Abstract
A rapid and specific method was developed for simultaneous quantification of hydrocortisone 21 acetate (HCA), dexamethasone (DEX), and fluocinolone acetonide (FCA) in whitening cream formulations using reversed-phase high-performance liquid chromatography. The effect of the composition of the mobile phase, analysis temperature, and detection wavelength was investigated to optimize the separation of studied components. The analytes were finally well separated using ACE Excel 2, C18 AR column having 150 mm length, 3 mm internal diameter, and 2 µm particle size at 35°C using methanol with 1% formic acid and double-distilled deionized water in the ratio of 60:40 (v/v), respectively, as the mobile phase in isocratic mode. Ten microliters of sample were injected with a flow rate of 0.5 mL/min. The specificity, linearity, accuracy, precision, recovery, limit of detection (LOD), limit of quantification (LOQ), and robustness were determined to validate the method as per International Conference on Harmonization guidelines. All the analytes were simultaneously separated within 8 min, and observed retention times of HCA, DEX, and FCA were 4.5, 5.5, and 6.9 min, respectively. The proposed method showed good linearity with the correlation coefficient, R2 = 0.999 over the range of 1–150 µg/mL for all standards. The linear regression equations were y = 12.7x + 118.7 (r = 0.999) for HCA, y = 12.9x + 106.8 (r = 0.999) for DEX, and y = 12.9x + 96.8 (r = 0.999) for FCA. The LOD was 0.25, 0.20, and 0.08 µg/mL for HCA, FCA, and DEX and LOQ was 2.06, 1.83, and 1.55 µg/mL for HCA, FCA, and DEX, respectively. The recovery values of HCA, DEX, and FCA ranged from 100.7–101.3, 102.0–102.6, and 100.2–102.0%, respectively, and the relative standard deviation for precision (intra- and interday) was less than 2, which indicated repeatability and reproducibility. The novelty of the method was described by forced degradation experimentation of all analytes in the combined form under acidic, basic, oxidative, and thermal stress. The proposed method was found to be simple, rapid, and reliable for the simultaneous determination of HCA, DEX, and FCA in cosmetics.
1 Introduction
The misuse of topical corticosteroids (TCs) as cosmetics has now become a trend. Corticosteroids are frequently abused as fairness creams. This abuse and addiction of TCs especially on the face as cosmetic cream formulation have developed many skin diseases particularly dermatitis [1]. Brisk whitening effects, easy access, cheapness, inappropriate marketing, ignoring side effects, and society’s attitude toward fair skin color are considered as significant reasons for consumers (mostly females) toward the use of whitening cosmetic creams. The TCs are generally safe when used rationally while significant morbidity among people can arise if used excessively. According to the data of the World Health Organization (WHO), most pharmaceuticals are inappropriately prescribed, which leads to misuse of medication among the majority of the world population [2].
It has become evident that TC is being misused by prescribers and people use them in various parts of the globe [3]. The use of TCs over the face for skin lightening is a very common practice in Pakistan. Only after getting approval from official regulator, corticosteroids should be used in drugs. Recently, some prohibited corticosteroids have been detected in commercial cosmetics. To increase the pharmacological efficiency, steroids are being illegally incorporated into cosmetics, and such practice may lead to compromise the health of end-user due to the hazardous side effects of TCs [4]. A study revealed that in Lahore (Pakistan), many patients have suffered from acne due to the use of skin whitening commercial products containing illegal steroids especially glucocorticoids [5]. The extent of skin diseases as a result of the side effects of skin whitening agents depends on the type of chemical, source (cream, ointment, lotion, or gel), and application method [6]. Burning sensation, itching, irritation, dryness, redness at the application site, atrophy of the skin, steroidal rosacea, acne, and perioral dermatitis are commonly observed as adverse effects of the use of TCs [7].
Hydrocortisone 21 acetate (HCA) is commonly used for treating different skin disorders by its topical application [8]. It is one of those chemicals that are added in skincare cosmetics as illicit agents [9]. Regular or long-term exposure to HCA can cause hypertension and irreversible skin atrophy [10].
Dexamethasone (DEX) as glucocorticoid has been used for the treatment of inflammation, and its treatment can lead to steroid diabetes [11]. Susceptibility of skin cancer is increased due to the use of DEX. Its high concentration in cosmetics can lead to hyperglycemia and hypertension as well as malignancies [12,13].
Fluocinolone acetonide (FCA) is among the highly potent ingredients in cosmetics. It is commonly used for treating eczema in addition to a composite of commercial cosmetics [14]. The chemical structures of the studied components are shown in Figure 1.
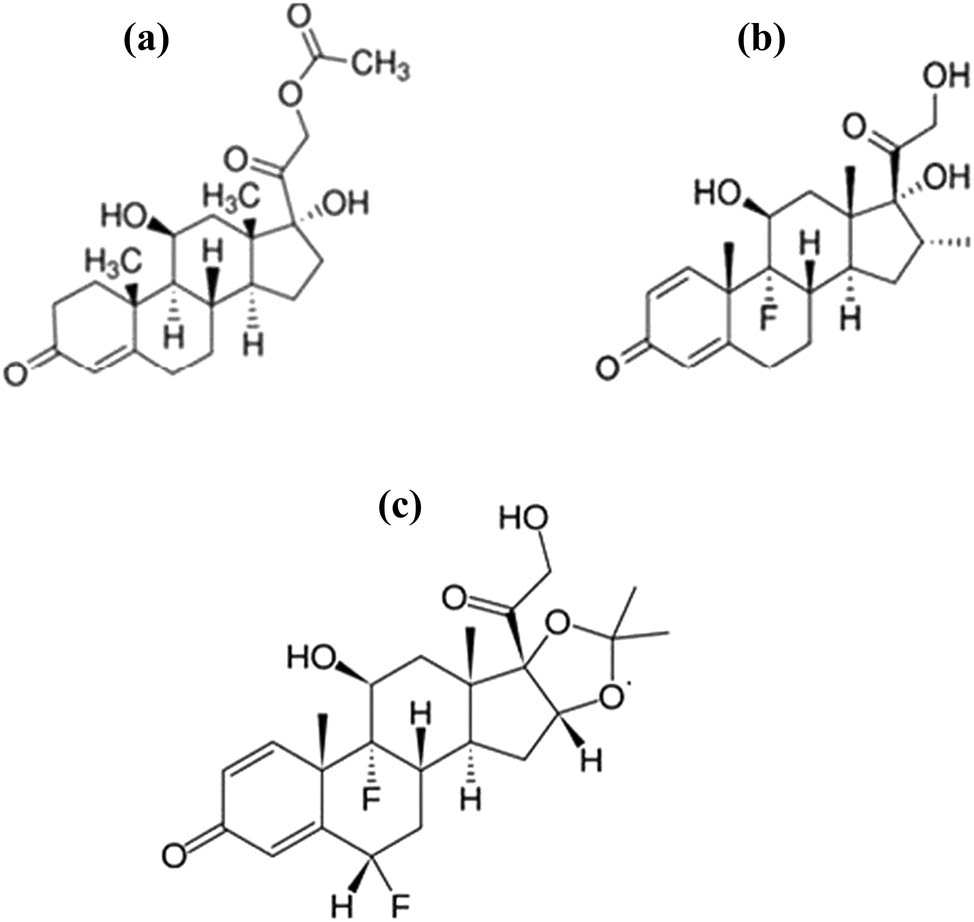
Chemical structure of (a) HCA, (b) DEX, and (c) FCA.
