The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
-
Katarína Ondreičková
, Michaela Piliarová
Abstract
Current problems with sewage sludge (SS) disposal could be solved by application to agricultural land considering its fertilizer properties and ability to improve soil condition. However, SS may contain heavy metals as well as pathogenic microorganisms. In this study, molecular analysis of partial 18S rRNA gene was used to study the impact of SS application into the soil on the genetic diversity of fungal communities, especially arbuscular mycorrhizal fungi in the rhizosphere and roots of barley. These samples were collected on three dates from the control soil without SS and from the soil with the addition of SS at the concentrations of 5 and 15 t ha−1. Fungal alpha diversity in the rhizosphere of barley was affected by SS differently than in barley roots. In addition, principal component analysis and cluster analysis revealed that fungal communities were strongly influenced by the SS addition into the soil, sample type, and the sampling date. This approach was complemented by an evaluation of the basic parameters of barley production and the response of these parameters to the presence of SS in the soil. The plant height increased with increasing SS concentration and the thousand seed weight significantly increased at the concentration of 5 t ha−1 SS but significantly decreased in 15 t ha−1.
1 Introduction
Globally, the amount of sewage sludge (SS) as a byproduct of the wastewater treatment process has been constantly increasing. Therefore, there is a serious problem with its disposal. On the contrary, there are several ways to dispose SS in the European Union (EU). Available data from Eurostat about the disposal of SS in the EU in 2018 indicate that 20.2% was applied to agricultural soil, 16.9% was composted, 13.7% was landfilled, 26.7% was incinerated, and 22.6% was disposed of through other means [1]. However, it should be noted that not all EU countries have provided data on SS disposal and therefore are not included in these statistics. In the Slovak Republic, the situation is different from this European average. In 2018, 45.5% of SS was composted, 20.2% was landfilled, 20.9% was incinerated, and 13.5% was used in other applications. In contrast to the EU, where the most SS was applied to agricultural soil, in the Slovak Republic it was 0%, and this trend remains from 2014 to the present [1]. The advantage of the application of SS to agricultural soil is the content of plant micro- and macronutrients and organic matter [2], which makes it is valuable for its fertilizing and soil conditioning properties [3]. On the contrary, SS may be a source of chemical (heavy metals) and biological contamination (thermo-tolerant coliform bacteria, fecal streptococci, and others) [3,4,5]. The concentrations of heavy metals, as well as pathogenic microorganisms, can simultaneously limit the acceptability for the application of SS to agricultural land. Therefore, its usage by farmers in the EU is defined by Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, particularly of the soil when SS is used in agriculture [6]. In 2014, this directive was evaluated in Final Report [7] which also evaluated four other waste stream directives and subsequently amended by Decision (EU) 2018/853 [8]. Certain SS materials (e.g., precipitated phosphate salts or materials exclusively obtained through the thermochemical conversion under non-oxygen and oxygen-limiting conditions [9]) may be part of the EU fertilizing products as defined by Regulation (EU) 2019/1009 of the European Parliament and the Council of 5 June 2019 [10]. This new and revised form of EU Regulation (EC) No 2003/2003 [11] extends the previous scope to secondary-raw-material-based fertilizing products. Furthermore, the European Commission describes the introduction of further measures to reduce waste and ensure that the EU has a well-functioning internal market for high-quality secondary raw materials [12]. At the same time, this Circular Economy Action Plan [12] is a part of the European Green Deal [13] that provides a roadmap for action to promote resource efficiency through a transition to a clean circular economy as well as biodiversity restoration and pollution reduction. These measures, as well as other measures not mentioned here, have been introduced by the European Commission to protect, preserve, and improve the environment in Europe for present and future generations.
As already mentioned, SS application to agricultural land appears to be a suitable solution considering its fertilizing properties and ability to improve the soil’s physical, chemical, and biological conditions [2,3,14]. Thus, sludge alters soil properties, which can subsequently affect soil microorganisms as well as plants. In general, better-quality soil usually has high microbial biomass content and enzyme activity, and so soil microorganisms can be used as indicators of soil quality [15]. Soil microorganisms play a crucial role in various biogeochemical cycles, as well as in the formation of soil structure, the decomposition of soil organic matter, and the recycling of nutrients [16]. In the rhizosphere, near the root–soil interface, there are high biological and chemical activities [17]. The plant roots excrete their products from photosynthesis as root exudates in the form of soluble sugars, amino acids, or secondary metabolites [18]. Therefore, the development of the microbial community in the rhizosphere is highly correlated with the root exudates of the host plant [17]. In addition, approximately 80% of terrestrial plant species form some type of mycorrhizal symbiosis, from which arbuscular mycorrhiza is the most widespread and predominant type [19]. Arbuscular mycorrhiza fungi (AMF) belonging to the phylum Glomeromycota [20] are obligate symbionts dependent on the host plant [21]. Plants colonized by AMF have improved resistance to environmental stresses, such as drought, cold, and pollution [22], and also are better able to overcome attacks by bacterial and fungal pathogens [23]. Moreover, they are useful in decreasing pollutants in the biosphere, including heavy metals, organic compounds, and radionuclides [24].
This study aimed to evaluate the dynamics of fungal communities, especially AMF, in the rhizosphere and roots of barley sown in soil with the addition of SS as a soil amendment at concentrations of 5 and 15 t ha−1. The molecular analysis of partial 18S rRNA gene was used for this evaluation, and we assumed that the presence of SS in the soil would affect the genetic diversity of fungal communities both in the rhizosphere and in the roots of barley. In addition, the effect of SS on selected parameters of barley production was determined. We supposed that the sludge would affect these parameters, and this would be more pronounced in the higher concentration of 15 t ha−1.
