easy, sensitive, and fast technique, the electrochemical method could become a suitable option for evaluating the antioxidant content and the reducing ability of mixture [7]. Based on a specific redox reaction, there was no need for difficult sample preparation, and the disturbance in the detection was much less [8].
Graphene was a kind of carbon nanomaterial with high-specific surface area and conductivity, which has been used in electrochemical sensing [9]. Heteroatom doping and functionalization of graphene could make an improved performance in gravimetric capacitance and magnetic response by increasing conductivity, pseudo-capacitive behavior, electron transfer, and so on [10]. Recently, nitrogen-doped graphene (NG) showed advantages in electrocatalysis owing to good chemical stability and high-electrical conductivity [11]. It has attracted particular research interests in electrocatalyst [12], semiconductor [13], lithium-ion batteries [14], and other fields [15]. Therefore, it could be expected that NG could exhibit enhanced performance in chemical and electrical properties.
Thionine was a phenothiazine redox dye with good solubility in water and ethanol [16]. Polythionine (PTH) was widely applied in the modification of electrochemical sensors because of its good redox reversibility, fast charge transfer capacity, and good stability [17]. An electroactive and conductive PTH membrane could be easily accomplished on the surface of the electrode using electrochemical polymerization. This kind of fabrication showed advantages in the thickness of the film, response time, and charge transfer rate than the suspension coating method [18,19]. It has been reported that PTH-modified electrode was applied for the determination of endocrine-disrupting compounds, hydrogen peroxide, hepatitis B, and nicotinamide adenine dinucleotide [20,21,22,23].
In this study, NG and PTH membranes were consequently modified on glassy carbon electrode (PTH/NG/GCE) to combine the excellent properties of them in electrical conductivity and adsorption ability. Then, guanine was immobilized on PTH/NG/GCE (G/PTH/NG/GCE) and used as a stable G/PTH/NG/GCE electrochemical sensor in the evaluation of antioxidant capacity. During the detection procedures, the hydroxyl radicals could make damage guanine on electrode, resulting in the decrease of oxidation peak current in square wave voltammetry (SWV). When antioxidants existed in the reaction system, the number of hydroxyl radicals would decrease due to the antioxidant activity, and there would be less damages on guanine. As a result, a higher peak current in SWV was observed and the following calculation could be completed. After modification, the proposed G/PTH/NG/GCE showed higher oxidation peak current, which meant higher sensitivity for evaluation. It could be directly used in the determination of antioxidant capacity for compounds and mixture without extra processes and reagents. Therefore, this modified electrode could provide a fast and cheap sensor for the analysis of antioxidants with promising prospects in the food and chemical industries.
2 Experimental
2.1 Chemicals and apparatus
Ethylenediaminetetraacetic acid (EDTA), guanine, and thionine were purchased from Merck KGaA (Darmstadt, Germany). Ammonium hydroxide, hydrogen peroxide, sulfuric acid, potassium permanganate, urea, and nitric acid were purchased from Sinopharm Chemical Reagent (Shanghai, China). Graphite flakes were purchased from Nanjing XFNANO Materials Tech Co., Ltd (Nanjing, China). Myricetin, kaempferol, and galangin were acquired from Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China). Ultrapure water (18.25 MΩ cm) was obtained from a Millipore system (Millipore Corporation, Milford, MA, USA). All other chemicals were of analytical grade without further purification.
The electrochemical measurements were accomplished on a CHI 660E Electrochemical Workstation (Shanghai Chenhua Instrument Company, Shanghai, China) using a standard three-electrode system. The characterizations of materials were conducted by transmission electron microscope (TEM) and scanning electron microscope (SEM). The observations were completed by a JSM 6610Lv scanning electron microscopy (JEOL, Tokyo, Japan) operated at 25 kV and a JEM-2100 electron microscope (JEOL, Akishima, Japan).
2.2 Preparation of NG
Graphene oxide (GO) was prepared according to the Hummers method with some modifications [24]. About 2.0 g of graphite flakes and 8.0 g of KMnO4 were added into 100 mL of ice-cold concentrated H2SO4. The mixture was kept in an ice bath and stirred for 3 h. Then, 400 mL of water containing 20 mL of 30% H2O2 was slowly added. The produced golden mixture was filtrated and the solid was washed with water and 5% HCl three times, respectively. The finally obtained solid was dried in vacuum at 60°C for 24 h.
For the preparation of NG, 0.1 g of GO was dispersed into 100 mL of water. About 2.0 g of urea was added into GO solution as the source of nitrogen and ultrasonically treated for 2 h. Then, the solution was transferred into a stainless-steel autoclave for the reaction at 180°C for 12 h. Finally, the products were washed with lots of water and dried in vacuum at 50°C for 24 h.
2.3 Fabrication of G/PTH/NG/GCE
The G/PTH/NG/GCE was fabricated by depositions of NG, guanine, and electropolymerization of thionine. Specifically, NG suspension (0.5 mg mL−1) was sonicated for 30 min prior to use. After sonication, 6 µL of NG suspension was dropped on the surface of a GCE and dried at room temperature. Then, the electrode was immersed into 4.4 mol L−1 acetic acid containing 0.5 mmol L−1 thionine solution and scanned from −0.4 to 1.4 V by cyclic voltammetry for 30 loops with the scanning rate at 80 mV s−1. The electrode was washed with water, dried in air, and marked as PTH/NG/GCE. Finally, 6 µL of guanine solution (1.0 mg mL−1) was dropped on to the surface of PTH/NG/GCE. After drying, the G/PTH/NG/GCE was obtained and kept for further use.
2.4 Electrochemical determination of antioxidant capacity
The antioxidant capacity assay was measured as our previous report with some modifications [25]. First, a certain concentration of test sample was mixed with freshly prepared Fe2+–EDTA solution in phosphate buffer saline (PBS, 50 mmol L−1, pH 3.5). Then, the fabricated G/PTH/NG/GCE was immersed into the mixture for 240 s. After incubation, the G/PTH/NG/GCE was washed with water and immersed in PBS (50 mmol L−1, pH 1.5) to perform the SWV. The oxidation peak of guanine in SWV was recorded, and the measurements were completed three times consecutively to obtain the average values. The same amount of PBS was used as a control instead of samples. The antioxidant capacity of the sample was calculated using the following equation:
where I0 is the oxidation peak current before damage in Fenton solution, and Ic and Is are the oxidation peak currents of control and samples after damage in Fenton solution, respectively.