Literature review reveals that various analytical methods exist for rapid screening of cosmetic products especially whitening creams; however, stability-indicating high-performance liquid chromatography (HPLC) method based on forced degradation studies has been rarely reported for the quantification of the mixture of glucocorticoids (HCA, DEX, and FCA) in skin whitening creams. Therefore, the objective of this research work was to develop a stability-indicating HPLC method for the simultaneous quantification of HCA, DEX, and FCA in skin whitening commercial creams. Conditions for quick, accurate, and precise separation of analytes were developed, and the effect of forced degradation on studied components in the combined form under acidic, basic, oxidative, and thermal stress was also investigated as per International Conference on Harmonization (ICH) guidelines. The method was practically tested for the detection of HCA, DEX, and FCA in cosmetic samples.
2 Experimental
2.1 Standards and reagents
Reference standards of HPLC grade HCA, DEX, and FCA (99.99% purity) were purchased from Sigma Aldrich, New York, USA. For chromatographic analysis, HPLC grade methanol, acetonitrile, and double-distilled deionized water were also purchased from Sigma Aldrich, New York, USA, and formic acid 98–100% was purchased from Merck, Munich, Germany.
2.2 Sample collection
Thirty skin whitening cosmetic creams belonging to different local and imported brands were purchased from local markets of Lahore (Pakistan) and online. Sample codes were used to ensure the confidentiality of the manufacturers of respective creams.
2.3 Sample preparation
About 500 mg as a uniform amount of each cream was diluted using 10 mL of the solvent mixture comprising methanol, water, and acetonitrile (45:45:10 v/v). The samples were homogenized at 70°C for 20 min on a digital hot plate with stirring at 1,500 rpm. The preparations were stored at room temperature for 1.5 h. Fats and waxes were precipitated and filtered through 0.45 µm polytetrafluoroethylene syringe filters. The filtrate was refiltered and diluted to 10 mL using solvent as described above followed by spinning on the Mini spin plus (4,000 rpm) for 3 min. The supernatants were collected and used as samples for HPLC analysis.
2.4 Standard preparation
The stock solutions of HPLC grade HCA, DEX, and FCA standards were prepared with a concentration of 1,000 µg/mL in a solvent comprising methanol with 1% formic acid and double-distilled deionized water (60:40 v/v). Further serial dilutions were made from the freshly prepared stock solution to make concentrations in the range of 1–150 µg/mL.
2.5 Chromatographic conditions
All separations were carried out on GPC LC-20 AD (Shimadzu, Kyoto, Japan) with the CBM-20A module equipped with autosampler, and chromatographic software (Lab Solutions) was used for acquiring and interpretation of results. A reversed-phase column (ACE Excel 2, C18 AR) having 150 mm length, 3 mm internal diameter, and 2 µm particle size was used for the separation of analytes.
Two different combinations of mobile phase were tested to separate the sample mixtures. Initially, methanol and double-distilled deionized water were used as mobile phases in the ratio of 60:40 (v/v), respectively, and the separation result was compared with the second mobile phase comprising methanol with 1% formic acid and double-distilled deionized water in the ratio of 60:40 (v/v), respectively. The effect of temperature on the separation efficiency of the column was studied by using a different range of column incubation temperatures (25, 30, 35, 40, 45, and 50°C). The detection wavelength was also varied in the range of 210–300 nm using a diode array detector to observe maximum absorption (lambda max) of a mixture of three standards followed by their separation in the column.
After optimization of chromatographic conditions, standard and sample analysis was performed for 12 min using isocratic mode; however, the run time was decreased in accordance with the observed maximum retention time of the analytes. The flow rate was set to 0.5 mL/min, and 10 µL of the sample was injected into the HPLC system for simultaneous detection of analytes in standards and samples.
2.6 Method validation
The specificity, accuracy, precision, reproducibility, linearity, limit of detection (LOD), limit of quantification (LOQ), and robustness were determined to validate the method as per ICH guidelines [15]. The linearity of method was studied for all standards by the analysis of solutions with different concentrations (1–150 µg/mL) in triplicate. The values of LOD and LOQ were determined through the signal-to-noise ratio.
For determination of recovery and accuracy, 5, 15, and 25 µg/mL solution of HCA, DEX, and FCA standards were added in the cosmetic samples, and analysis was performed with three replicates to observe percentage recovery and relative standard deviation (RSD). Accuracy was calculated as the difference between the theoretically added amount and the practically obtained amount. While for the determination of precision, five injections of different concentrations (20, 40, and 60 µg/mL) of all standards were administered, and precision was calculated through observation of found concentrations of analytes on the same day of injection and other days (days 1, 2, and 3). Moreover, flow rate, wavelength, mobile phase composition, and column temperature were the determining factors of the robustness of the method [15].