2 Materials and methods
2.1 Study description and SS used
Municipal SS used in this experiment was obtained from the wastewater treatment plant Tavos, a.s., Piešťany, Slovak Republic that collects wastewater from more than 9,000 households. This sludge was concentrated, anaerobically digested, dewatered, dried, and mechanically homogenized to a fine powder. Elemental analyses of macroelements and heavy metals (heavy metals did not exceed the limits permitted by the Directive 86/278/EEC [6]) in SS are shown in Table 1. Plants of spring barley (Hordeum vulgare, L.), cultivar Levan, were used in this experiment. The seeds were sown in the pots filled with 7 kg of arable soil supplemented with anaerobically digested SS at a concentration of 15.7 or 47.1 g of SS per pot, which corresponded to the application of SS at a concentration of 5 or 15 t ha−1, and the control plants were in soil without any SS supplement. Pots were placed in the greenhouse conditions (under natural light conditions with a photoperiod of 15 h light/9 h dark and temperature of 25°C day/18°C night) and irrigated as needed. At the same time, water-holding plates were placed under each pot to reuse the percolated water and thus to prevent the nutrients from leaching out of the pots. The soil type used was Luvi-Haplic Chernozem on loess with pH (KCl) 6.3 and a humus content of 1.77%, and the content of macroelements is shown in Table 1. The total contents of N and C were determined according to the Dumas method using a CNS analyzer (TruMac; LECO, St. Joseph, MI, USA). Other macroelements were measured by Microwave Plasma-Atomic Emission Spectroscopy (MP-AES 4100; Agilent, Santa Clara, CA, USA) after extraction from the samples by a Mehlich III solution and microwave digestion of the extracts (system Ethos 1, Milestone, Sorisole, Italy). The heavy metal content was analyzed by X-ray fluorescence spectrometry in an accredited laboratory (Department of Inorganic Analyses Laboratory, Division of Geoanalytical Laboratories, Regional Centre Spišská Nová Ves, State Geological Institute of Dionýz Štúr, Slovak Republic) with three CRMs (Certified Reference Materials) of SS with different contents of trace elements, the certificates of which were issued by the Slovak Institute of Metrology, Bratislava, Slovak Republic. Similarly, CRM GSD 12 (river sediment) was measured alongside our samples, which monitors the long-term stability of the instrument. The GSD 12 certificate was issued by the National Analysis Center for Iron & Steel, Beijing, China.
Content of macroelements in soil and anaerobically digested SS and microelements/heavy metals in SS, and the conversion of heavy metal content to 1 kg of soil supplemented with SS at the concentrations of 5 and 15 t ha−1
| Macroelements | Amount in soil (g kg−1) | Amount in SS (g kg−1) |
|---|---|---|
| N | 0.958 | 35.10 |
| P | 0.097 | 16.663 |
| K | 0.196 | 2.663 |
| C | 10.30 | 334.0 |
| Ca | 2.940 | 36.395 |
| Mg | 0.280 | 6.444 |
| Microelements/heavy metals | Amount in SS (g kg−1) | SS (g kg−1) soil in 5 t ha−1 | SS (g kg−1) soil in 15 t ha−1 | EU limit values* in SS (g kg−1) |
|---|---|---|---|---|
| As | 0.003 | 0.00001 | 0.00002 | NA |
| Cd | <0.001 | <0.000002 | <0.00001 | 0.020–0.040 |
| Cr | 0.036 | 0.00008 | 0.00024 | NA |
| Cu | 0.224 | 0.00050 | 0.00151 | 1.000–1.750 |
| Ni | 0.022 | 0.00005 | 0.00015 | 0.300–0.400 |
| Pb | 0.046 | 0.00010 | 0.00031 | 0.750–1.200 |
| Zn | 1.269 | 0.00285 | 0.00854 | 2.500–4.000 |
*Council directive 86/278/EEC of 12 June 1986 on the protection of the environment, particularly of the soil when SS is used in agriculture [6].
NA, not applicable.
2.2 Rhizosphere and root sampling
The rhizosphere and root samples from barley plants were collected in three stages of barley growth – GS29 in May, GS75 in June, and GS92 in July 2015 [25], and each sample was taken individually from separate pots. Three pots (three individual samples) were considered as controls with arable soil only, three pots (three individual samples) were supplemented with anaerobically digested SS with the concentration of 5 t ha−1, and three pots (three individual samples) with the concentration of 15 t ha−1. Therefore, nine samples from the rhizosphere and nine samples from the roots in each of the three developmental stages of barley were collected. Together 27 samples from the rhizosphere and 27 samples from the roots were analyzed. The rhizosphere was collected by taking the plants out of the soil, gently removing the soil residues from the roots, and then scraping the rhizosphere soil from the roots with a sterile scalpel without damaging the roots. The samples were then cooled and stored before analysis at 4°C. The remaining roots were gently rinsed in sterile water to remove any residual soil and dried at room temperature. On the sampling day, immediately after the roots had dried, they were used with the rhizosphere samples for DNA isolation.
2.3 DNA extraction and molecular analysis of partial 18S rRNA gene
The metagenomic DNA (mgDNA) from the rhizosphere was extracted from 0.25 g of rhizosphere samples using the PowerSoilTM DNA Isolation kit (Qiagen, Hilden, Germany). The mgDNA from roots was extracted from 0.1 g of dried roots using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). Extracted DNA from rhizosphere and roots was dissolved in 50 μL of nuclease-free water. The quantity and purity of DNA were measured spectrophotometrically with a NanoDrop-1000 Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA), and samples were diluted to the same final concentration (20 ng μL−1) and stored at −20°C. Molecular analysis of partial 18S rRNA gene using two conserved primer pairs NS1 with NS4 [26] and NS31 with AM1 [27] in terminal restriction fragment length polymorphism (T-RFLP) was performed according to our previous study [28]. The second primer pair is specific for AMF; however, it should be noted that it also amplifies DNA from Ascomycota and Basidiomycota to a limited extent [28,29,30].