2.5 DPPH radical scavenging assay
The DPPH radical scavenging assay was measured according to Sridhar’s report with some modifications [26]. Briefly, 2 mL of DPPH solution (0.05 mg/mL in methanol) and 1.0 mL of the sample with different concentrations were mixed. Then, the mixture was kept in dark for 5 min and transferred into a UV2700 UV-Vis Spectrophotometer (Shimadzu, Kyoto, Japan) for testing the absorbance at 517 nm. The same amount of water was used as a control. The scavenging rate of DPPH radicals was calculated by the following equation:
where As and A0 are the absorbance of sample and control, respectively. The DPPH radical scavenging rate of sample was expressed as the concentration of sample needed to scavenge 50% of DPPH (EC50).
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussions
3.1 Characterization of NG
TEM and SEM were used to characterize the morphology of prepared NG. The images of NG are shown in Figure 1. In the TEM image of NG (Figure 1a), the typical characteristics of NG such as smooth surface, sheet-like structure, and wrinkled edge could be found [27]. It could be seen the structure of NG was not influenced during nitrogen-doped procedure. The NG agglomerated into particles with nanoscale could be found in SEM image (Figure 1b). This kind of agglomeration might occur in the drying process of samples. The elemental mapping images of NG verified the existence of carbon and nitrogen elements in the materials (Figure 1c–e). In elemental mapping images, the green spots denoting nitrogen atoms and red spots denoting carbon atoms were well distributed over the NG [28]. It demonstrated that nitrogen atoms were uniformly doped in graphene, and the atomic percentage of nitrogen to carbon was about 5.59%. These characterization images could be considered as evidences that NG was successfully prepared in this research.
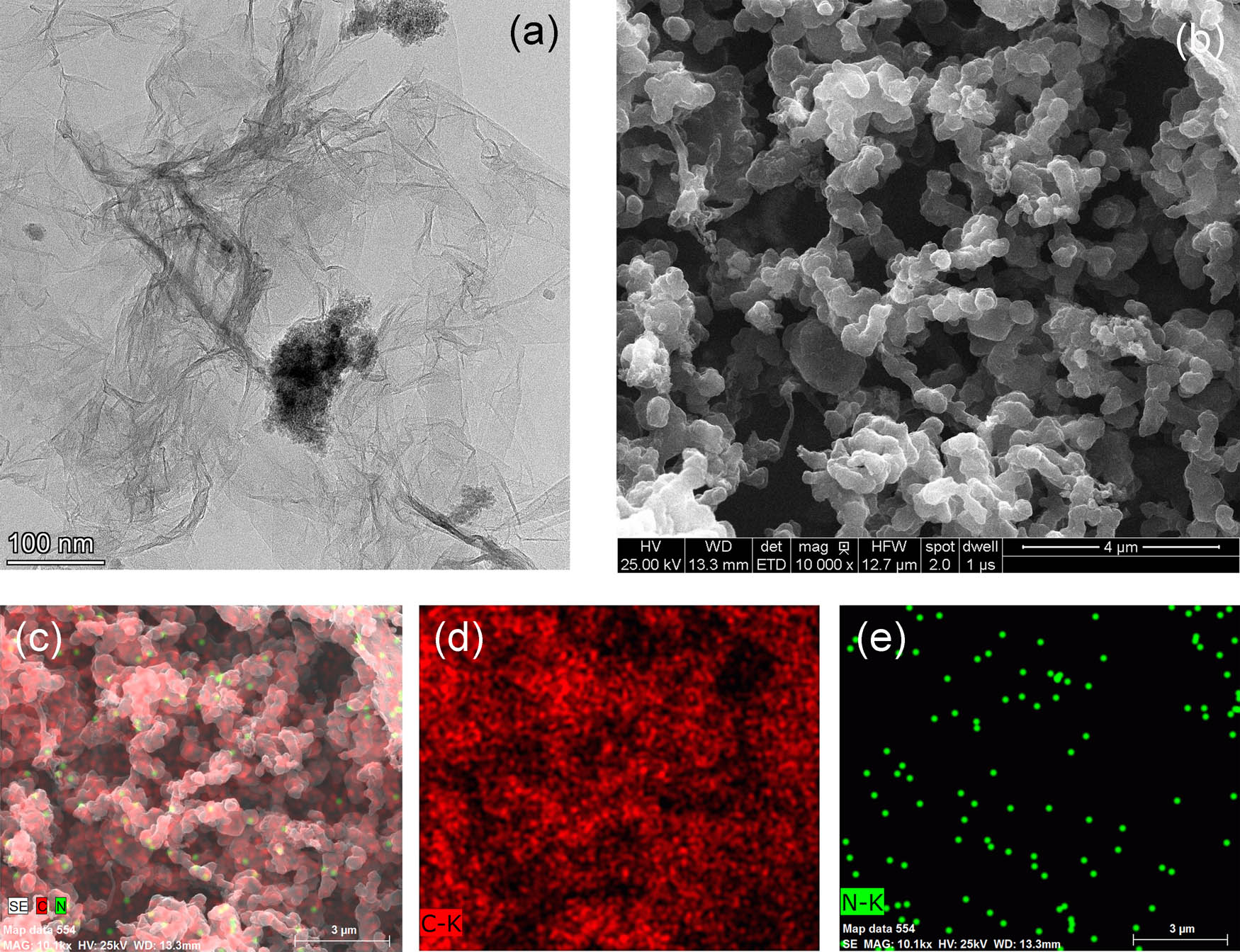
(a) TEM, (b) SEM, and (c–e) elemental mapping images of NG.
3.2 Fabrication of G/PTH/NG/GCE
The cyclic voltammograms for the electrochemical polymerization process of PTH on electrode with consecutive cyclic scanning are shown in Figure 2a. With the increasing scanning times, a pair of redox peaks could be found from 0 to 0.3 V, and the peak currents increased as well. Finally, a thin blue membrane could be observed on the surface of electrode, which showed that thionine was successfully polymerized on the electrode [29]. The experimental results showed that a stable and uniform blue PTH membrane could be obtained with a satisfied response through 30 loops consecutive cyclic scanning in 4.4 mol L−1 acetic acid containing 0.5 mmol L−1 thionine solution with a scanning potential range from −0.4 to 1.4 V and a scanning rate at 80 mV s−1. The oxidation peak current of modified electrodes during the fabrication process was compared (Figure 2b). After the modifications of NG and PTH, the oxidation peak currents of electrodes were increased. Moreover, when NG and PTH were both modified on the electrode, the oxidation peak current was higher than that of electrode modified using any single material. It could be assumed that good electrical conductivities and electrocatalytic properties of NG and PTH played important roles in this sensor [30].