2.7 Forced degradation studies
Forced degradation studies were performed to evaluate the stability of the proposed method by treating the studied components with various conditions of stress including acid, alkali, chemical oxidant, and heat stress. The main purpose of the stability testing was to look over how the quality of a drug product changes with respect to time under the influence of environmental factors [16]. The interference produced by the degradation products was noted. Forced degradation study in the basic medium was performed by addition of 0.1 N NaOH into 5 mL of stock solution of HCA, DEX, and FCA in a 25 mL volumetric flask and stored at ambient temperature for a period of 6 h. Then, the solution was neutralized with an acid and further diluted to a final concentration of 10 μg/mL of a mixture of HCA, DEX, and FCA. Similarly, degradation experiments were performed in the acidic medium by using 0.1 N HCl. For the purpose of oxidative degradation, the prepared standard solution was treated with 3% hydrogen peroxide (H2O2) for 6 h. For heat degradation, the standard solution was subjected to heat in the oven at 80°C for 6 h. Finally, all solutions were diluted to obtain a 10 μg/mL solution of a mixture of HCA, DEX, and FCA and injected into the system.
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Optimization of chromatographic conditions
Among different compositions of the mobile phase, the first mobile phase comprising methanol and double-distilled deionized water without formic acid showed poor separation of analytes as distortion was observed in baseline along with broad peaks (Figure 2). However, the best separation was achieved using the second combination of solvents as the mobile phase comprising methanol with 1% formic acid and double-distilled deionized water with a ratio of 60:40 (v/v). This composition of the mobile phase exhibited more polarity-based compatibility for the separation of analytes leading to sharp and more symmetrical peaks (Figure 3c). Among various ranges of temperatures (25, 30, 35, 40, 45, and 50°C), the best separation was observed at 35°C with 0.5 mL/min flow rate and 10 µL injection volume. Among different tested wavelengths in the range of 210–300 nm, three values (254, 260, and 280 nm) were selected for final testing based on maximum absorption of standards through DAD detector; however, 254 nm was found to be the optimum wavelength for the simultaneous detection of HCA, DEX, and FCA as maximum peak height and area were observed at 254 nm wavelength when compared with 260 and 280 nm wavelength detection (Figure 3a–c). The ACE Excel 2 C18 AR (150 mm × 3 mm, 2 µm) column yielded well-defined sharp peaks of both standards and sample analytes. The mixture of standards of HCA, DEX, and FCA was simultaneously eluted at 4.5, 5.5, and 6.9 min, respectively, using the above-mentioned optimized chromatographic conditions with a reduced run time of 8 min (Figure 3c).

Effect of composition of mobile phase on the separation of standards. The chromatogram is showing broad peaks and distorted baseline using a mobile phase comprising methanol and double-distilled deionized water in the ratio of 60:40 (v/v), respectively, without the addition of formic acid.
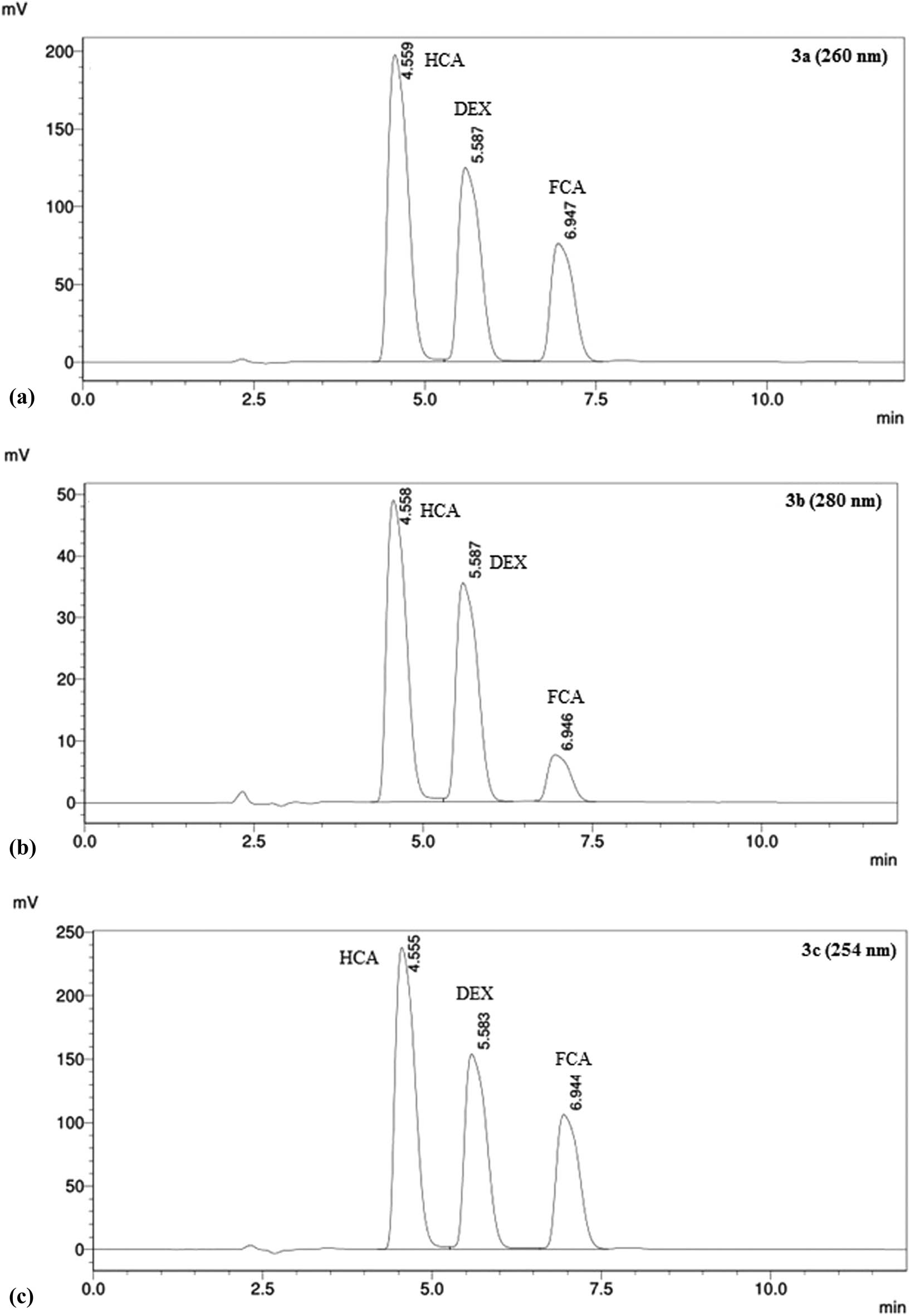
Effect of different wavelengths on detection of standards having concentration of 10 µg/mL, (a = 260 nm, b = 280 nm, c = 254 nm) using DAD detector under the same experimental conditions. More peak height and area were observed at 254 nm wavelength when compared with other wavelengths
3.2 Method validation
3.2.1 Specificity
The system suitability parameters including retention time (tR), number of theoretical plates (N), capacity factor (k′), tailing factor (Tf), and resolution (Rs) were determined, and their values indicated good specificity of the analytical method for the determination of stability of HCA, DEX, and FCA standard solutions, as per acceptance criteria listed in Table 1. The proposed method was found specific as the results of all validating parameters were in accordance with expectations for the determination of all the three analytes. HCA, DEX, and FCA were completely separated without the formation of interfering excipient peaks with the peaks of analytes, and chromatograms were free from baseline noise as shown in Figure 3c.