2.4 Parameters of barley production
Barley plants were allowed to grow until full maturity stage (GS92). One hundred plants per treatment (control – soil without any supplement and soil supplemented with anaerobically digested SS at the concentration of 5 and 15 t ha−1) were used for the evaluation of barley production. Four parameters were evaluated: plant height in centimeters, the number of grains per plant, the weight of grains per plant in grams, and thousand seed weight in grams.
2.5 Statistical analyses
Statistically significant differences among samples were tested using analysis of variance (ANOVA) and subsequently using post hoc pairwise comparisons based on Fisher’s least significant difference (LSD) procedure at the 95% confidence level, using the software Statgraphics x64 (Statpoint Technologies, Inc., Warrenton, VA, USA). Diversity indices such as the Simpson index, Shannon’s diversity index, and Pielou evenness index for the evaluation of alpha diversity also were calculated according to our previous study [28]. Species richness, Simpson, and Shannon indices were also used for SHE analysis [31]. T-RFLP profiles of fungal communities in different samples were subsequently used for principal component analysis (PCA) using the scores of the first two principal components, for the scree plot, and also for neighbor-joining cluster analysis with Euclidean distance measure. Euclidean distance measure with 9999 permutations was also used for PERMANOVA analysis using scores from the first six principal components. SHE analysis, PCA, cluster analysis, and PERMANOVA were evaluated using PAST (PAleontological Statistics) software version 3.19 [32]. Graphical multifactorial ANOVA was made using the scores of principal components 1 (PC1) and 2 (PC2) from the PCA. Analysis of means plot with 95% decision limit was made using values from parameters of barley production. Multifactorial ANOVA and analysis of means plot were created using the software Statgraphics x64 (Statpoint Technologies, Inc., Warrenton, VA, USA).
3 Results
3.1 The influence of SS on the alpha diversity in the rhizosphere and roots of barley
Fungal alpha diversity in the rhizosphere and roots of barley was evaluated as three diversity indices: Simpson, Shannon, and Evenness, in May, June, and July 2015 (Figure 1). The alpha diversity because of SS was otherwise manifested in the rhizosphere and otherwise in barley roots during 3 months. In the rhizosphere in May, the alpha diversity remained statistically unchanged because of the SS concentration, while in June the SS caused a statistically significant decline in Shannon and between control and 5 t ha−1 SS in Simpson diversity. Subsequently, in July, the Simpson and Shannon indices were statistically stable in the rhizosphere, but Evenness had a significant downward trend because of the SS concentration. On the contrary, in barley roots, the Simpson and Shannon indices in May significantly increased with increasing SS concentration. In June, diversity in the roots was not affected by the presence of SS in the soil but subsequently in July, the Simpson and Shannon diversity were statistically decreased in barley roots with SS. Evenness in the roots in all three dates was without differences between the control and SS samples. Comparing the diversity differences between rhizosphere and root, a statistically significant difference was detected in seven cases (Figure 1). In general, the mean values of Simpson and Shannon diversity were statistically higher in the rhizosphere than in the roots, but Evenness in July was statistically higher in the roots. There was a significant difference twice in May between control samples in the Simpson and Shannon diversity indices, and five times in July in all three indices but only between samples with the addition of SS to the soil.

The violin and box plots of alpha diversity indices ((a) Simpson, (b) Shannon, and (c) Evenness) of fungal communities detected in the rhizosphere (RH, gray) and root (RT, white) of barley in control (RH0/RT0) and samples with SS at concentrations of 5 t ha−1 (RH5/RT5) and 15 t ha−1 (RH15/RT15). The different lowercase and capital letters denote the statistically significant differences among samples in the rhizosphere and root, respectively; values and corresponding letters indicating statistical significance go in sequence a/A < b/B < c/C; each sampling date and differences between RH and RT were evaluated separately (LSD, α = 0.05); *statistically significant difference between RH and RT (LSD, α = 0.05).
SHE analysis showed that samples from the rhizosphere and roots of barley contained substantially homogeneous fungal populations despite the presence or absence of SS in the soil and sampling date. In the rhizosphere, there was a gradual increase in species and diversity with an increasing number of samples, but Evenness declined subtly (Figure 2a). In barley roots, the number of species and the diversity increased with an increasing number of samples, while the Evenness remained at the same level. Furthermore, the curves that correspond to ln S, H, and ln E from roots did not run as smoothly as the curves from the rhizosphere (Figure 2b).

The SHE analysis of fungal diversity for rhizosphere (a) and root (b) of barley. ln S, natural logarithm of species richness; ln E, natural logarithm of evenness; H, Shannon diversity index; ln N, natural logarithm of counted individuals (in our case the height of fluorescence in individual OTUs).
3.2 The influence of SS on the composition of fungal communities in the rhizosphere and roots of barley
Based on PCA, samples from the rhizosphere and roots of barley were mostly separated from each other, although a small overlap between the two groups was recorded (Figure 3). Fungal communities from barley roots were slightly more similar based on PC1 than rhizosphere fungal communities. Furthermore, PC1 divided only four samples from roots and root samples were mostly distributed in the left side from this first axis. As the similarity or difference of the fungal communities could not be evidently demonstrated from the PCA plot between the rhizosphere and root, or between the controls and SS concentrations, or among the three sampling dates, ANOVA using these three factors (sample type, SS concentration, and date) was performed using the scores for PC1 and PC2 (Figure 4). It is obvious from the multifactorial ANOVA that PC1 and PC2 significantly divided rhizosphere fungal communities from root fungal communities as well as fungal communities in control samples from fungal communities in soil with SS addition. Regarding the sampling date, PC1 statistically separated May from June and July, but PC2 separated May and June from July.