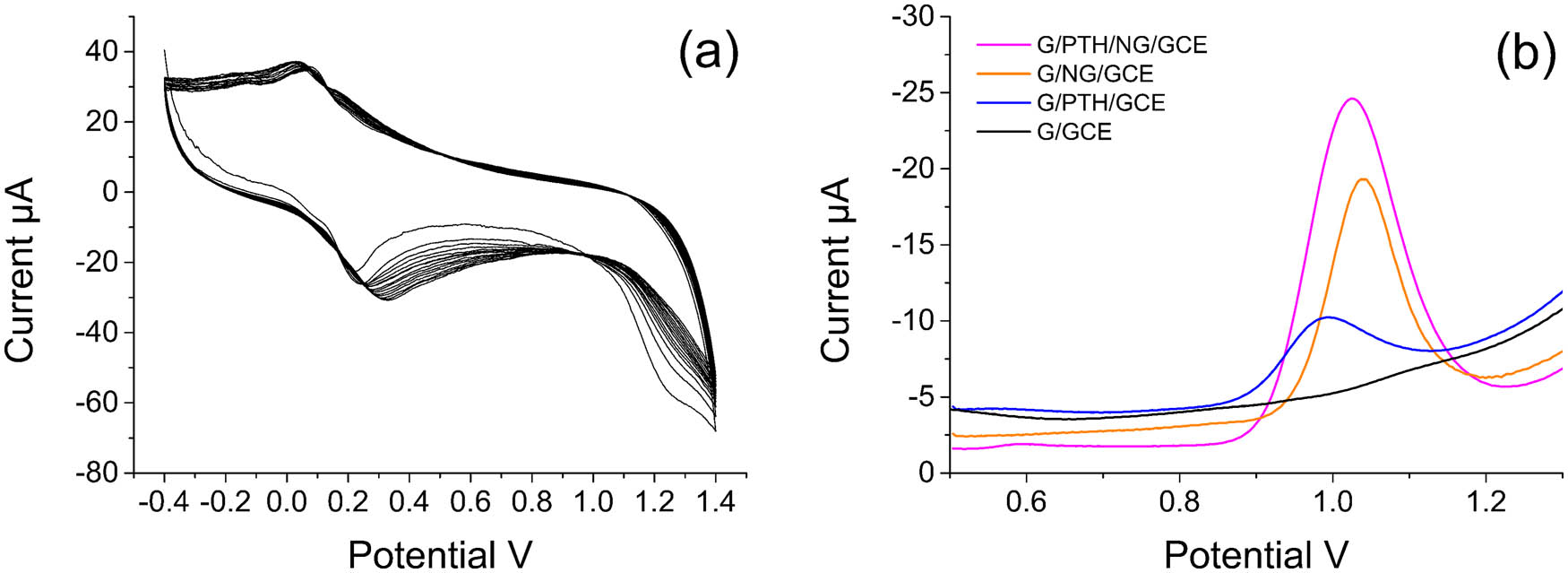
(a) The cyclic voltammograms for electrochemical polymerization of PTH on the electrode. (b) The oxidation peak current of several modified electrodes using SWV detection.
3.3 Optimization of fabrication and detection parameters
3.3.1 Effect of pH
The effect of pH on the oxidation peak of G/PTH/NG/GCE was studied. As shown in Figure 3a, with the growth of pH values (from 1.5 to 7.0), the peak currents decreased gradually and finally reached to a stable status. As a result, the peak current reached the maximum when pH was 1.5. Therefore, pH 1.5 was selected for electrolyte used in this study.
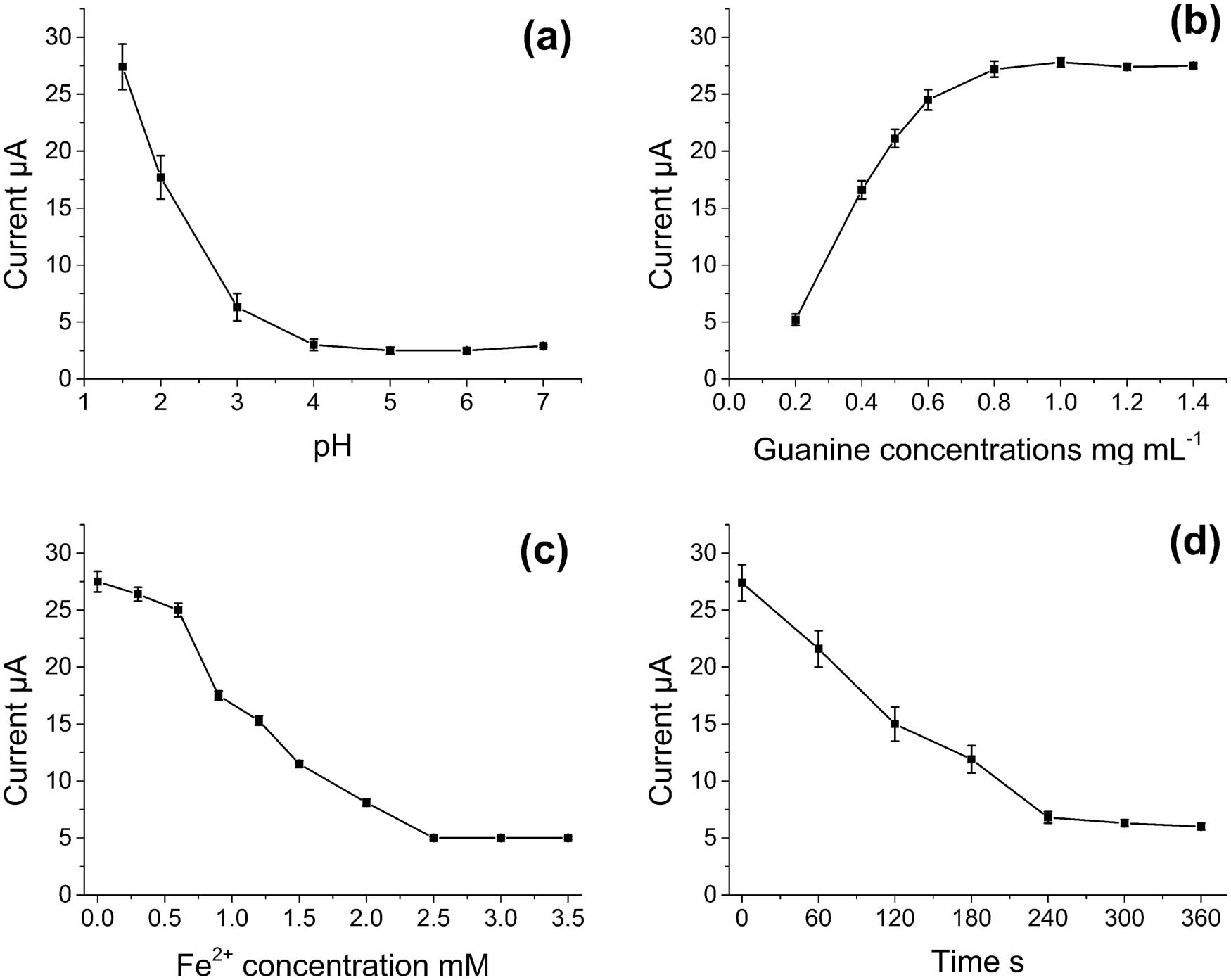
Effects of (a) pH, (b) guanine concentration, (c) Fe2+ concentration, and (d) incubation time on the oxidant peak currents of G/PTH/NG/GCE.
