Abstract
Fisetin is a polyphenolic compound with anti-inflammatory and antioxidant properties. Inflammation and reactive oxygen species play a major role in the pathophysiology of the dry eye syndrome (DES). Patients with DES undergo symptomatic treatment using eye drops known as artificial tears. Addition of fisetin into the eye drops could result in a better recovery of the eye surface. This experimental study examines the stability of fisetin in selected eye drops (Arufil, Hypromelóza-P, Ocutein, Refresh). Absorption spectra of fisetin were measured in selected eye drops, dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS) during a period of four weeks. The fisetin absorption maximum was placed at 350 – 390 nm depending on the solvent. Good stability of fisetin solutions were observed in DMSO and deionized water. The highest stability of fisetin in selected eye drops was observed in Hypromelóza-P. Irreversible fisetin structural changes were detected in Arufil, Ocutein, Refresh and NSS. For further clinical evaluation, fisetin solution in Hypromelóza-P could be examined.
Graphical Abstract

1 Introduction
Flavonols have attracted attention as new therapeutic agents thanks to their antioxidant and anti-inflammatory characteristics. They show a broad spectrum of biological activities emerging from their structure. They possess the ability to donate hydrogen of hydroxyl groups or an electron from aromatic system and, in this manner, interact with the reactive oxygen species (ROS) in their close vicinity. Delocalization of a radical electron prevents formation of another free radical [1]. Furthermore, they work as a chelation agent. Its binding of Fe2+ leads to the reduction of Fenton reaction products (reactive hydroxyl radicals) [2]. Moreover, they serve as enzyme and transcription factor activators that play an important role in prevention and treatment of numerous diseases [3, 4, 5]. Their stability is influenced by environmental factors. Light [6], high temperature and alkaline pH enhance degradation [7]. Hydroxylation of flavonols plays a major role in their stability. Glycosylation of these hydroxyl groups also increased stability of flavonols [7]. Moreover, the presence of aromatic system causes specific coloration and enables detection of structural changes using UV-Vis spectral methods. Flavonols provide characteristic absorption bands with the maximum of approximately 380 nm.
Fisetin is a natural 3, 7, 3′, 4′ – tetrahydroxyflavone (Figure 1). It possesses wide range of bioactive properties: anti-inflammatory [8], antioxidant [9], antiviral [10], antiaging [11], anticancer [12] and neuroprotective [13]. It also affects glucose metabolism [14] and angiogenesis [15]. Hytti et al. (2017) [16] proved that fisetin has anti-inflammatory effects on retinal pigment epithelium and it also prevents ischemia-induced cell death of retinal ganglion cells [17]. Furthermore, it inhibits UV-radiation induced oxidative stress in lens epithelium [18].
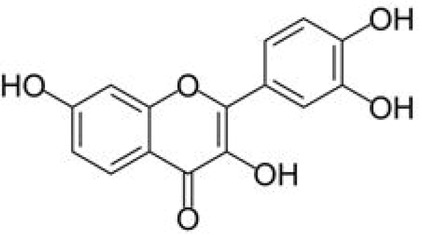
Fisetin structure. Fisetin consists of two phenyl rings and one heterocyclic ring with hydroxyl groups on C3, C7, C3′, C4′.
Currently, eye drops enriched by various antioxidants (vitamin A, C, E and others) are available. Therefore, flavonoids have started to gain attention in the treatment of ocular surface. They help to nourish avascular cornea and are used for prevention or treatment of the dry eye syndrome (DES). DES is highly prevalent worldwide and reduces life quality [19]. It has impact on daily tasks such as reading and driving and the treatment available can only ameliorate the symptoms. The more potent substance without side effects is needed to fight inflammatory processes and the formation of reactive oxygen species which takes part in pathogenesis of DES. In the eye, the fisetin anti-inflammatory effect persists in various model systems [16].
The new active pharmaceutical ingredient undergoes a series of tests. Stability is the fundamental parameter in the establishment of new agents into the pharmaceutical practice. In the field of ophthalmology, active ingredients’ stability in eye drops varies. It could be limited to several days in the case of antibiotic agents, but 30-days persistence has been observed in other types of eye drops. Stability tests include physical, chemical and microbiological assessment. Physical assessment includes olfactory and visual analysis, chemical evaluation comprises monitoring of active compound concentration, degradation products, pH and osmolality and microbiological examination where bacterial growth is excluded. After meeting these criteria, incorporation of an active ingredient and creation of eye drops is possible under the aseptic conditions required for the topical eye application products, given that an eye is a sensitive organ.