The PCA constructed from fluorescent data of fungal communities from the rhizosphere and roots of barley collected on three sampling dates in control samples and samples with SS at concentrations of 5 and 15 t ha−1. PCA graph explained a total of 39.2% of the variability in the data.
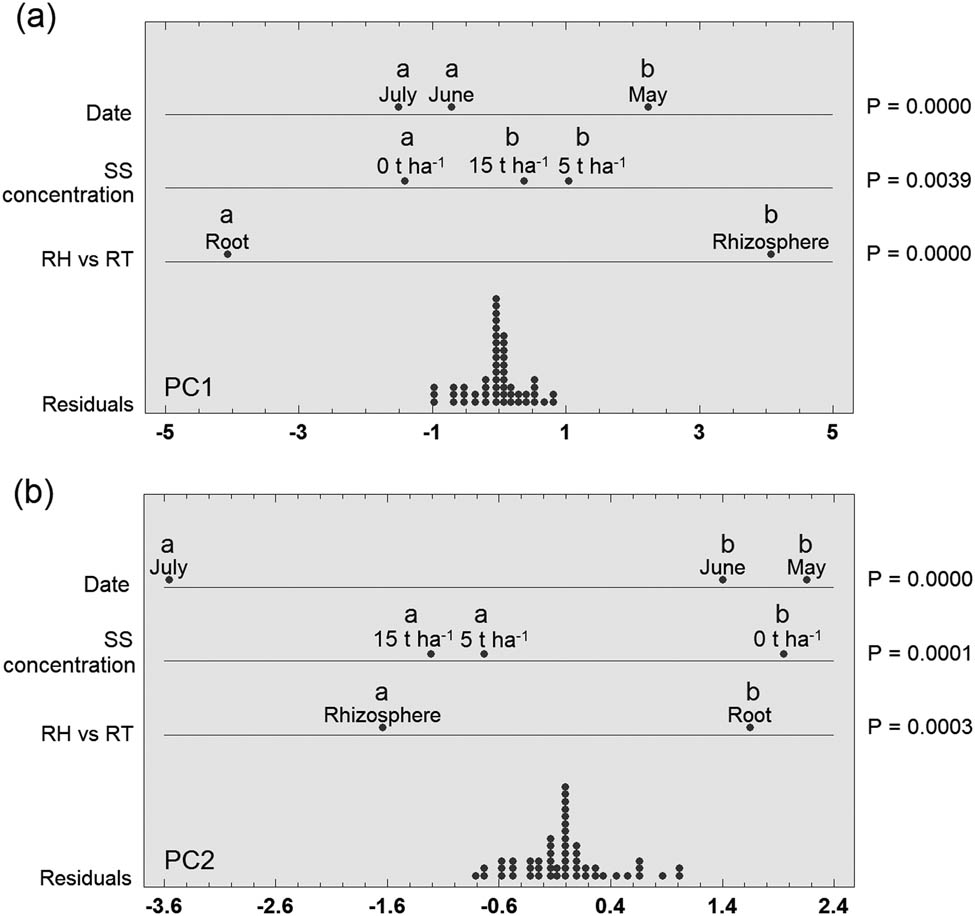
Effect of sewage sludge concentration, sample type, and sample date on the composition of fungal communities in the rhizosphere and roots of barley. Graphical multifactorial ANOVA for principal component 1 (PC1; a) and principal component 2 (PC2; b) derived from PCA in Figure 3. The different letters denote statistically significant differences among samples (LSD, α = 0.05). RH, rhizosphere; RT, root; SS, sewage sludge.
The previous PCA resulted in 53 principal components expressing a specific degree of variability. PC1 determined the greatest variability, which decreased as the number of components increased (Figure 5). However, not all these components were significant. Based on the scree plot, it could be assessed that the first six components were significant for a thorough evaluation of the PCA. PC1–PC6 explained a total of 61.7% of the variability and are sufficient for this evaluation. Therefore, scores of the first six significant components were used to determine the impact of two factors (rhizosphere vs root, SS concentration) on the genetic diversity of fungal communities using two-way PERMANOVA (Table 2). It was confirmed that both evaluated factors had a significant impact on the genetic diversity of fungal communities. The date as a factor was evaluated separately using one-way PERMANOVA and also, in this case, a statistically significant difference was confirmed (P = 0.0001, data not shown).
![Figure 5
Influence of SS concentration on the composition of fungal communities in the rhizosphere and roots of barley. Scree plot indicating the percentage of eigenvalues for all 53 principal components (dotted line) derived from PCA from Figure 3. Eigenvalues under dashed line may represent nonsignificant components [33]. The first six significant components explained a total of 61.7% of the variability.](/document/doi/10.1515/biol-2021-0024/asset/graphic/j_biol-2021-0024_fig_005.jpg)
Influence of SS concentration on the composition of fungal communities in the rhizosphere and roots of barley. Scree plot indicating the percentage of eigenvalues for all 53 principal components (dotted line) derived from PCA from Figure 3. Eigenvalues under dashed line may represent nonsignificant components [33]. The first six significant components explained a total of 61.7% of the variability.