3.3.2 Effect of guanine concentration
The amount of guanine on the modified electrode could influence the voltammetric response (Figure 3b). As a result, the peak currents increased with guanine concentrations from 0.2 to 1.4 mg mL−1 and reached a stable state when guanine concentration was higher than 1.0 mg mL−1. This behavior might be due to the fact that when the concentration of guanine reached a certain amount, the guanines adsorbed on the surface of electrode were saturated. More guanines could not be immobilized and were washed off during the washing procedure. Therefore, 1.0 mg mL−1 of guanine solution was selected as the optimum concentration of guanine solution for modification of the electrode.
3.3.3 Effect of Fe2+ concentration
It was reported that the concentration of Fe2+ affected the generation of hydroxyl radical and following damage to guanine [31]. The effect of Fe2+ concentration on the oxidation peak current of G/PTH/NG/GCE was studied. As shown in Figure 3c, the peak currents decreased with increasing of Fe2+ concentration. When the Fe2+ concentration was higher than about 2.5 mmol L−1, there was only a little change of peak current and the damage to guanine reached the maximum. Therefore, 2.5 mmol L−1 of Fe2+ ions was chosen as the optimum Fe2+ concentration for this study.
3.3.4 Effect of incubation time
The effect of incubation time on oxidation peak current of G/PTH/NG/GCE was also investigated (Figure 3d). As the incubation time increased to 240 s, the peak currents decreased gradually. When the incubation time was longer than 240 s, the peak current remained constant, which showed the 240 s of incubation was enough for the damage to guanine. Therefore, 240 s incubation time was chosen as the optimum incubation time.
3.4 Determination of antioxidant capacity using G/PTH/NG/GCE
The fabricated G/PTH/NG/GCE was used in the detection of antioxidant capacity for investigated samples. As a well-known antioxidant, ascorbic acid was selected for the determination of antioxidant capacity using G/PTH/NG/GCE [32]. The prepared G/PTH/NG/GCE was processed with Fenton solution containing different concentrations of ascorbic acid and then detected using SWV. It was observed that the peak current increased with the growing concentration of ascorbic acid (Figure 4). A good linear relationship between peak current and ascorbic acid concentration could be found when the ascorbic acid concentration ranged from 0.5 to 3.0 mg L−1. The corresponding linear function was fitted as Ip (μA) = −7.7526 × CAA − 2.7169 with a correlation coefficient at 0.999. The detection limit was 0.21 mg L−1 (S/N = 3).
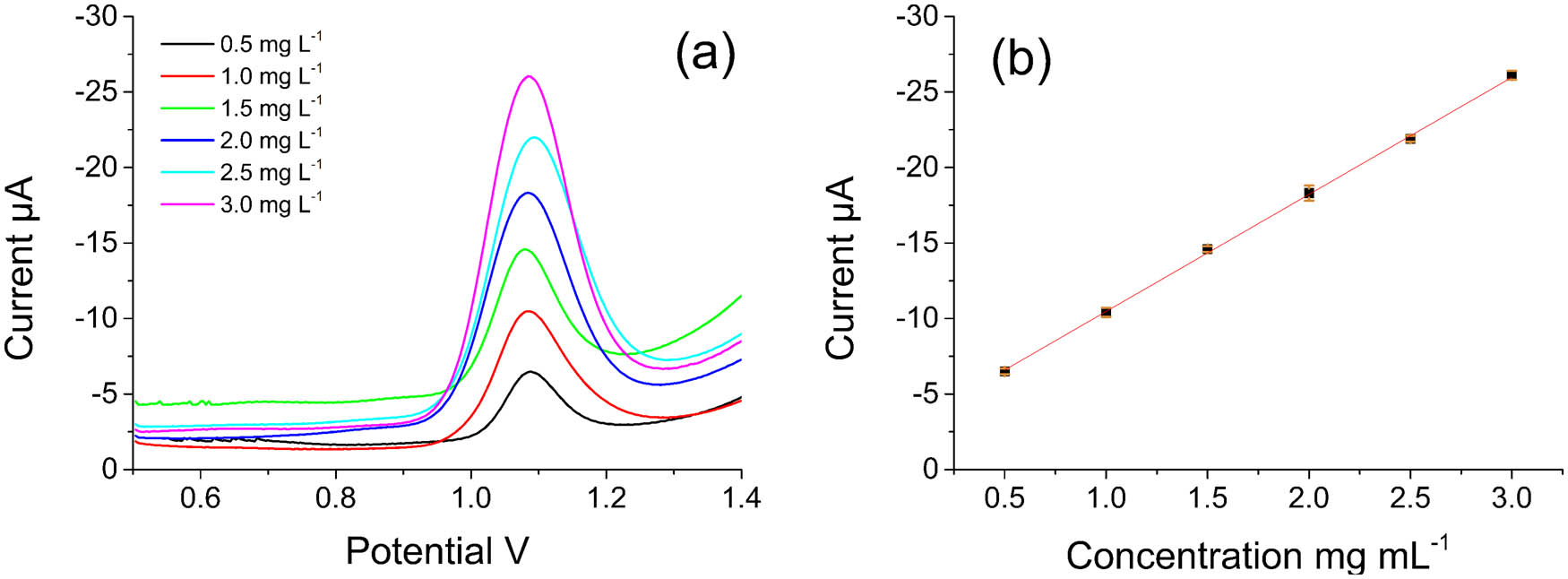
(a) The oxidation peak current of G/PTH/NG/GCE after incubation in Fenton solution with different concentration of ascorbic acid (a–i): 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg L−1. (b) The calibration plot of oxidant peak current against the concentration of ascorbic acid.