Dimethylsulphoxide (DMSO) is an aprotic clear odourless solvent which solubilizes the wide range of both polar and non-polar compounds. As a variety of pharmaceutical active agents are poorly soluble in water, the medical use of DMSO as a solvent and carrier has started to rise. It was applied therapeutically for the first time to women with interstitial cystitis and since then, it has been tested for skin, oncological and cardiovascular diseases [20]. However, despite its beneficial effect, the total amount of DMSO in a drug must be strictly determined. While the use of low DMSO content did not result in a significant negative outcome on cell viability, morphology and inflammatory status [21, 22], its high content (>10%) promoted harmful events and biological membranes disruption [23]. The effect on the eye surface is also controversial. While Altan and Oğurtan (2017) [24] showed that 40% DMSO induced reepithelization on eye burns, Galvao et al. (2014) [25] demonstrated that even 2-4% DMSO induced cell apoptosis and represents a potential risk. In the cell assays, the save and commonly used total DMSO content is under 0.5%. Careful examinations should be conducted during establishment of DMSO as an ingredient for topical eye treatments.
This study deals with the stability of fisetin dissolved in DMSO in various eye drops and represents the possible strategy of fisetin administration to the eye.
2 Methods
2.1 General Experimental Procedures
UV-Vis spectra measurements were conducted on SPECORD S300 UV VIS (Analytik Jena). Hanna Instruments HI 2211 pH/ORP Meter was used to measure pH.
Fisetin was purchased from Santa Cruz Biotechnology, Inc. (Germany). Dimethylsulphoxide was obtained from Merck KGaA (Germany), normal saline solution from Bieffe Medital (Italy).
Arufil (povidonum, benzalkonii chloridum, dinatrii edetas dihydricus, dinatrii hydrogenphosphate dodecahydrate, natrii dihydrogenphosphate dihydrate, natrii chloridum, acqua ad injectabilia) was acquired from Bausch&Lomb (USA), Hypromelóza-P (hypromellosum, dexpantenolum, benzalkonii chloridum, natrii chloridum, dinatrii edetas dihydricus, aqua ad injectabilia) from Unimed Pharma (Slovakia), Ocutein (acqua, acidum boricum, natrii chloridum, polyhexamethylene biguanid, borax, dinatrii dihydro-ethylendiamintetraacetas, natrii hyaluronas) from Simply You Pharmaceuticals (Czech republic), Refresh (natrii carboxymethelcellulosum, PURITE®) from Allegran (Ireland). All eye drops were stored according to manufacturer instruction.
Resistivity of deionized water was 10 MΩ.cm.
2.2 Preparation of fisetin solutions
The 10 mM fisetin stock solution was prepared in DMSO. Subsequently, the respective volume of eye drops and fisetin stock solution were mixed to obtain final fisetin concentrations of 25 μM and 50 μM with the total DMSO content of 0.5%. The solutions were stored in dark at ambient temperature for a period of four weeks.
2.3 UV-Vis spectrophotometry
UV-Vis spectra measurements were conducted with the following settings: Wavelength range was 200-600 nm, integration time 442 ms and the number of accumulations 10. Dark current correction was used and spectrum mode was selected. Spectra were measured in quartz cuvette with optical path length of 1 cm at 25°C. Each sample was measured on the day of preparation and then after 7, 14, 21 and 28 days and spectral changes were monitored.
2.4 Reversibility test of fisetin changes in eye drops
For testing whether the observed spectral shifts correspond to reversible or irreversible fisetin structural changes in eye drops, the spectra of fisetin in Arufil, Ocutein and Refresh were measured after 1:1 dilution of 50 μM fisetin sample with DMSO or deionized water at 25°C and in the presence of oxygen. After mixing up, a sample of 25 μM fisetin and 50% DMSO or water content were obtained and subsequent spectral changes were observed.
2.5 pH measurements and buffer solutions
Glass body refillable pH semi-micro electrode with Ag/AgCl reference was used (Hanna Instruments HI 1131 B). For pH-dependent spectral changes observations 200 mM phosphate buffers (Sigma-Aldrich, Germany) with pH 6.12 and 7.99 (23°C) were used.
2.6 Data analysis
The data analysis was performed using the OriginPro 8.5 software (OriginLab Corp.).
Ethical approval: The conducted research is not related to either human or animal use.
3 Results
During four weeks, a negligible shift appeared in the wavelength of fisetin absorbance maximum in DMSO (after 0 days: 363 nm, after 7, 14, 21 and 28 days: 362 nm), Hypromelóza-P (after 0 days: 362 nm, after 7, 14, 21 and 28 days: 360 nm) and deionized water (after 0 days: 359 nm, after 7 days: 358 nm, after 14, 21 and 28 days: 357 nm) for both fisetin concentrations and in NSS (after 0 days: 358 nm, after 7, 14, 21 and 28 days: 357 nm) for 50 mM fisetin concentration (Figure 2-3). In these solutions, fisetin shows good stability manifested also by small decrease in absorbance maximum (Table 1-2).