Results of the two-way PERMANOVA calculated from the obtained data using the first six significant component scores derived from the PCA in Figure 3 and the scree plot in Figure 5
| Similarity index | Euclidean distance |
|---|---|
| Permutation N | 9999 |
| P-value | |
| RH vs RT | 0.0001 |
| SS concentration | 0.0248 |
| Interaction | 0.2095 |
RH, rhizosphere; RT, root; SS, sewage sludge.
Further analysis was done to determine the effect of SS on the fungal genetic diversity in the rhizosphere and barley roots, but the sampling date was removed as a factor. Cluster analysis separated rhizosphere samples from roots and also control samples from samples with SS addition into the soil (Figure 6). However, the fungal communities were not separated based on the amount of SS in the soil. Fungal communities in control samples from barley roots formed a separate cluster, while the three rhizosphere control samples did not form a separate cluster. One control sample from the rhizosphere was on a separate cluster, although it was located near the other controls.
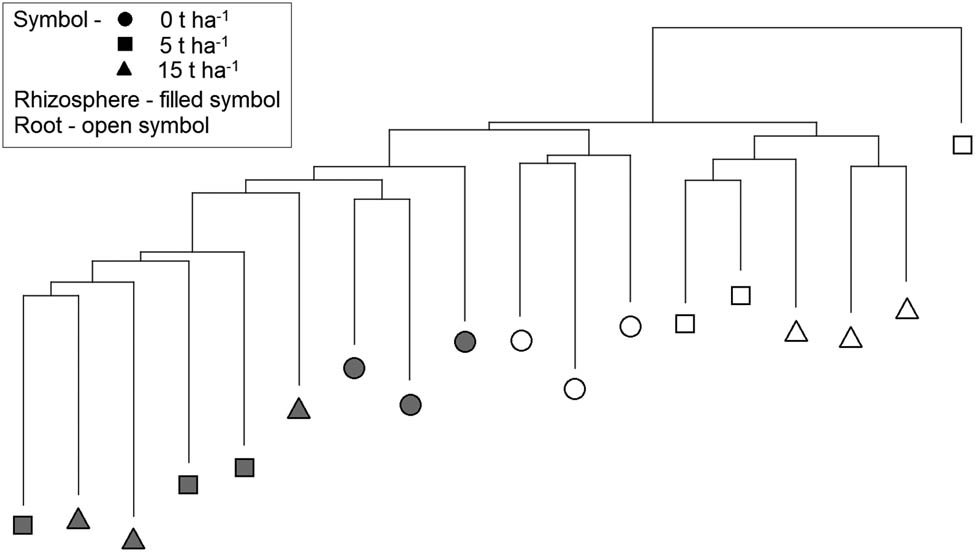
Neighbor-joining cluster analysis with Euclidean distance measure constructed from fluorescent data of fungal communities from the rhizosphere and roots of barley collected in control samples and samples with SS at concentrations of 5 and 15 t ha−1.
3.3 The influence of SS on the barley production parameters
Barley production was evaluated by four parameters: plant height, number of grains per plant, the weight of grains per plant, and thousand seed weight (Figure 7). Plant height was significantly affected by SS concentration and plants were clearly the largest at the concentration of 15 t ha−1. The number of grains per plant and the weight of grains per plant were not statistically affected by the SS concentration. However, there was a trend that these two parameters decreased at the concentration of 15 t ha−1 compared to the control samples. The last parameter, thousand seed weight, was influenced by the presence of SS in the soil at the concentration of 5 t ha−1. Interestingly, this parameter at 5 t ha−1 was not statistically different from the control samples but differed from the samples at the 15 t ha−1 concentration. Besides, the value of this parameter at 15 t ha−1 was the lowest and well below the overall average (central limit, Figure 7).

Analysis of means plot with 95% decision limit for the effect of SS concentration (0, 5, and 15 t ha−1) on parameters of barley production including plant height, number and weight of grains per plant, and thousand seed weight. The different letters denote statistically significant differences among samples (LSD, α = 0.05). UDL, upper decision limit; CL, central limit; LDL, lower decision limit.
4 Discussion
Using biological approaches to determine the ecological effects of land application of municipal SS has been preferred widely for decades [4,28]. Alpha diversity [34] indicates the diversity that exists within a sample/location, and it is measured by the number of species or the richness of species but other diversity indices are often used, such as Shannon and Simpson [35]. The results of the present study indicate that the application of sludge to the soil influenced the alpha diversity of fungal communities in the rhizosphere and barley roots differently. The Simpson and Shannon indices from the rhizosphere of barley collected on three sampling dates had the following significant trend from control to sludge samples: without change (May) – downward trend (June) – without change (July). In the roots of barley, these indices were as follows: upward trend (May) – without change (June) – downward trend (July). These figures evidence that in the final tillering stage (GS29) [25] in May, the root fungal diversity was significantly affected by the presence of SS in the soil, which caused an increase in the diversity in barley roots compared to the control. Subsequently, in the barley late milk development stage (GS75) [25] in June, the SS significantly decreased fungal diversity in the rhizosphere but was without a change in the roots. On the contrary, in the barley final ripening stage (GS92) [25] in July, the SS significantly decreased the root fungal alpha diversity compared to the control. Similarly, the impact of SS spreading under field conditions on AMF species in the soil and within root systems of Medicago truncatula was studied by Jacquot-Plumey et al. [36], who detected the different impact of SS on AMF diversity between the soil and the root systems of M. truncatula. While in the soil the effect of composted SS on AMF diversity was manifested by its increase, the diversity of AMF in the roots was lower in the presence of SS and significantly higher in the control. In addition to SS such as in our case, high ash applications also enhanced mycorrhizal status in barley compared to the control and triple superphosphate application [37]. Figueiredo et al. [38] and Yusif et al. [39] came to a similar finding when they used SS biochar, which increased the mycorrhizal colonization of corn roots in relation to the control [38] or enhanced root colonization in tomato genotypes [39]. Even, increasing the heavy metal content in the soil, which often occurs following SS application, may increase mycorrhizal colonization of the plant roots [40,41].