System suitability parameters for HCA, DEX, and FCA standard solutions (n = 5)
| Parameter | HCA | DEX | FCA | Acceptance criteria |
|---|---|---|---|---|
| Retention time (tR in min) | 4.5 | 5.5 | 6.9 | — |
| Resolution (Rs) | 6.82 | 6.94 | 6.98 | >2 |
| Tailing factor (Tf) | 0.609 | 1.223 | 1.372 | <2 |
| Capacity factor (k′) | 3.86 | 7.15 | 6.23 | >1.0 |
| Plate count (N)/m | 3,56,309 | 6,48,617 | 92,482 | >1,000 |
3.2.2 Linearity, LOD, and LOQ
A linear dynamic range of six concentrations in the range of 1–150 µg/mL of standard solutions were analyzed for the determination of linear regression values. The value of the correlation coefficient, R2, was 0.999, which shows the linearity of the developed method for respective ranges of standards. A linear calibration curve in the form of y = ax + b was obtained by plotting the peak area (y) in triplicate against the concentration (x) at different concentrations of HCA, DEX, and FCA, whereas b is the intercept and a is the slope of the calibration curve. The values of resultant parameters of regression analysis are listed in Table 2. The LOD and LOQ were determined through signal-to-noise ratio 3:1 and 10:1, respectively. The LOD of HCA, DEX, and FCA was found to be 0.25, 0.08, and 0.20 μg/mL, respectively, while LOQ of HCA, DEX, and FCA was found to be 2.06, 1.55, and 1.83 μg/mL, respectively (Table 2).
Linearity, correlation coefficient, LOD, and LOQ of standards
| Standards | Linear equation | Correlation coefficient | Slope | SE of Slope | Intercept | Standard error of intercept | Linear range (µg/mL) | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| HCA | y = 12.7x + 118.7 | 0.999 | 12.74 | 0.172 | 118.7 | 16.20 | 1–150 | 0.25 | 2.06 |
| DEX | y = 12.9x + 106.8 | 0.999 | 12.92 | 0.209 | 106.8 | 19.70 | 1–150 | 0.08 | 1.55 |
| FCA | y = 12.9x + 96.81 | 0.999 | 12.92 | 0.190 | 96.81 | 17.90 | 1–150 | 0.20 | 1.83 |
3.2.3 Recovery and accuracy
Different concentrations of standard solutions were added to the cosmetic samples to test the recovery and accuracy. The values of recovery were determined at different concentrations of standards by mean recovery and RSD as listed in Table 3. The detected amounts of standards (added in samples) were found to be significant as RSD was less than 2 which is in accordance with ICH guidelines. HCA, DEX, and FCA were recovered in the range of 100.7–101.3, 102.0–102.6, and 100.2–102.0%, respectively (Table 3). The sufficient yield of recovery in an overall range of 100.2–102.6% indicates the accuracy of the method.
Recovery and accuracy results for detection of HCA, DEX, and FCA
| Studied component | Concentration after spikinga (µg/mL) | Concentration foundb (µg/mL), mean ± SD; RSD | Recovery (%) |
|---|---|---|---|
| HCA | 10 | 10.08 ± 0.19; 1.88 | 100.8 |
| 20 | 20.15 ± 0.40; 1.98 | 100.7 | |
| 30 | 30.40 ± 0.10; 0.32 | 101.3 | |
| DEX | 10 | 10.26 ± 0.10; 0.97 | 102.6 |
| 20 | 20.40 ± 0.38; 1.86 | 102.0 | |
| 30 | 30.70 ± 0.25; 0.81 | 102.3 | |
| FCA | 10 | 10.20 ± 0.15; 1.47 | 102.0 |
| 20 | 20.05 ± 0.33; 1.64 | 100.2 | |
| 30 | 30.30 ± 0.20; 0.66 | 101.0 |
- a
Actual concentration of each standard was 5 µg/mL.
- b
Three replicates were run for each sample. The value of the RSD was less than 2%.
3.2.4 Precision, repeatability, and reproducibility
The observed results of intra- (same day) and interday (Day 1, 2, and 3) injections at different concentrations of 20, 40, and 60 µg/mL (n = 5) are listed in Table 4 indicating precision, repeatability, and reproducibility. The significant concentrations of injected standards showed reliable repeatability (intraday) and reproducibility (interday) of the developed method. The value of RSD was also acceptable for each run as per ICH guidelines.