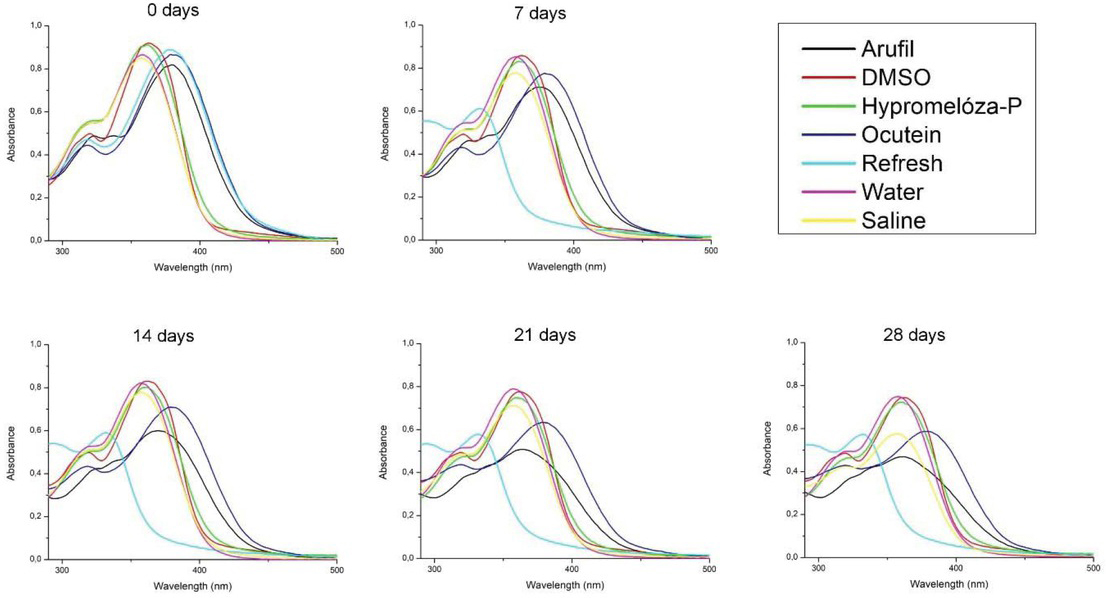
Absorption spectra of 50 M fisetin in eye drops (Arufil, Hypromelóza-P, Ocutein, Refresh), dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS) during four weeks. Fisetin was dissolved in DMSO (stock solution) and the solution was mixed with the respective volume of eye drops, DMSO, deionized water or NSS to obtain final 50 M fisetin concetration. Absorption spectra of fisetin in eye drops, DMSO, deionized water and NSS have been measured on the day of solutions preparation (0 days), after 7, 14, 21 and 28 days.
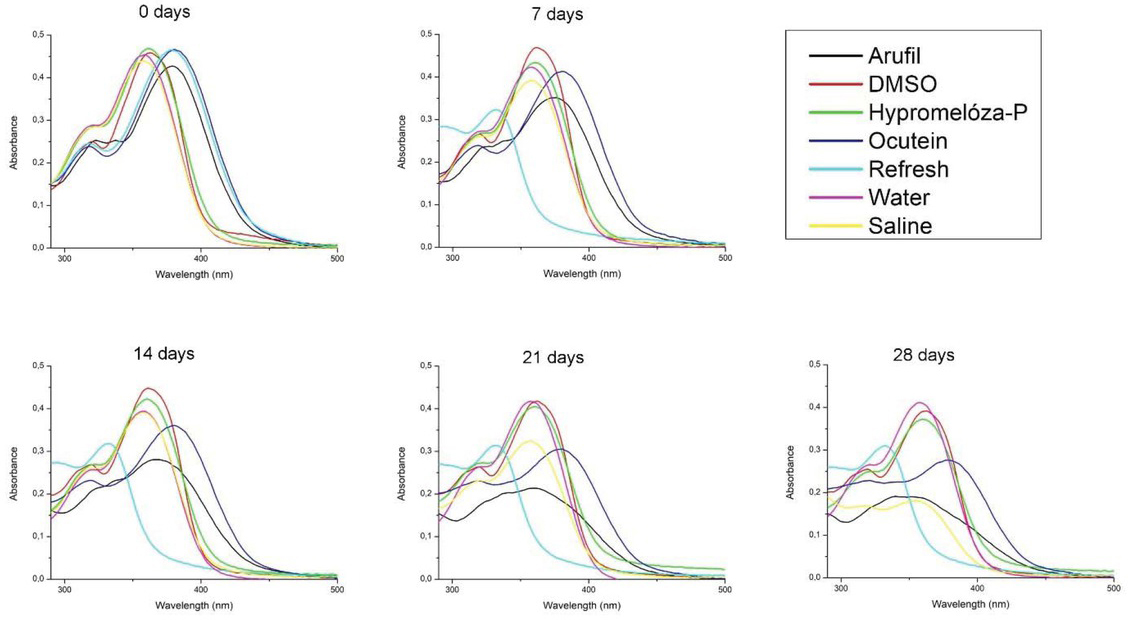
Absorption spectra of 25 M fisetin in eye drops (Arufil, Hypromelóza-P, Ocutein, Refresh), dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS) during four weeks. Fisetin was dissolved in DMSO (stock solution) and the solution was mixed with the respective volume of eye drops, DMSO, deionized water or NSS to obtain final 25 M fisetin concetration. Absorption spectra of fisetin in eye drops, DMSO, deionized water and NSS have been measured on the day of solutions preparation (0 days), after 7, 14, 21 and 28 days.