Shannon information function (H), species richness (S), and Evenness (E) also can be used in ecological studies for SHE analysis, which examines the relationship among S, H, and E in the samples [31]. In this case, H = ln S + ln E and this decomposition formula expresses that relationship in one plot with three variables/curves that are plotted against the abundance (N) of the sample [42,43,44]. At the same time, these variables form linear trends on a log scale, and when N accumulates with each sample, S usually increases [42]. There are several ways in which the curves for H and ln E can proceed (increasing, constant, or decreasing trends) but simultaneously, any departures from linear trends indicate a mixture of communities [42,45]. In this study, SHE analysis showed that rhizosphere fungal communities formed more homogenous communities than those in the roots. On the contrary, it should be noted that although the curves for ln S, H, and ln E from the roots did not run as smoothly as those from the rhizosphere, they still maintained a linear trend on a log scale without departures. These results suggest that, from an ecological perspective, fungal communities from roots and the rhizosphere formed homogeneous communities even in the presence of SS in the soil at two different concentrations.
The PCA and cluster analysis revealed that fungal communities were strongly influenced by the SS addition to the soil, sample type, and the sampling date. Generally, SS is composed of organic compounds, micro- and macronutrients, non-essential trace metals, organic micropollutants, and microorganisms [3]. Therefore, SS added to the soil can modify soil structure, moisture, porosity, humus content, pH, electrical conductivity, or cation exchange capacity. [4,46,47,48]. Besides, SS also changes soil microbial communities [4,49,50], which was the case with our study as well. In particular, several studies have focused on monitoring changes in the dynamics of the microbial community because of the addition of sludge containing higher concentrations of potentially toxic elements/heavy metals such as Cu, Zn, Cd, and others [51,52,53,54,55,56,57,58]. Interestingly, Gomes et al. [54] found that soil fungal communities were influenced by the quality and amount of SS soil application but not by Cd and Zn at higher concentrations. Similar results were also obtained by Anderson et al. [53] who observed that sludge type had the greatest effect on the soil fungal communities rather than SS rich in Cd, Cu, or Zn. Likewise, the results of Lloret et al. [59] were interesting, in a comparative study of the effect of SS addition on two different sludge stabilization processes on soil bacterial, archaeal, and fungal communities, while heavy metal concentrations were below the limits set by the EU. From various fungal communities, only the relative abundance of Glomeromycota was significantly increased in all amendments. Our current results regarding the addition of SS to the soil and the observed changes in the fungal communities in the rhizosphere and roots of barley are in agreement with these abovementioned studies. On the contrary, we previously observed different results in our parallel experiment with the same concentrations of SS added to the soil and monitoring the dynamics of the fungal communities in the Arundo donax rhizosphere [28]. In this published paper, we used the same methodology and similar statistical evaluation, but the SS did not cause a shift in the overall rhizosphere fungal communities through PCA and analysis of similarities (ANOSIM) analysis. Only sequencing of 18S rDNA showed that more various fungal taxa were detected in the sample with SS than in the control. We assume that these different results between our two experiments were mainly because of the plant species, i.e., barley (Hordeum vulgare, L.) vs A. donax. This assumption reflects the fact that plant roots release a wide range of chemicals in the form of root exudates into the soil, which subsequently determine the plant–microbe interaction in the rhizosphere [60]. Furthermore, the quantity and quality of root exudates depend on the plant species, plant developmental stage, and various biotic and abiotic factors. Together, all these factors play a pivotal role in determining specifically the strength and type of microorganisms present in the rhizosphere [18,61].
Currently, there is an increasing interest worldwide in the use of SS in agriculture because of the possibility of recycling valuable components such as organic matter, N, P, and other plant nutrients [4]. A minor part of the current study was monitoring the effect of SS as a soil amendment on some selected parameters of barley production. The plant height increased with increasing concentration of SS. In general, the addition of SS to agricultural soil increases the growth and production of cultivated plants [3]. SS also increased yield parameters of barley and also enhanced protein content compared to unfertilized soil [62] as well as growth and N uptake [63]. Kępka et al. [64] observed that spring barley yield was mainly influenced by N coming from the SS applied. At the same time, they mentioned that their applied SS at a concentration of 5.34 t ha−1 dry matter met the nutrient requirements of N by spring barley, and along with the applied concentration of SS, more than 118 kg N ha−1 was introduced. Antolín et al. [47] investigated the effects of SS on the relationships between barley physiology and some soil properties during a 3-year period. They detected that repeated yearly application of SS to barley crops resulted in increased grain and dry matter yields and leaf protein concentrations. Application of SS also improved soil chemical, microbiological, and biochemical properties, which were reflected in an increase in barley yield. However, they detected a significant increase in heavy metal concentrations in barley grains. Similarly, Fernandez et al. [65] evaluated the effects of composted and thermally dried SSs with different frequencies (single or yearly applications) and at two application concentrations (20 and 80 t ha−1) on the yield of barley during a 3-year period. They observed that in the cumulative experiment high concentrations of both SSs caused a significant decrease in crop yield, but in contrast, cumulative applications of both types of SS at low concentrations showed, in general, better barley yield parameters. Moreover, Eid et al. [66] studied the impact of different SS concentrations (10, 20, 30, 40, and 50 g kg−1) on soil properties and barley yield. The best results with enhanced barley growth were achieved at the 40 g kg−1 concentration of SS, while all barley growth parameters were decreased at the concentration of 50 g kg−1. We observed a similar effect of different SS concentrations on one of the measured barley parameters – thousand seed weight. This parameter was significantly increased at the SS concentration of 5 t ha−1 but significantly decreased at 15 t ha−1. Eid et al. [66] explained these findings result from the fact that the high concentration of SS is composed of high levels of some heavy metals that may affect plant metabolic activities as well as plant growth [67,68,69].