Precision, repeatability, and reproducibility results of HCA, DEX, and FCA
| Standards | Intraday (n = 5) | Interday (n = 5) conc. found (µg/mL) ± SD; RSD | |||
|---|---|---|---|---|---|
| Conc. (µg/mL) | Conc. found (µg/mL) ± SD; RSD | Day 1 | Day 2 | Day 3 | |
| HCA | 20 | 19.93 ± 0.30; 1.51 | 19.98 ± 0.03; 0.15 | 19.96 ± 0.30; 1.50 | 19.98 ± 0.20; 1.00 |
| 40 | 39.96 ± 0.70; 1.75 | 39.92 ± 0.40; 1.00 | 39.92 ± 0.60; 1.50 | 40.05 ± 0.50; 1.24 | |
| 60 | 59.91 ± 0.20; 0.33 | 59.94 ± 0.30; 0.50 | 59.96 ± 0.30; 0.50 | 60.03 ± 0.02; 0.03 | |
| DEX | 20 | 19.97 ± 0.30; 1.50 | 19.95 ± 0.10; 0.50 | 19.93 ± 0.20; 1.00 | 19.98 ± 0.05; 0.25 |
| 40 | 39.94 ± 0.60; 1.50 | 39.97 ± 0.40; 1.00 | 39.98 ± 0.10; 0.25 | 39.95 ± 0.40; 1.00 | |
| 60 | 59.95 ± 0.50; 0.83 | 59.97 ± 0.20; 0.33 | 59.98 ± 0.40; 0.66 | 60.02 ± 0.20; 0.33 | |
| FCA | 20 | 19.92 ± 0.30; 1.51 | 19.93 ± 0.03; 0.15 | 19.96 ± 0.20; 1.00 | 20.04 ± 0.10; 0.49 |
| 40 | 39.95 ± 0.40; 1.00 | 39.96 ± 0.20; 0.50 | 39.95 ± 0.50; 1.25 | 39.99 ± 0.20; 0.50 | |
| 60 | 59.96 ± 0.20; 0.33 | 59.98 ± 0.40; 0.66 | 59.94 ± 0.30; 0.50 | 59.98 ± 0.30; 0.50 | |
3.2.5 Robustness
Experiments for robustness were performed by inducing careful changes in the chromatographic conditions. The newly developed method was found to be robust as no significant effects were observed by variation in the flow rate (±0.1 mL/min), wavelength (±2 nm), mobile phase composition (±5.0 mL), and column temperature (±5°C) as listed in Table 5.
Robustness results for HCA, DEX, and FCA
| RSD (%) | |||
|---|---|---|---|
| Conditions | HCA | DEX | FCA |
| Flow rate (0.6 mL/min) | 1.50 | 0.83 | 1.52 |
| Flow rate (0.4 mL/min) | 1.20 | 1.04 | 1.00 |
| Wave length (256 nm) | 1.75 | 0.25 | 1.25 |
| Wave length (252 nm) | 1.02 | 0.33 | 0.35 |
| Mobile phase (65:35) | 0.33 | 0.67 | 0.66 |
| Mobile phase (55:45) | 1.51 | 0.50 | 0.50 |
| Column temperature (40°C) | 1.23 | 0.99 | 1.47 |
| Column temperature (30°C) | 0.15 | 0.51 | 0.15 |
3.3 Forced degradation studies
The stability-indicating capacity of the proposed method was assessed by the forced degradation experimentation performed under different conditions of stress. The extent of degradation of HCA, DEX, and FCA by different stress conditions is tabulated in Table 6. When compared with the control (Figure 4a), under acidic stress, HCA was very slightly degraded and non-significant degradation was observed for DEX and FCA with the formation of three degradation products at the retention time of 2.33, 2.76, and 3.12 min (Figure 4b). However, alkaline treatment significantly degraded HCA, DEX, and FCA with the remaining concentration of 35, 26, and 20%, respectively, and with one degradation product observed at 2.13 min (Figure 4c). The effect of oxidative stress was significant on the degradation of HCA and FCA while DEX was slightly degraded and four degradation products were observed at the retention time of 2.35, 2.62, 2.91, and 3.68 min (Figure 4d). During thermal stress, HCA and FCA were slightly degraded when compared with the degradation of DEX, and the formation of four degradation products was observed at the retention time of 2.34, 2.53, 2.95, and 3.48 min (Figure 4e). As per the results of degradation studies, the proposed method was found to be specific for the determination of HCA, DEX, and FCA.
Results of forced degradation of HCA, DEX, and FCA by HPLC analysis; each analysis was performed in three replicates
| Nature of degradation | Time (h) | Remaining amount mean ± SD (%) | Extent of degradation | ||||
|---|---|---|---|---|---|---|---|
| HCA | DEX | FCA | HCA | DEX | FCA | ||
| 0.1 N HCl | 6 | 93.62 ± 0.22 | 89.47 ± 0.12 | 70.36 ± 0.29 | Slight | Non-significant | Non-significant |
| 0.1 N NaOH | 6 | 35.49 ± 0.16 | 26.28 ± 0.73 | 20.44 ± 0.52 | Significant | Significant | Significant |
| 3% H2O2 | 6 | 10.27 ± 0.12 | 96.57 ± 0.28 | 15.82 ± 0.17 | Significant | Slight | Significant |
| 80°C | 6 | 92.84 ± 0.31 | 83.72 ± 0.56 | 90.39 ± 0.28 | Slight | Non-significant | Slight |

Chromatograms of HCA, DEX, and FCA under (a) non-stressed condition, (b) acidic stress, (c) basic stress, (d) oxidative stress, and (e) thermal stress.
Although different methods have been described by researchers for the determination of studied components, forced degradation studies of the reported work describe its novelty in comparison to the existing procedures (Table 7).