To further evaluate the validity of determination for antioxidant capacity using G/PTH/NG/GCE, the samples containing ascorbic acid were measured by the standard addition method [33]. The analytical results are listed in Table 1. It could be found the ascorbic acid could be detected with a recovery rate of 98.7–101.5%, and the relative standard deviation (RSD) was less than 1.6%. These results illustrated that the determination of antioxidant capacity using G/PTH/NG/GCE was effective with good linearity and high accuracy.
Determination of ascorbic acid at different addition (n = 5)
| Samples | Concentrations (mg L−1) | Added (mg L−1) | Found (mg L−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Ascorbic acid | 2.0 | 0.25 | 2.28 | 101.5 | 0.91 |
| 2.0 | 0.5 | 2.47 | 98.7 | 0.47 | |
| 2.0 | 1.0 | 2.98 | 99.2 | 1.59 |
3.5 The performances of G/PTH/NG/GCE
In order to evaluate the selectivity of G/PTH/NG/GCE in determination of antioxidant capacities, some samples or substances were used for this comparison. Figure 5 shows the peak currents for these samples. It could be seen that the currents of substances, such as potassium chloride, sodium chloride, calcium chloride, and aluminum nitrate, were very low, which mean that these samples did not show antioxidant activities. However, the currents of ascorbic acid and galangin were relatively higher, which illustrated that these samples have antioxidant capacities. The antioxidant capacities of ascorbic acid and galangin would be verified in the following study. This comparison showed that the determination of antioxidant capacity using G/PTH/NG/GCE has a good selectivity of antioxidants and showed a certain anti-interference ability.
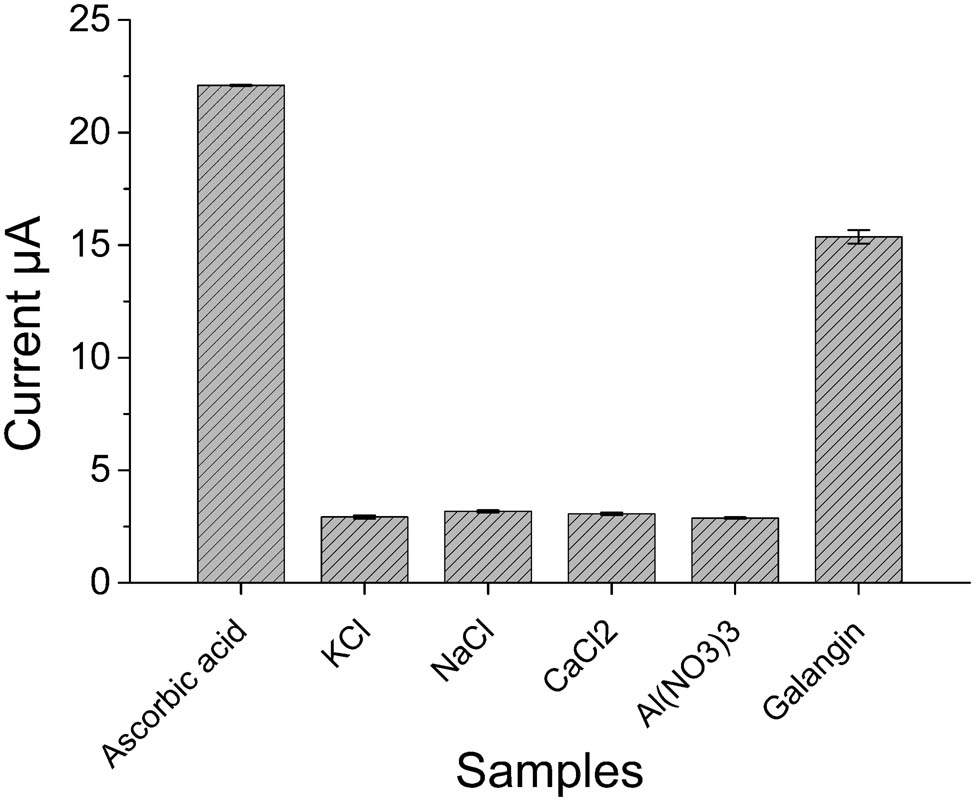
The selectivity of G/PTH/NG/GCE in the detection of samples (KCl, NaCl, CaCl2, Al(NO3)3 and galangin: 5.0 μmol L−1).
The reproducibility and stability of G/PTH/NG/GCE were evaluated as well. Several electrodes were used for parallel measurements to evaluate the reproducibility of test. The results showed a good reproducibility in 10 times tests with the RSD value at 3.1% (shown in Figure 6a). After one week of storage at 25°C for a certain electrode, the peak current reduced about 5.2% (shown in Figure 6b). These results demonstrated that the modified electrode was stable for determination at least for one week.
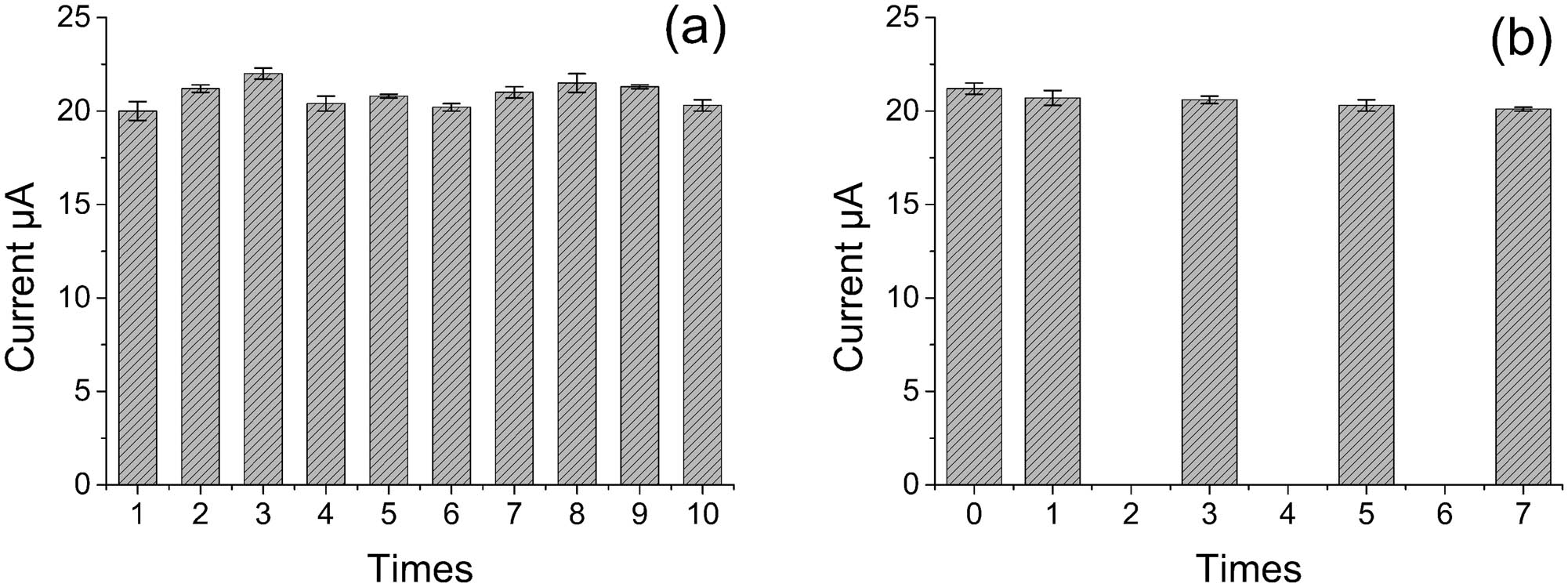
The (a) reproducibility and (b) storage stability of G/PTH/NG/GCE in the detection of ascorbic acid.