Absorbance of 50 μM fisetin main peak in the eye drops (Arufil, Refresh, Ocutein, Hypromelóza-P), dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS) after 0, 7, 14, 21 and 28 days.
| Solvent | 0 days | 7 days | 14 days | 21 days | 28 days |
|---|---|---|---|---|---|
| Arufil | 0.818 | 0.710 | 0.599 | 0.507 | 0.468 |
| DMSO | 0.919 | 0.857 | 0.829 | 0.776 | 0.743 |
| Hypromelóza-P | 0.911 | 0.830 | 0.801 | 0.747 | 0.723 |
| Ocutein | 0.866 | 0.775 | 0.709 | 0.633 | 0.586 |
| Refresh | 0.888 | 0.611 | 0.590 | 0.578 | 0.572 |
| Deionized water | 0.865 | 0.852 | 0.821 | 0.789 | 0.748 |
| Normal saline solution | 0.847 | 0.777 | 0.777 | 0.711 | 0.574 |
Absorbance of 25 μM fisetin main peak in the eye drops (Arufil, Refresh, Ocutein, Hypromelóza-P), dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS).
| Solvent | 0 days | 7 days | 14 days | 21 days | 28 days |
|---|---|---|---|---|---|
| Arufil | 0.426 | 0.351 | 0.280 | 0.213 | 0.190 |
| DMSO | 0.458 | 0.469 | 0.447 | 0.417 | 0.391 |
| Hypromelóza-P | 0.467 | 0.433 | 0.404 | 0.404 | 0.371 |
| Ocutein | 0.466 | 0.412 | 0.360 | 0.305 | 0.276 |
| Refresh | 0.464 | 0.323 | 0.321 | 0.313 | 0.309 |
| Deionized water | 0.453 | 0.422 | 0.394 | 0.417 | 0.411 |
| Normal saline solution | 0.439 | 0.391 | 0.391 | 0.323 | 0.180 |
In Arufil, Ocutein, Refresh for both fisetin concentrations and NSS for 25 mM fisetin concentration, changes in fisetin spectra shape and shifts of the absorbance maximum wavelength occurred (Figure 2-3). Fisetin absorbance spectra in Arufil, Ocutein and Refresh were red-shited for both fisetin concentrations at the beginning of the experiment (after 0 days). In the following four weeks, fisetin spectrum in Arufil changed for both fisetin concentrations. The main peak at 379 nm shifted to 378 nm after the first week, to 366 nm after the second week, to 360 nm after the third week and to 340 nm after the fourth week for 25 mM fisetin concentration. For the 50 mM fisetin concentration, the main peak at 380 nm shifted to 373 nm after the first week, to 370 nm after the second week, to 364 nm after the third week and to 362 nm after the fourth week. A new shoulder also appeared for both fisetin concentrations. Fisetin spectrum in Ocutein eye drops did not show any shifts in the absorbance maximum or the shape during four weeks for both fisetin concentrations. Fisetin spectrum in Refresh showed significant blueshift after the first week for both fisetin concentrations. The spectrum of 25 mM fisetin in NSS changed markedly after four weeks. This change was not observed for the 50 mM fisetin concentration. Furthermore, considerable decrease in the absorbance of fisetin main peak occurred in the same solvents for both fisetin concentrations (Table 1-2).
This is in accordance with the examination of reversibility of fisetin spectral changes (Figure 4). The addition of DMSO to the freshly prepared fisetin solution in Arufil, Ocutein and Refresh shifted the spectrum towards higher wavelengths in all three eye drops types. Addition of deionized water did not result in spectrum shift.
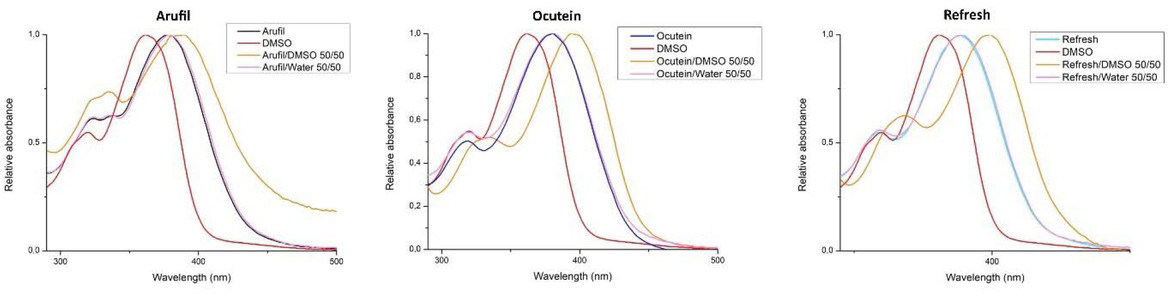
Reversibility of fisetin spectral changes. Fisetin spectra in dimethylsulphoxide (DMSO), Arufil, Ocutein and Refresh before and after addition of DMSO or water in 50/50 ratio to particular eye drops. The spectra of fisetin in Arufil, Ocutein and Refresh were measured after 1:1 dilution of 50 μM fisetin sample with DMSO or deionized water. After mixing up, the spectra of final 25 μM fisetin and 50% DMSO or deionized water content were obtained. Spectral shifts of fisetin absorption peak in Arufil, Ocutein or Refresh to wavelength relative to DMSO or deionized water were not observed suggesting irreversibility of the detected changes.