Comparison of some existing analytical methods for quantification of HCA, DEX, and FCA in cosmetic samples with reported research work
| Technique | Sample preparation/analysis time | Mobile phase (v/v) | Column/stationary phase | Analytes/matrix | LOD | LOQ | Forced degradation studies | Reference no. |
|---|---|---|---|---|---|---|---|---|
| HPLC-UV | Solvent extraction/50 min | Methanol:water (1:1) | Reversed-phase zorbax phenyl (250 mm × 4.6 mm, 5.0 µm) | 43 analytes including HCA, DEX, FCA/oil, creams, milk, and soaps | (%) | Not reported. Qualitative analysis | No | [17] |
| 0.01 to 0.05 | ||||||||
| UHPLC | Solvent extraction/12 min | Water with 0.025 M ammonium borate buffer (pH = 10):acetonitrile | Waters acquity BEH shield RP18 (2.1 mm × 100 mm, 1.7 µm) | 12 analytes including HCA, DEX, FCA/creams, lotions, and soaps | (ng/mL) | (ng/mL) | No | [18] |
| HCA = 55.76 | HCA = 111.53 | |||||||
| DEX = 65.55 | DEX = 157.32 | |||||||
| FCA = 49.72 | FCA = 132.60 | |||||||
| HPTLC | Solvent extraction/30 min | n-Hexane:ethyl acetate (1:9) | Silica gel aluminum plate 60F254, (10 × 10 cm) | 2 analytes including FCA/ointment | (ng/spot) | (ng/spot) | Yes | [19] |
| FA = 11.54 | FA = 34.97 | |||||||
| LC/DAD/MS | Solvent extraction/27 min | Acetonitrile:methanol: water (gradient mode) | Phenomenex prodigy ODS-3, (100 mm × 2 mm, 3 µm) | 13 analytes including HCA, DEX, FCA/phytocosmetics, and ointments | No data | No data | No | [20] |
| LC-ESI-MS/MS | Solvent extraction/10 min | Water:acetonitrile (both with 0.1% formic acid | Thermo scientific hypersil gold PFP RP-UHPLC (100 mm, 2.1 mm, 1.9 μm) | 10 analytes including DEX, FCA/Gels, ointments, and creams | (mg/kg) | (mg/kg) | No | [21] |
| DEX = 0.109 | DEX = 0.117 | |||||||
| FCA = 0.093 | FCA = 0.107 | |||||||
| HPLC-PDA | Solvent extraction/28 min | 0.1% Formic acid in water:acetonitrile (gradient mode) | Waters X bridge C18 column (4.6 mm × 250 mm, 5 μm) | 6 analytes including HCA, DEX/creams | (µg/mL) | (µg/mL) | No | [22] |
| HCA = 2.003 | HCA = 6.676 | |||||||
| DEX = 1.96 | DEX = 6.54 | |||||||
| LC/MS/MS | Solvent extraction/25 min | 0.1% formic acid in water:acetonitrile (60:40) gradient mode | Atlantis T3 column (4.6 mm × 150 mm, 3 μm) | 11 analytes including HCA, DEX, FCA/creams | (ng/mL) | (ng/mL) | No | [23] |
| HCA = 1 | HCA = 3 | |||||||
| DEX = 0.25 | DEX = 0.75 | |||||||
| FCA = 1 | FCA = 3 | |||||||
| UPLC-MS/MS | Solvent extraction/25 min | 0.1% formic acid in distilled water:0.1% formic acid in acetonitrile gradient mode | Waters acquity UPLC BEH C18 column (2.0 mm × 100 mm, 1.7 µm) | 43 analytes including HCA, DEX, FCA/lotions, creams, solutions, gels, and powders | (ng/mL) | (ng/mL) | No | [24] |
| HCA = 5 | HCA = 15 | |||||||
| DEX = 10 | DEX = 30 | |||||||
| FCA = 7.50 | FCA = 22.50 | |||||||
| UPLC-TOF-MS | Solvent extraction/6 min | Aqueous ammonia 0.01%:acetonitrile | Column C18 (10 cm × 2.1 mm, 1.7 µm) | 43 analytes including HCA, DEX, FCA/cream, lotion, soap, oil, gel, and serum | (pg/mL) | No data | No | [25] |
| HCA = 732 | ||||||||
| DEX = 102 | ||||||||
| FCA = 102 | ||||||||
| HPLC-DAD | Solvent extraction/<8 min | Methanol with 1% formic acid:double-distilled deionized water (60:40) | ACE Excel 2, C18 AR column (150 mm × 3 mm, 2 µm) | 3 analytes HCA, DEX, FCA/cosmetics, creams | (µg/mL) | (µg/mL) | Yes | This work |
| HCA = 0.25 | HCA = 2.06 | |||||||
| DEX = 0.08 | DEX = 1.55 | |||||||
| FCA = 0.20 | FCA = 1.83 |
3.4 Quantification of HCA, DEX, and FCA in cosmetic samples
The applicability of the proposed method was checked by the evaluation of commercial cosmetic creams for the presence of studied components. Sharp and well-separated peaks of HCA, DEX, and FCA were obtained in sample mixtures at 254 nm. A typical chromatogram of sample analysis has been shown (Figure 5) in which all of the three analytes have been well-separated and eluted at their specific retention times in accordance with standards under the proposed optimized conditions. HCA as one of the undeclared illicit whitening agents was detected in six samples (Figure 6) with a maximum amount of 0.67 µg/mL found in C27 while the least amount was detected in C2 (0.04 µg/mL). DEX was present in 16 samples (Figure 7) with a maximum concentration of 56.38 µg/mL in C17 and a minimum concentration (0.03 µg/mL) in C9. FCA was found in 13 samples with a maximum concentration of 5.24 µg/mL (C20) and the least amount (0.09 µg/mL) in C29 (Figure 8).

Chromatogram of a typical sample (cosmetic cream) for the detection of HCA, DEX, and FCA under optimized chromatographic conditions as described in Section 3.1.
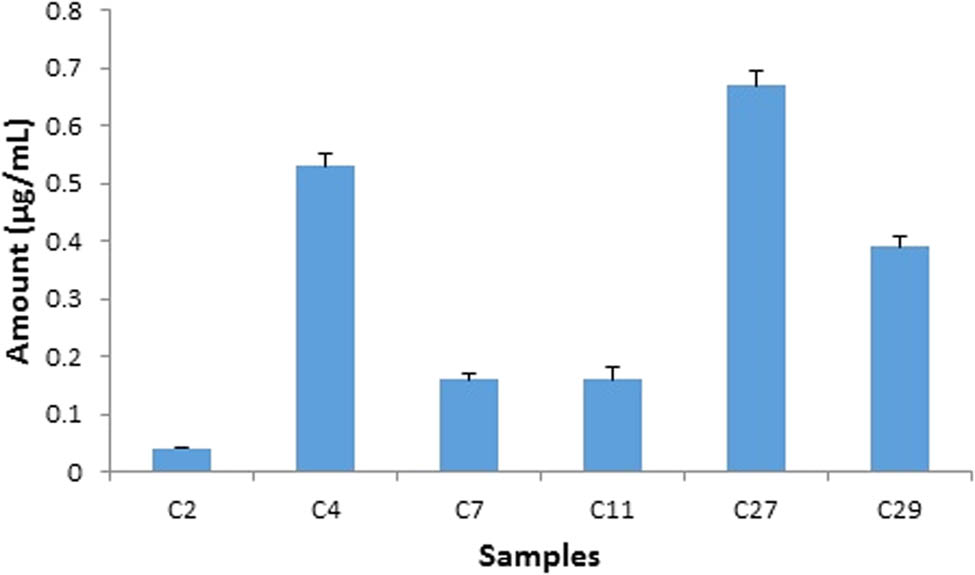
Assay result of HCA in cosmetic creams by HPLC analysis. HCA was detected in six samples (n = 30) with a maximum amount of 0.67 µg/mL (C27).