3.6 Comparison of antioxidant capacity using G/PTH/NG/GCE and DPPH method
The antioxidant capacities of three flavonoids were analyzed by fabricated G/PTH/NG/GCE. The concentration of three flavonoids was unified at 6.0 μmol L−1, and the results of the three flavonoids and ascorbic acid are listed in Table 2. Ascorbic acid displayed the highest antioxidant capacity (80.4 ± 2.5%), then followed by myricetin (73.1 ± 3.7%), kaempferol (63.2 ± 1.2%), and galangin (60.7 ± 3.1%). Simultaneously, the antioxidant capacities of myricetin, kaempferol, and galangin were detected by the DPPH assay, and the activities were presented as EC50 values in Table 2. For the DPPH assay, the lower EC50 values mean higher antioxidant capacities of the sample [34]. The rank of three flavonoids from high antioxidant activity to low was myricetin, kaempferol, and galangin. These results were in accordance with that obtained through the G/PTH/NG/GCE method.
Determination of antioxidant capacities of myricetin, kaempferol, and galangin compared to ascorbic acid using G/PTH/NG/GCE and DPPH method
| Flavonoids | Antioxidant capacity | |
|---|---|---|
| G/PTH/NG/GCE (%) | DPPH method (µmol L−1) | |
| Myricetin | 73.1 ± 3.7 | 10.5 ± 0.4 |
| Kaempferol | 63.2 ± 1.2 | 23.5 ± 1.8 |
| Galangin | 60.7 ± 3.1 | 28.4 ± 2.4 |
| Ascorbic acid | 80.4 ± 2.5 | 5.5 ± 0.5 |
As reported in references, the antioxidant activities of flavonoids were affected by structure and substituent groups [35,36,37]. The main structure of myricetin, kaempferol, and galangin was the same with different hydroxyl groups on B ring (Figure 7). The number of hydroxyl groups on B ring mostly affected the antioxidant activities of flavonoids, and more hydroxyl groups on B ring showed higher antioxidant activities [38]. According to this principle, myricetin should have the best antioxidant ability among these three flavonoids, while galangin would have the lowest antioxidant capacity. The antioxidant capacities detected by G/PTH/NG/GCE and DPPH methods confirmed this principle, which showed that this kind of detection followed reported principle and could be considered effective in the detection of antioxidant capacities.
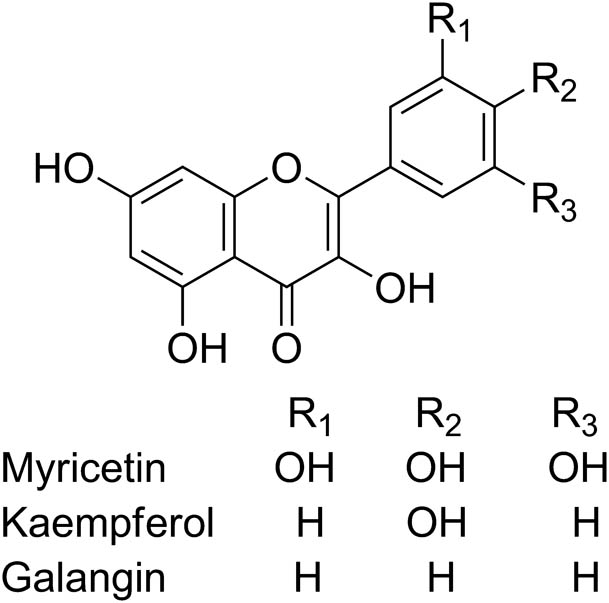
The chemical structures of myricetin, kaempferol, and galangin.
3.7 Antioxidant capacities of real samples
In order to explore the application of G/PTH/NG/GCE for real samples, the prepared G/PTH/NG/GCE was utilized to evaluate the antioxidant capacities of fruit juices and plant extracts. Three kinds of fruit juices including grape juice, guava juice, and orange juice were bought from the local supermarket. Three plant extracts were made in our labs including jute leaves extract, ramie leaves extract, and hemp leaves extract. All of the samples were filtered to remove insoluble substances and set the concentration at 2.0 mg L−1. As shown in Figure 8, all of the three juices showed antioxidant capacities from 56% to 68%, whereas the plant extracts showed relatively higher antioxidant capacities (from 85% to 92%) than those of juices. It might be due to fact that the plant extracts contain more active compounds and antioxidants. Based on these results, the fabricated G/PTH/NG/GCE showed predictable ability in determination of antioxidant capacities of real samples.