For examination of the pH effect on the fisetin structure, pH values of the solvents were measured (Table 3) together with absorption spectra of 50 mM fisetin solution in phosphate buffers at pH 6.12 and 7.99 (Figure 5). The main peak of fisetin in phosphate buffer pH 6.12 was at 357 nm and for pH 7.99 was at 381 nm.
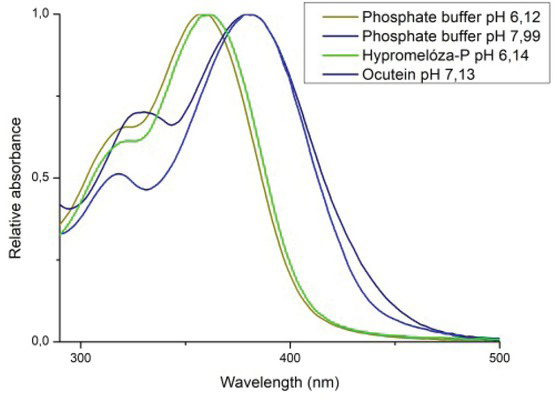
Fisetin pH-dependent spectral changes. Comparison of fisetin spectra in phosphate buffers pH 6.12, 7.99, Hypromelóza-P and Ocutein. For pH-dependent spectral changes observations, 200 mM phosphate buffers with pH 6.12 and 7.99 were used. Fisetin absorption spectra in phosphate buffer pH 6.12 and 7.99 shifted to the wavelength of Hypromelóza-P 6.14 and Ocutein 7.13, respectively.
pH values of eye drops (Arufil, Refresh, Ocutein, Hypromelóza-P), deionized water and normal saline solution (NSS).
| Solvent | pH | Temperature (°C) |
|---|---|---|
| Arufil | 7.34 | 21.6 |
| Hypromelóza-P | 6.14 | 21.6 |
| Ocutein | 7.13 | 21.5 |
| Refresh | 7.14 | 21.6 |
| Deionized water | <6 | 21.7 |
| Normal saline solution | 5.72 | 21.8 |
4 Discussion
The data concerning the therapeutic effect of natural compounds with weak solubility in aqueous solutions are inconsistent [26, 27, 28]. One of the reasons for such inconsistency is different preparation procedures with subsequent different bioavailability. In the study, we focused on fisetin which belongs to compounds weakly soluble in aqueous solutions (in water 10.45 mg/ml, in ethanol ~5 mg/ml and in dimethylsulphoxide (DMSO) (~30 mg/ml)) [29]. To dissolve fisetin in eye drops, DMSO was chosen for preparation of fisetin stock solution. Although high DMSO concentration is harmful for living organisms, a positive cellular response was observed up to 0.5 % [30]. In our formulation, the total DMSO content did not exceed this level.
The fisetin spectra in selected eye drops differed. The rationale for redshifts in absorption spectra are changed electron transitions. Fisetin consists of aromatic rings rich on π electrons, undergoes keto-enol tautomerism and forms radicals during elimination of ROS [31]. All such electron rearrangements are manifested as changes in the absorption spectrum [32, 33]. The fisetin electron changes are influenced by nearby surroundings [7, 34], therefore, solvent contribution on the electron distribution in fisetin is crucial. Solvent ionic strength, pH and polarity influence the resulting spectrum [35]. However, all eye drops used in this study were water-based, hence, their polarity did not differ significantly. Ionic strength of the eye drops is required to strictly match the tear fluid values. However, the eye can handle a wider pH range, compared to blood. Also, pH of eye drops differs, as it was shown in this study (Table 3). The most considerable fisetin spectral changes were observed in Arufil, Ocutein and Refresh eye drops with pH 7.34, 7.13 and 7.14, respectively. These values are close to fisetin pKa of OH group at C7 position (pKa = 7.27 in water environment) [36] suggesting higher concentration of deprotonated fisetin species (Figure 6) in comparison to solvents with lower pH. Friedman and Jürgens [37] explained in detail that various polyphenolic compounds are sensitive to pH changes due to their OH groups. They described the changes as time-dependent and irreversible which is in accordance with results of this study (Figure 4). Also Jurasekova et al. [38] studied fisetin at different pH using UV Vis absorption spectroscopy, confirming fisetin oxidation products at pH ≥ 9.2 by Raman and surface-enhanced Raman scattering spectroscopy (SERS). Therefore, we suppose that our conditions did not provide fisetin oxidation products, but deprotonated species.