5 Conclusions
The present study showed that short-term application of SS to the soil at the concentrations of 5 and 15 t ha−1 affected fungal communities, especially AMF in the rhizosphere and roots of barley. Both concentrations of SS affected these fungal communities to a comparable extent without a significant difference between them, while the yield of barley was positively affected by SS at the concentration of 5 t ha−1. However, other similar studies need to be conducted to understand the interactions and feedback between the application of SS to the agricultural soil and the structure and function of microbial communities in the rhizosphere and plant roots to develop an optimal and safe use of SS in agriculture.
-
Funding: This work was supported by the Slovak Research and Development Agency under the contract numbers APVV-17-0150 and APVV-14-0055 and by the Operational Programme Research and Development: “Development and installation of lysimeters equipment for the rational farming on land in sustainable crop production” (ITMS 26220220191).
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] ec.europa.eu [Internet]. Eurostat: the Statistical Office of the European Union; c2020 [cited 2020 Nov 2]. Available from: https://ec.europa.eu/eurostatSuche in Google Scholar
[2] Moffet BF, Nicholson FA, Uwakwe NC, Chambers BJ, Harris JA, Hill TCJ. Zinc contamination decreases the bacterial diversity of agricultural soil. FEMS Microbiol Ecol. 2003;43(1):13–9.10.1111/j.1574-6941.2003.tb01041.xSuche in Google Scholar PubMed
[3] Singh RP, Agrawal M. Potential benefits and risks of land application of sewage sludge. Waste Manage. 2008;28(2):347–58.10.1016/j.wasman.2006.12.010Suche in Google Scholar PubMed
[4] Usman K, Khan S, Ghulam S, Khan MU, Khan N, Khan MA, et al. Sewage sludge: an important biological resource for sustainable agriculture and its environmental implications. Am J Plant Sci. 2012;3(12):1708–21.10.4236/ajps.2012.312209Suche in Google Scholar
[5] Kumar V, Chopra AK, Kumar A. A review on sewage sludge (biosolids) a resource for sustainable agriculture. Arch Agric Environ Sci. 2017;2(4):340–7.10.26832/24566632.2017.020417Suche in Google Scholar
[6] EUR-Lex. Council Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off J Eur Commun. 1986;L181:6–12.Suche in Google Scholar
[7] ec.europa.eu [Internet]. European Commission: ex-post evaluation of certain waste stream directives – final report; c2020 [cited 2020 Aug 3]. Available from: https://ec.europa.eu/environment/waste/pdf/target_review/Final%20Report%20Ex-Post.pdfSuche in Google Scholar
[8] EUR-Lex. Decision (EU) 2018/853 of the European Parliament and of the council of 30 May 2018 amending regulation (EU) No 1257/2013 and directives 94/63/EC and 2009/31/EC of the European Parliament and of the council and council directives 86/278/EEC and 87/217/EEC as regards procedural rules in the field of environmental reporting and repealing council directive 91/692/EEC. Off J Eur Commun. 2018;L150:155–61.Suche in Google Scholar
[9] Huygens D, Saveyn HGM, Tonini D, Eder P, Delgado Sancho L. Technical proposals for selected new fertilising materials under the fertilising products regulation (regulation (EU) 2019/1009) – process and quality criteria, and assessment of environmental and market impacts for precipitated phosphate salts and derivates, thermal oxidation materials and derivates and pyrolysis and gasification materials, EUR 29841 EN. Luxembourg: Publications Office of the European Union; 2019.Suche in Google Scholar
[10] EUR-Lex. Regulation (EU) 2019/1009 of the European Parliament and of the council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending regulations (EC) no 1069/2009 and (EC) no 1107/2009 and repealing regulation (EC) no 2003/2003. Off J Eur Commun. 2019;L170:1–114.Suche in Google Scholar
[11] EUR-Lex. Regulation (EC) no 2003/2003 of the European Parliament and of the council of 13 October 2003 relating to fertilisers. Off J Eur Commun. 2003;L304:1–194.Suche in Google Scholar
[12] ec.europa.eu [Internet]. European Commission: a new circular economy action plan for a cleaner and more competitive Europe; c2020 [cited 2020 Aug 3]. Available from: https://ec.europa.eu/environment/circular-economy/pdf/new_circular_economy_action_plan.pdfSuche in Google Scholar
[13] ec.europa.eu [Internet]. European Commission: the European green deal; c2020 [cited 2020 Aug 3]. Available from: https://eur-lex.europa.eu/resource.html? uri = cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format = PDFSuche in Google Scholar
[14] Skowrońska M, Bielińska EJ, Szymański K, Futa B, Antonkiewicz J, Kołodziej B. An integrated assessment of the long-term impact of municipal sewage sludge on the chemical and biological properties of soil. Catena. 2000;189:104484.10.1016/j.catena.2020.104484Suche in Google Scholar
[15] Tang J, Zhang J, Ren L, Zhou Y, Gao J, Luo L, et al. Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J Env Manage. 2019;242:121–30.10.1016/j.jenvman.2019.04.061Suche in Google Scholar PubMed