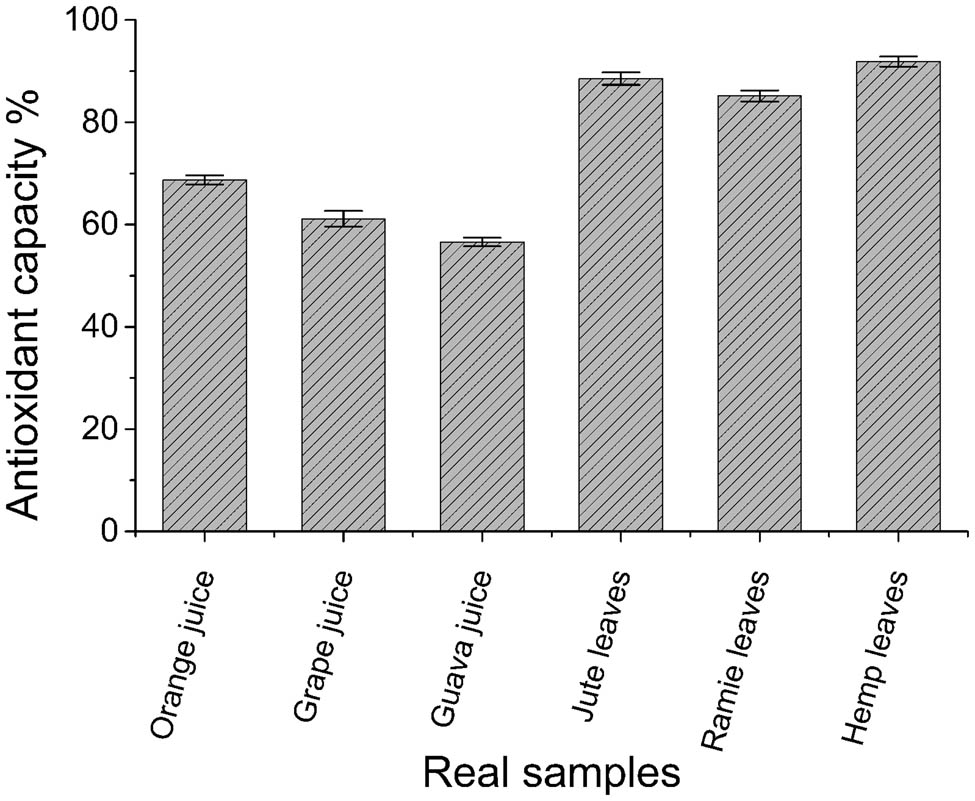
Determination of antioxidant capacities of fruit juices and plant extracts using G/PTH/NG/GCE.
4 Conclusions
In this research, the G/PTH/NG/GCE was fabricated and used for the electrochemical determination of antioxidant capacity. After the modification, the related oxidation peak current on G/PTH/NG/GCE was improved. The factors including pH, guanine concentration, Fe2+ concentration, and incubation time were optimized for the optimum performance of the modified electrode. The G/PTH/NG/GCE showed good linearity and selectivity, satisfied reproducibility, and storage stability. Three flavonoids were selected as the samples for the antioxidant capacity tests using G/PTH/NG/GCE and DPPH methods, and the results have a good agreement. Some fruit juices and plant extracts were also tested as real samples. The proposed G/PTH/NG/GCE provided to be a rapid and efficient sensor for the evaluation of antioxidants in food and natural chemical fields.
Acknowledgments
This work was supported by the Central Public-interest Scientific Institution Basal Research Fund (No. Y2019PT22-02 and 1610242020005), Open project of key laboratory of biology and processing for bast fiber crops, MARA and National Agricultural Science and Technology Innovation Project (Characteristic fruit and vegetable innovation team, ASTIP-IBFC05).
Conflict of Interest: The authors declare no conflict of interest.
References
[1] Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30(11):1191–212.10.1016/S0891-5849(01)00480-4Suche in Google Scholar
[2] Hashkavayi AB, Hashemnia S, Osfouri S, Zarei S. Electrochemical study of antioxidant capacity of gracilaria pygmaea macro-algae based on the green synthesis of gold nanoparticles: assessment of its cytotoxic effect on four cancer cell lines. J Electrochem Soc. 2019;166(12):B969–77.10.1149/2.0951912jesSuche in Google Scholar
[3] Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118–26.10.4103/0973-7847.70902Suche in Google Scholar PubMed PubMed Central
[4] de Araújo Rodrigues I, Gomes SMC, Fernandes IPG, Oliveira-Brett AM. Phenolic composition and total antioxidant capacity by electrochemical, spectrophotometric and HPLC-EC evaluation in Portuguese red and white wines. Electroanalysis. 2019;31(5):936–45.10.1002/elan.201800842Suche in Google Scholar
[5] Ren R, Cai G, Yu Z, Zeng Y, Tang D. Metal-polydopamine framework: an innovative signal-generation tag for colorimetric immunoassay. Anal Chem. 2018;90(18):11099–105.10.1021/acs.analchem.8b03538Suche in Google Scholar PubMed
[6] Lai W, Wei Q, Xu M, Zhuang J, Tang D. Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens Bioelectron. 2017;89:645–51.10.1016/j.bios.2015.12.035Suche in Google Scholar PubMed
[7] Veton H, Avni B, Fetah IP. Electrochemical modification of platinum and glassy carbon surfaces with pyridine layers and their use as complexing agents for copper (ii) ions. Open Chem. 2019;17(1):722–7.10.1515/chem-2019-0084Suche in Google Scholar
[8] Hoyos-Arbeláez J, Vázquez M, Contreras-Calderón J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: a review. Food Chem. 2017;221:1371–81.10.1016/j.foodchem.2016.11.017Suche in Google Scholar PubMed
[9] Zhou M, Zhai Y, Dong S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem. 2009;81(14):5603–13.10.1021/ac900136zSuche in Google Scholar PubMed
[10] Pang Y, Luo K, Tang L, Li X, Song Y, Li CY, et al. Preparation and application of magnetic nitrogen-doped rGO for persulfate activation. Environ Sci Pollut R. 2018;25(30):30575–84.10.1007/s11356-018-2974-2Suche in Google Scholar PubMed
[11] Wang B, Likodimos V, Fielding AJ, Dryfe RAW. In situ electron paramagnetic resonance spectroelectrochemical study of graphene-based supercapacitors: Comparison between chemically reduced graphene oxide and nitrogen-doped reduced graphene oxide. Carbon. 2020;160:236–46.10.1016/j.carbon.2019.12.045Suche in Google Scholar
[12] Zhang LS, Liang XQ, Song WG, Wu ZY. Identification of the nitrogen species on N-doped graphene layers and Pt/NG composite catalyst for direct methanol fuel cell. Phys Chem Chem Phys. 2010;12(38):12055–9.10.1039/c0cp00789gSuche in Google Scholar
[13] Wang X, Li X, Zhang L, Yoon Y, Weber PK, Wang H, et al. N-Doping of graphene through electrothermal reactions with ammonia. Science. 2009;324(5928):768.10.1126/science.1170335Suche in Google Scholar
[14] Cho YJ, Kim HS, Im H, Myung Y, Jung GB, Lee CW, et al. Nitrogen-doped graphitic layers deposited on silicon nanowires for efficient lithium-ion battery anodes. J Phys Chem C. 2011;115(19):9451–7.10.1021/jp201485jSuche in Google Scholar
[15] Jeong HM, Lee JW, Shin WH, Choi YJ, Shin HJ, Kang JK, et al. Nitrogen-doped graphene for high-performance ultracapacitors and the importance of nitrogen-doped sites at Basal planes. Nano Lett. 2011;11(6):2472–7.10.1021/nl2009058Suche in Google Scholar
[16] Yin Z, Wu J, Yang Z. A sensitive mercury (ii) sensor based on CuO nanoshuttles/poly(thionine) modified glassy carbon electrode. MicrochimActa. 2010;170(3):307–12.10.1007/s00604-010-0359-4Suche in Google Scholar