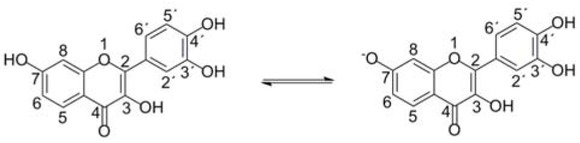
Fisetin protonated and deprotonated species. Fisetin pKa of OH group at C7 position is 7.27 in water environment. Various eye drops differ in pH values resulting in the different ratio between protonated and deprotonated fisetin species.
It is not possible to completely exclude the incompatibility of excepients interactions with fisetin. For example, boric acid, the component of Ocutein, undergoes Wilson’s reaction with flavonoids forming complexes [39]. However, we can keep out the substances of Arufil, Ocutein and Refresh which were common with Hypromelóza-P: preservative benzalkonium chloride and salts natrium chloride and natrium edeticum.
The decrease in fisetin absorbance maximum was presumably caused by a drop in fisetin concentration with time. The most significant declines in fisetin concentration (Arufil, Ocutein, Refresh, NSS) correlate with the spectral analysis changes.
Although fisetin is considered to have slightly weaker antioxidant capacity in comparison with quercetin [40], current studies stress the contribution brought by different experimental conditions. Fisetin showed more significant inhibition of lipid peroxidation in lung homogenates [41] and was the most potent senolytic agent in comparison with other 10 flavonoids [42]. Therefore, it is recommended to perform more tests for its utility as an active pharmaceutical ingredient in eye drops against dry eye syndrome.
To sum up, fisetin is a natural compound with high potential as a pharmacologically active substance. Its biological activity is dependent on its structure which undergoes changes in the environment of various solvents. In our study, we demonstrated changes in fisetin stability in the eye drops (Arufil, Hypromelóza-P, Ocutein, Refresh), dimethylsulphoxide (DMSO), deionized water and normal saline solution (NSS) during four weeks for 25 mM and 50 mM fisetin solutions. High stability of fisetin solutions (both concentrations) occurred in DMSO, deionized water and Hypromelóza-P. The irreversible structural changes were observed in Arufil, Ocutein, Refresh and NSS. For further ophthalmological clinical studies, fisetin solution in Hypromelóza-P is recommended.
Acknowledgement
The authors would like to express sincere thanks to prof. Ing. Marián Antalík, DrSc., Principal of Department of Biochemistry, for providing us with all necessary facilities. This work was supported by vvgs-2018-747, vvgs-pf-2019-1054, vvgs-2019-1071 grant, VEGA 1/0559/18 and VEGA 1/0333/20 grant.
Conflict of interest: The authors declare no conflict of interest. Research was conducted without any commercial or financial relationships to eye drops producers.
References
[1] Gil ES, Couto RO. Flavonoid electrochemistry: a review on the electroanalytical applications. Rev. Bras. Pharmacognon. 2013;23(3):542–58.10.1590/S0102-695X2013005000031Suche in Google Scholar
[2] Dimitrić Marković JM, Marković ZS, Brdarić TP, Filipović ND. Comparative spectroscopic and mechanistic study of chelation properties of fisetin with iron in aqueous buffered solutions. Implications on in vitro antioxidant activity. Dalton Trans. 2011 May;40(17):4560–71.10.1039/c0dt01834aSuche in Google Scholar PubMed
[3] Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006 Oct;103(44):16568–73.10.1073/pnas.0607822103Suche in Google Scholar PubMed PubMed Central
[4] Kim S, Choi KJ, Cho SJ, Yun SM, Jeon JP, Koh YH, et al. Fisetin stimulates autophagic degradation of phosphorylated tau via the activation of TFEB and Nrf2 transcription factors, Sci. Rep., 2016, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844953/10.1038/srep24933Suche in Google Scholar PubMed PubMed Central
[5] Wang Y, Wang B, Lu J, Shi H, Gong S, Wang Y, et al. Fisetin provides antidepressant effects by activating the tropomyosin receptor kinase B signal pathway in mice. J Neurochem. 2017 Dec;143(5):561–8.10.1111/jnc.14226Suche in Google Scholar PubMed
[6] Dall’Acqua S, Miolo G, Innocenti G, Caffieri S. The photodegradation of quercetin: relation to oxidation. Molecules. 2012 Jul;17(8):8898–907.10.3390/molecules17088898Suche in Google Scholar PubMed PubMed Central