Assay result of DEX in cosmetic creams by HPLC analysis. DEX was detected in 16 samples (n = 30) with a maximum amount 56.38 µg/mL (C17).
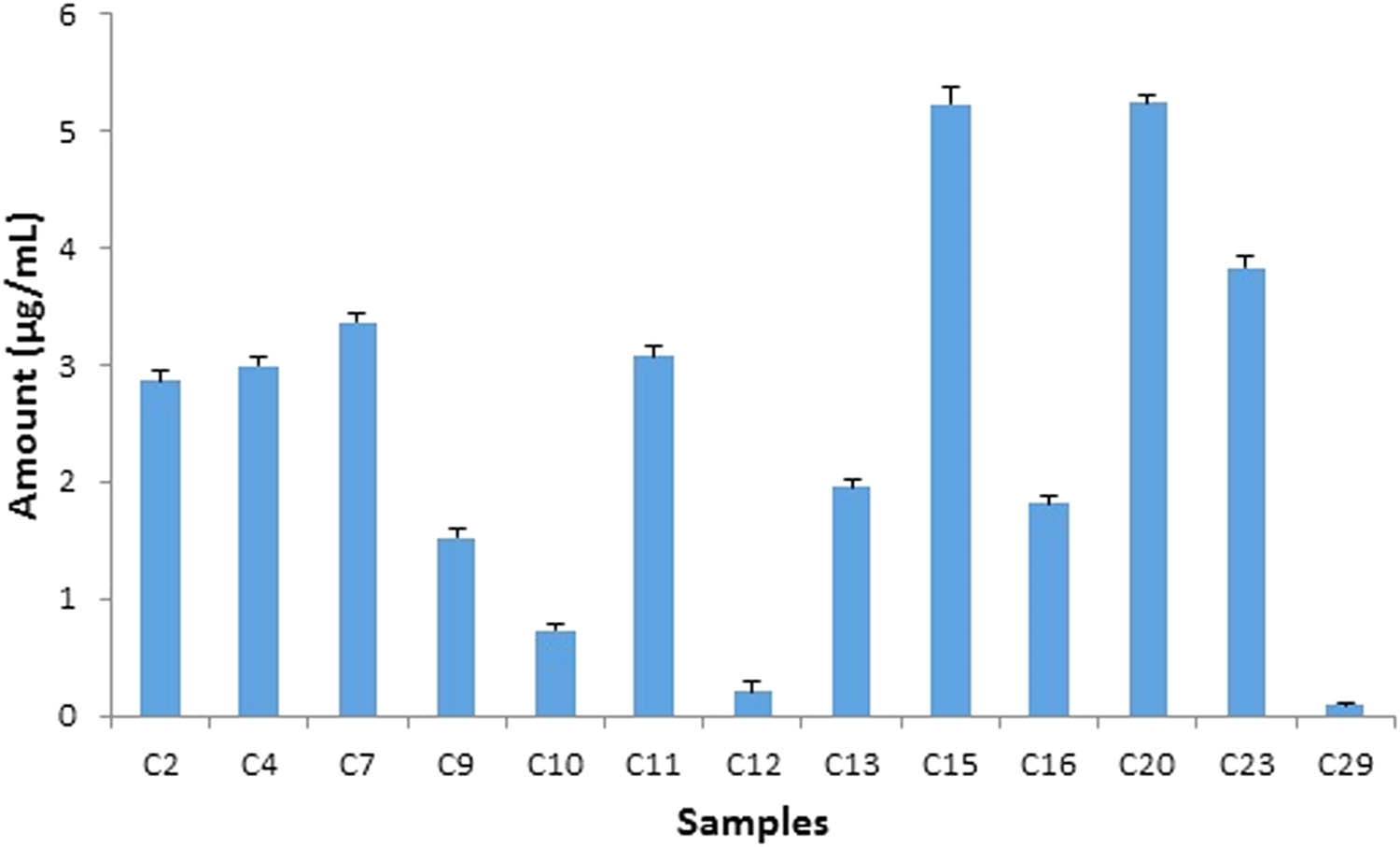
Assay result of FCA in cosmetic creams by HPLC analysis. FCA was detected in 13 samples (n = 30) with a maximum 5.24 µg/mL (C20).
4 Conclusion
A selective, sensitive, and rapid HPLC method was developed and validated for the simultaneous determination of HCA, DEX, and FCA in commercial cosmetics. All of the analytes were quantified with precision, accuracy, and robustness within 8 min. The separation of analytes was obtained with good resolution under optimized chromatographic conditions. Forced degradation behavior of the studied components in the combined form as per ICH guidelines further confirmed the stability of assay. The method was found to be suitable for the routine analysis of HCA, DEX, and FCA in commercial cosmetics as RSD of all parameters was observed within limits (<2).
Acknowledgments
This research work was funded by the Institute of Chemistry, University of the Punjab, Quaid-i-Azam Campus, Lahore, Pakistan.
Conflict of interest: The authors declare no conflicts of interest.