[17] Gao Q, Cui X, Yang F, Ma Y, Yang X. Preparation of poly(thionine) modified screen-printed carbon electrode and its application to determine NADH in flow injection analysis system. Biosens Bioelectron. 2003;19(3):277–82.10.1016/S0956-5663(03)00212-4Suche in Google Scholar
[18] Dohno C, Stemp EDA, Barton JK. Fast back electron transfer prevents guanine damage by photoexcited thionine bound to DNA. J Am Chem Soc. 2003;125(32):9586–7.10.1021/ja036397zSuche in Google Scholar PubMed
[19] Ohsaka T, Tanaka K, Tokuda K. Electrocatalysis of poly(thionine)-modified electrodes for oxidation of reduced nicotinamide adenine dinucleotide. Chem Commun. 1993;3:222–4.10.1039/c39930000222Suche in Google Scholar
[20] Dempsey E, Diamond D, Collier A. Development of a biosensor for endocrine disrupting compounds based on tyrosinase entrapped within a poly(thionine) film. Biosens Bioelectron. 2004;20(2):367–77.10.1016/j.bios.2004.02.007Suche in Google Scholar PubMed
[21] Wu S, Zhong Z, Wang D, Li M, Qing Y, Dai N, et al. Gold nanoparticle-labeled detection antibodies for use in an enhanced electrochemical immunoassay of hepatitis B surface antigen in human serum. Microchim Acta. 2009;166(3):269–75.10.1007/s00604-009-0184-9Suche in Google Scholar
[22] Liu Y, Hu LM, Yang SQ. Amplification of bioelectrocatalytic signalling based on silver nanoparticles and DNA-derived horseradish peroxidase biosensors. Microchim Acta. 2008;160(3):357–65.10.1007/s00604-007-0817-9Suche in Google Scholar
[23] Huo HY, Luo HQ, Li NB. Electrochemical sensor for heparin based on a poly(thionine) modified glassy carbon electrode. Microchim Acta. 2009;167(3):195.10.1007/s00604-009-0240-5Suche in Google Scholar
[24] Cen Y, Xiao AP, Chen XQ, Liu LL. Screening and separation of alpha-amylase inhibitors from Solanum nigrum with amylase-functionalized magnetic graphene oxide combined with high-speed counter-current chromatography. J Sep Sci. 2017;40(24):4780–7.10.1002/jssc.201700333Suche in Google Scholar PubMed
[25] Tang P, Tang XS, Mei SY, Xie YX, Liu LL, Ren LC. Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE). Open Chem. 2020;18(1):1–9.10.1515/chem-2020-0003Suche in Google Scholar
[26] Sridhar K, Charles AL. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: estimation methods for EC50 using advanced statistical programs. Food Chem. 2019;275:41–9.10.1016/j.foodchem.2018.09.040Suche in Google Scholar PubMed
[27] Liu L, Yu J, Chen X. Enhanced stability and reusability of alcohol dehydrogenase covalently immobilized on magnetic graphene oxide nanocomposites. J Nanosci Nanotechnol. 2015;15(2):1213–20.10.1166/jnn.2015.9024Suche in Google Scholar PubMed
[28] Son M, Chee SS, Kim SY, Lee W, Kim YH, Oh BY, et al. High-quality nitrogen-doped graphene films synthesized from pyridine via two-step chemical vapor deposition. Carbon. 2020;159:579–85.10.1016/j.carbon.2019.12.095Suche in Google Scholar
[29] Liu Y, Zhang HL, Lai GS, Yu AM, Huang YM, Han DY. Amperometric NADH biosensor based on magnetic chitosan microspheres/poly(thionine) modified glassy carbon electrode. Electroanalysis. 2010;22(15):1725–32.10.1002/elan.200900544Suche in Google Scholar
[30] Liu C, Huang J, Wang L. Electrochemical synthesis of a nanocomposite consisting of carboxy-modified multi-walled carbon nanotubes, polythionine and platinum nanoparticles for simultaneous voltammetric determination of myricetin and rutin. Microchim Acta. 2018;185(9):414.10.1007/s00604-018-2947-7Suche in Google Scholar PubMed
[31] Hájková A, Barek J, Vyskočil V. Electrochemical DNA biosensor for detection of DNA damage induced by hydroxyl radicals. Bioelectrochemistry. 2017;116:1–9.10.1016/j.bioelechem.2017.02.003Suche in Google Scholar PubMed
[32] Duarte TL, Lunec J. Review: when is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res. 2005;39(7):671–86.10.1080/10715760500104025Suche in Google Scholar PubMed
[33] Haitham A, Atef H, Ahmed B, Gamal AEM. Cyclodextrin potentiometric sensors based on selective recognition sites for procainamide: Comparative and theoretical study. Open Chem. 2019;17(1):1222–34.10.1515/chem-2019-0131Suche in Google Scholar
[34] Ling LT, Palanisamy UD, Cheng HM. Prooxidant/antioxidant ratio (ProAntidex) as a better index of net free radical scavenging potential. Molecules. 2010;15(11):7884–92.10.3390/molecules15117884Suche in Google Scholar PubMed PubMed Central
[35] Murni Nazira S, Qamar Uddin A, Siti Zaiton Mat SA, Alhassan Muhammad A, Suganya M, Vikneswari P, et al. Antioxidant and antidiabetic effects of flavonoids: a structure-activity relationship based study. BioMed Res Int. 2017;2017:8386065.Suche in Google Scholar
[36] Ferreira RdQ, Greco SJ, Delarmelina M, Weber KC. Electrochemical quantification of the structure/antioxidant activity relationship of flavonoids. Electrochim Acta. 2015;163:161–6.10.1016/j.electacta.2015.02.164Suche in Google Scholar
[37] Mendes APS, Borges RS, Neto AMJC, de Macedo LGM, da Silva ABF. The basic antioxidant structure for flavonoid derivatives. J Mol Model. 2012;18(9):4073–80.10.1007/s00894-012-1397-0Suche in Google Scholar PubMed
[38] Glevitzky I, Dumitrel GA, Glevitzky M, Pasca B, Otrisal P, Bungau S, et al. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev Chim. 2019;70(9):3103–7.10.37358/RC.19.9.7497Suche in Google Scholar
© 2020 Yafen Fu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