[7] Wang J, Zhao XH. Degradation kinetics of fisetin and quercetin in solutions affected by medium pH, temperature and co-existing proteins. J Serb Chem Soc. 2016;81(3):243–53.10.2298/JSC150706092WSuche in Google Scholar
[8] Gutiérrez-Venegas G, Contreras-Sánchez A, Ventura-Arroyo JA. Anti-inflammatory activity of fisetin in human gingival fibroblasts treated with lipopolysaccharide. J Asian Nat Prod Res. 2014 Oct;16(10):1009–17.10.1080/10286020.2014.932351Suche in Google Scholar PubMed
[9] Prasath GS, Sundaram CS, Subramanian SP. Fisetin averts oxidative stress in pancreatic tissues of streptozotocin-induced diabetic rats. Endocrine. 2013 Oct;44(2):359–68.10.1007/s12020-012-9866-xSuche in Google Scholar PubMed
[10] Lani R, Hassandarvish P, Shu MH, Phoon WH, Chu JJ, Higgs S, et al. Antiviral activity of selected flavonoids against Chikungunya virus. Antiviral Res. 2016 Sep;133:50–61.10.1016/j.antiviral.2016.07.009Suche in Google Scholar PubMed
[11] Azminah OA, Hayun LE, Yanuar A. Virtual Scsreening of Indonesian Herbal Database to Find Sirtuin 1 Activators Using the Docking Method. Asian J Pharm Clin Res. 2017;10(17):158– 62.10.22159/ajpcr.2017.v10s5.23121Suche in Google Scholar
[12] Kim JA, Lee S, Kim DE, Kim M, Kwon BM, Han DC. Fisetin, a dietary flavonoid, induces apoptosis of cancer cells by inhibiting HSF1 activity through blocking its binding to the hsp70 promoter. Carcinogenesis. 2015 Jun;36(6):696–706.10.1093/carcin/bgv045Suche in Google Scholar PubMed
[13] Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO. Neuroprotective Effect of Fisetin Against Amyloid-Beta-Induced Cognitive/Synaptic Dysfunction, Neuroinflammation, and Neurodegeneration in Adult Mice. Mol Neurobiol. 2017 Apr;54(3):2269–85.10.1007/s12035-016-9795-4Suche in Google Scholar PubMed
[14] Prasath GS, Pillai SI, Subramanian SP. Fisetin improves glucose homeostasis through the inhibition of gluconeogenic enzymes in hepatic tissues of streptozotocin induced diabetic rats. Eur J Pharmacol. 2014 Oct;740:248–54.10.1016/j.ejphar.2014.06.065Suche in Google Scholar PubMed
[15] Bhat TA, Nambiar D, Pal A, Agarwal R, Singh RP. Fisetin inhibits various attributes of angiogenesis in vitro and in vivo—implications for angioprevention. Carcinogenesis. 2012 Feb;33(2):385–93.10.1093/carcin/bgr282Suche in Google Scholar PubMed
[16] Hytti M, Szabó D, Piippo N, Korhonen E, Honkakoski P, Kaarniranta K, et al. Two dietary polyphenols, fisetin and luteolin, reduce inflammation but augment DNA damage-induced toxicity in human RPE cells. J Nutr Biochem. 2017 Apr;42:37–42.10.1016/j.jnutbio.2016.12.014Suche in Google Scholar PubMed
[17] Maher P, Hanneken A. Flavonoids protect retinal ganglion cells from ischemia in vitro. Exp Eye Res. 2008 Feb;86(2):366–74.10.1016/j.exer.2007.11.009Suche in Google Scholar PubMed
[18] Yao K, Zhang L, Zhang Y, Ye P, Zhu N. The flavonoid, fisetin, inhibits UV radiation-induced oxidative stress and the activation of NF-kappaB and MAPK signaling in human lens epithelial cells. Mol Vis. 2008;14:1865–71.Suche in Google Scholar
[19] Stapleton F, Garrett Q, Chan C, Craig JP. The Epidemiology of Dry Eye Disease. Dry Eye: A Practical Approach. Berlin: Springer-Verlag; 2015. https://doi.org/10.1007/978-3-662-44106-0_210.1007/978-3-662-44106-0_2Suche in Google Scholar
[20] Madruga GM, Crivellenti LZ, Borin-Crivellenti S, Cintra CA, Gomes LG, Spiller RP. Comparative use of dimethyl sulphoxide (DMSO) in different animal species. Vet Med (Praha). 2017;62(4):179–85.10.17221/176/2015-VETMEDSuche in Google Scholar
[21] Hollebeeck S, Raas T, Piront N, Schneider YJ, Toussaint O, Larondelle Y, et al. Dimethyl sulfoxide (DMSO) attenuates the inflammatory response in the in vitro intestinal Caco-2 cell model. Toxicol Lett. 2011 Oct;206(3):268–75.10.1016/j.toxlet.2011.08.010Suche in Google Scholar PubMed
[22] Lee H, Park JB. Evaluation of the effects of dimethylsulphoxide on morphology, cellular viability, mRNA, and protein expression of stem cells culture in growth media. Biomed Rep. 2017 Oct;7(4):291–6.10.3892/br.2017.961Suche in Google Scholar PubMed PubMed Central
[23] Gurtovenko AA, Anwar J. Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J Phys Chem B. 2007 Sep;111(35):10453–60.10.1021/jp073113eSuche in Google Scholar PubMed