References
[1] Rathi SK, Kumrah L. Topical corticosteroid-induced rosacea-like dermatitis: a clinical study of 110 cases. Ind J Dermatol Venereol Lepro. 2011 Jan;77(1):42–6.10.4103/0378-6323.74974Suche in Google Scholar PubMed
[2] Yousefi N, Majdzadeh R, Valadkhani M, Nedjat S, Mohammadi H. Reasons for physicians’ tendency to irrational prescription of corticosteroids. Iran Red Cres Med J. 2012 Nov;14(11):713–8.10.5812/ircmj.2284Suche in Google Scholar PubMed PubMed Central
[3] Askari SH, Sajid A, Faran Z, Sarwar S. Skin-lightening practice among women living in Lahore; prevalence, determinants, and user’s awareness. 3rd Int. Conf. Business. Manage. 2017 Feb.Suche in Google Scholar
[4] Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clinical Immunol. 2013 Aug;9(1):30.10.1186/1710-1492-9-30Suche in Google Scholar PubMed PubMed Central
[5] Chohan SN, Suhail M, Salman S, Bajwa UM, Saeed M, Kausar S, et al. Facial abuse of topical steroids and fairness creams: A clinical study of 200 patients. J Pak Assoc Dermatol. 2016 Dec;24(3):204–11.Suche in Google Scholar
[6] Fischer DA. Adverse effects of topical corticosteroid use. Western J Med. 1995 Feb;162(2):123–6.Suche in Google Scholar
[7] Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Americ Acad Dermatol. 2006 Jan;54(1):1–15.10.1016/j.jaad.2005.01.010Suche in Google Scholar PubMed
[8] Ibrahim F, El-Deen AK, Shimizu K. Comparative study of two different chromatographic approaches for quantitation of hydrocortisone acetate and pramoxine hydrochloride in presence of their impurities. J Drug Anal. 2018 Jul;26(3):1160–70.10.1016/j.jfda.2017.12.008Suche in Google Scholar PubMed
[9] Olumide Y M, Akinkugbe AO, Altraide D, Mohammed T, Ahamefule N, Ayanlowo S, et al. Complications of chronic use of skin lightening cosmetics. Int J Dermatol. 2008 Mar; 47(4):344–53.10.1111/j.1365-4632.2008.02719.xSuche in Google Scholar PubMed
[10] Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy induced skin atrophy. Experiment Dermatol. 2006 May;15(6):406–20.10.1111/j.0906-6705.2006.00435.xSuche in Google Scholar PubMed
[11] Anitha KN, Reddy BS, Velmurugan C, Baig MA, Kumar BA. Pear fruit velocity of wound healing in dexamethasone delayed wound healing model in rats. Der Pharmacia Lettre. 2015;7(9):310–19.Suche in Google Scholar
[12] Amar MI, Shama IY, Enaia AA, Hind AE, Hager AM. Effects of various levels of oral doses dexamethasone (al-nagma) abused as cosmetic by Sudanese women on Wistar rats. J Med Sci. 2013 Aug;13(6):432–8.10.3923/jms.2013.432.438Suche in Google Scholar
[13] Uddin Siddiqui E, Qazi GI. Role of dexamethasone in meningitis. Meningitis. 2012 Mar:209–16. https://ecommons.aku.edu/pakistan_fhs_mc_emerg_med/161/.10.5772/29863Suche in Google Scholar
[14] Joshi MD, Patel S, Moin MK, Patel AK, Patel VM. Development and characterization of transdermal patch for controlled release of fluocinolone acetonide. J Club Pharmaceutic Sci. 2014 Aug;1(1):21–32.Suche in Google Scholar
[15] Guideline IH: Validation of analytical procedures: text and methodology Q2(R1). Int Conf Harmoniz. 2005 Nov;11. https://www.gmp-compliance.org/guidemgr/files/Q2(R1).pdf.Suche in Google Scholar
[16] Ivana S, Goran N, Valentina M, Ivan S. Monitoring of thermal and oxidation stability of sodium picosulfate by modified RP-HPLC method. Chem Ind Chem Eng Quar. 2010;16(1):103–10.10.2298/CICEQ090718009SSuche in Google Scholar
[17] Gimeno P, Maggio AF, Bancilhom M, Lassu N, Gornes H, Brenier C, et al. HPLC-UV method for the identification and screening of hydroquinone, ethers of hydroquinone and corticosteroids possibly used as skin-whitening agents in illicit cosmetic products. J Chromatogr Sci. 2016 Mar;54(3):343–52.10.1093/chromsci/bmv147Suche in Google Scholar PubMed
[18] Desmedt B, Rogiers V, Courselle P, De Beer JO, De Paepe K, Deconinck E. Development and validation of a fast chromatographic method for screening and quantification of legal and illegal skin whitening agents. J Pharmaceutic Biomedic Anal. 2013 Sep;83:82–8.10.1016/j.jpba.2013.04.020Suche in Google Scholar PubMed
[19] Patel AJ. Development and validation of stability-indicating HPTLC method for simultaneous estimation of fluocinolone acetonide and miconazole nitrate in ointment. Austin Chromatogr. 2014 Dec;1(5):1023–32.10.5958/2231-5675.2015.00009.5Suche in Google Scholar
[20] Hauri U, Hohl C. Determination of clandestine corticosteroids in cosmetics with LC/DAD/MS. Mitteilungen aus Lebensmitteluntersuchung und Hygien. 2004;95(5):466–76.Suche in Google Scholar
[21] Giaccone V, Polizzotto G, Macaluso A, Camilleri G, Ferrantelli V. Determination of ten corticosteroids in illegal cosmetic products by a simple, rapid, and high-performance LC-MS/MS method. Int J Anal Chem. 2017:1–12. 10.1155/2017/3531649.Suche in Google Scholar PubMed PubMed Central
[22] Rahmayuni E, Harmita H, Suryadi H. Development and validation method for simultaneous analysis of retinoic acid, hydroquinone and corticosteroid in cream formula by high-performance liquid chromatography. J Appl Pharmaceut Sci. 2018 Sep;8(9):87–92.10.7324/JAPS.2018.8913Suche in Google Scholar
[23] Golubovic JB, Otasevic BM, Protic AD, Stankovic AM, Zecevic ML. Liquid chromatography/tandem mass spectrometry for simultaneous determination of undeclared corticosteroids in cosmetic creams. Rapid Communicat Mass Spectromet. 2015 Nov;29(24):2319–27.10.1002/rcm.7403Suche in Google Scholar PubMed
[24] Kim NS, Yoo GJ, Lee JH, Park HJ, Cho S, Shin DW, et al. Determination of 43 prohibited glucocorticoids in cosmetic products using a simultaneous LC-MS/MS method. Anal Meth. 2017;9(13):2104–15.10.1039/C6AY03065CSuche in Google Scholar
[25] Desmedt B, Van Hoeck E, Rogiers V, Courselle P, De Beer JO, De Paepe K, et al. Characterization of suspected illegal skin whitening cosmetics. J Pharm Biomed Anal. 2014 Mar;90:85–91.10.1016/j.jpba.2013.11.024Suche in Google Scholar PubMed
© 2020 Saira Arif and Sadia Ata, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