[24] Altan S, Oğurtan Z. Dimethyl sulfoxide but not indomethacin is efficient for healing in hydrofluoric acid eye burns. Burns. 2017 Feb;43(1):232–44.10.1016/j.burns.2016.09.026Suche in Google Scholar PubMed
[25] Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014 Mar;28(3):1317–30.10.1096/fj.13-235440Suche in Google Scholar PubMed
[26] Ma Z, Zhang Y, Li Q, Xu M, Bai J, Wu S. Resveratrol improves alcoholic fatty liver disease by downregulating HIF-1α expression and mitochondrial ROS production, PLoS ONE. 2017, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.018342610.1371/journal.pone.0183426Suche in Google Scholar PubMed PubMed Central
[27] Izdebska M, Piątkowska-Chmiel I, Korolczuk A, Herbet M, Gawrońska-Grzywacz M, Gieroba R, et al. The beneficial effects of resveratrol on steatosis and mitochondrial oxidative stress in HepG2 cells. Can J Physiol Pharmacol. 2017 Dec;95(12):1442–53.10.1139/cjpp-2016-0561Suche in Google Scholar PubMed
[28] Poulsen MK, Nellemann B, Bibby BM, Stødkilde-Jørgensen H, Pedersen SB, Grønbaek H, et al. No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes Metab. 2018 Oct;20(10):2504–9.10.1111/dom.13409Suche in Google Scholar PubMed
[29] Mehta P, Pawar A, Mahadik K, Bothiraja C. Emerging novel drug delivery strategies for bioactive flavonol fisetin in biomedicine. Biomed Pharmacother. 2018 Oct;106:1282–91.10.1016/j.biopha.2018.07.079Suche in Google Scholar PubMed
[30] Timm M, Saaby L, Moesby L, Hansen EW. Considerations regarding use of solvents in in vitro cell based assays. Cytotechnology. 2013 Oct;65(5):887–94.10.1007/s10616-012-9530-6Suche in Google Scholar PubMed PubMed Central
[31] Jash SK, Mondal S. Bioactive flavonoid fisetin a molecule of pharmacological interest. J. Org. Biomol. Chem. 2014;2:89– 128.Suche in Google Scholar
[32] Zawadiak J, Mrzyczek M. UV absorption and keto-enol tautomerism equilibrium of methoxy and dimethoxy 1,3-diphenylpropane-1,3-diones. Spectrochim Acta A Mol Biomol Spectrosc. 2010 Feb;75(2):925–9.10.1016/j.saa.2009.12.040Suche in Google Scholar PubMed
[33] Zutterman F, Louant O, Mercier G, Leyssens T, Champagne B. Predicting Keto-Enol Equilibrium from Combining UV/Visible Absorption Spectroscopy with Quantum Chemical Calculations of Vibronic Structures for Many Excited States. A Case Study on Salicylideneanilines. J Phys Chem A. 2018 Jun;122(24):5370–4.10.1021/acs.jpca.8b03389Suche in Google Scholar
[34] Lemańska K, Szymusiak H, Tyrakowska B, Zieliński R, Soffers AE, Rietjens IM. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic Biol Med. 2001 Oct;31(7):869–81.10.1016/S0891-5849(01)00638-4Suche in Google Scholar
[35] Reichardt C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem Rev. 1994;94(8):2319–58.10.1021/cr00032a005Suche in Google Scholar
[36] Herrero-Martínez JM, Sanmartin M, Rosés M, Bosch E, Ràfols C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis. 2005 May;26(10):1886–95.10.1002/elps.200410258Suche in Google Scholar PubMed
[37] Friedman M, Jürgens HS. Effect of pH on the stability of plant phenolic compounds. J Agric Food Chem. 2000 Jun;48(6):2101– 10.10.1021/jf990489jSuche in Google Scholar PubMed
[38] Jurasekova Z, Domingo C, Garcia-Ramos JV, Sanchez-Cortes S. Effect of pH on the chemical modification of quercetin and structurally related flavonoids characterized by optical (UV-visible and Raman) spectroscopy. Phys Chem Chem Phys. 2014 Jul;16(25):12802–11.10.1039/C4CP00864BSuche in Google Scholar
[39] Wilson CW. A Study of the Boric Acid Color Reaction of Flavone Derivates. J Am Chem Soc. 1939;61(9):2303–6.10.1021/ja01878a011Suche in Google Scholar
[40] Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. Interaction of flavonoids with red blood cell membrane lipids and proteins: antioxidant and antihemolytic effects. Int J Biol Macromol. 2007 Jun;41(1):42–8.10.1016/j.ijbiomac.2006.12.003Suche in Google Scholar PubMed
[41] Khanduja KL, Bhardwaj A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J Biochem Biophys. 2003 Dec;40(6):416–22.Suche in Google Scholar
[42] Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018 Oct;36:18– 28.10.1016/j.ebiom.2018.09.015Suche in Google Scholar PubMed PubMed Central
© 2020 Kristína Krajčíková et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