[16] Garbeva P, van Veen JA, van Elsas JD. Microbial diversity in soil: selection microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol. 2004;42:243–70.10.1146/annurev.phyto.42.012604.135455Suche in Google Scholar PubMed
[17] Yuan J, Raza W, Shen Q. Root exudates dominate the colonization of pathogen and plant growth-promoting rhizobacteria. In: Giri B, Prasad R, Varma A, eds., Root biology. Cham, Switzerland: Springer; 2018. p. 167–80.10.1007/978-3-319-75910-4_6Suche in Google Scholar
[18] Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, Vivanco JM. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One. 2013;8(2):e55731.10.1371/journal.pone.0055731Suche in Google Scholar PubMed PubMed Central
[19] Garg N, Chandel S. Arbuscular mycorrhizal networks: process and functions. A review. Agron Sustain Dev. 2010;30:581–99.10.1051/agro/2009054Suche in Google Scholar
[20] Schüβler A, Schwarzott D, Walker C. A new fungal phylum, the Glomeromycota: phylogeny and evolution. Mycol Res. 2001;105(12):1413–21.10.1017/S0953756201005196Suche in Google Scholar
[21] Klironomos JN, Hart MM. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza. 2002;12(4):181–4.10.1007/s00572-002-0169-6Suche in Google Scholar PubMed
[22] Juniper S, Abbott LK. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza. 2006;16(5):371–9.10.1007/s00572-006-0046-9Suche in Google Scholar PubMed
[23] Selvaraj T, Chellappan P. Arbuscular mycorrhizae: a diverse personality. J Cent Eur Agric. 2006;7(2):349–58.Suche in Google Scholar
[24] Vivas A, Barea JM, Azcón R. Interactive effect of Brevibacillus brevis and Glomus mosseae both isolated from Cd contaminated soil, on plant growth, physiological mycorrhizal fungal characteristics and soil enzymatic activities in Cd polluted soils. Environ Pollut. 2005;134(2):257–66.10.1016/j.envpol.2004.07.029Suche in Google Scholar PubMed
[25] Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Res. 1974;14(6):415–21.10.1111/j.1365-3180.1974.tb01084.xSuche in Google Scholar
[26] Lee J, Lee S, Young JP. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol. 2008;65(2):339–49.10.1111/j.1574-6941.2008.00531.xSuche in Google Scholar PubMed
[27] Mummey DL, Rillig MC, Holben WE. Neighbouring plant influences on arbuscular mycorrhizal fungal community composition as assessed by T-RFLP analysis. Plant Soil. 2005;271:83–90.10.1007/s11104-004-2066-6Suche in Google Scholar
[28] Ondreičková K, Gubišová M, Piliarová M, Horník M, Matušinský P, Gubiš J, et al. Responses of rhizosphere fungal communities to the sewage sludge application into the soil. Microorganisms. 2019;7(11):505.10.3390/microorganisms7110505Suche in Google Scholar PubMed PubMed Central
[29] Clapp JP, Young JPW, Merryweather JW, Fitter AH. Diversity of fungal symbionts in arbuscular mycorrhizas from a natural community. N Phytol. 1995;130(2):259–65.10.1111/j.1469-8137.1995.tb03047.xSuche in Google Scholar
[30] Clapp JP, Fitter AH, Young JP. Ribosomal small subunit sequence variation within spores of an arbuscular mycorrhizal fungus, Scutellospora sp. Mol Ecol. 1999;8(6):915–21.10.1046/j.1365-294x.1999.00642.xSuche in Google Scholar PubMed
[31] Hayek LAC, Buzas MA. Surveying natural populations. Quantitative tools for assessing biodiversity. 2nd edn. New York, NY, USA: Columbia University Press; 1997.10.7312/haye14620Suche in Google Scholar
[32] Hammer Ø, Harper D. Paleontological data analysis. Oxford, UK: Blackwell Publishing; 2006.10.1002/9780470750711Suche in Google Scholar
[33] Jackson DA. Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology. 1993;74(8):2204–14.10.2307/1939574Suche in Google Scholar
[34] Whittaker RH. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol Monogr. 1960;30(3):279–338.10.2307/1943563Suche in Google Scholar
[35] Whittaker RH. Evolution and measurement of species diversity. Taxon. 1972;21(2/3):213–51.10.2307/1218190Suche in Google Scholar
[36] Jacquot-Plumey E, van Tuinen D, Chatagnier O, Gianinazzi S, Gianinazzi-Pearson V. 25S rDNA-based molecular monitoring of glomalean fungi in sewage sludge-treated field plots. Environ Microbiol. 2001;3(8):525–31.10.1046/j.1462-2920.2001.00219.xSuche in Google Scholar
[37] Cruz-Paredes C, López-García Á, Rubæk GH, Hovmand MF, Sørensen P, Kjøller R. Risk assessment of replacing conventional P fertilizers with biomass ash: residual effects on plant yield, nutrition, cadmium accumulation and mycorrhizal status. Sci Total Environ. 2017;575:1168–76.10.1016/j.scitotenv.2016.09.194Suche in Google Scholar
[38] Figueiredo CC, Farias WM, Coser TR, Monteiro de Paula A, Sartori da Silva MR, Paz-Ferreiro J. Sewage sludge biochar alters root colonization of mycorrhizal fungi in a soil cultivated with corn. Eur J Soil Biol. 2019;93:103092.10.1016/j.ejsobi.2019.103092Suche in Google Scholar
[39] Yusif SA, Habib MY, Hayatu NG. Impact of biochar and arbuscular mycorrhizal inoculation on root colonization and selected soil chemical properties in south western Nigeria. J Res Forest Wildl Environ. 2018;10(3):92–8.Suche in Google Scholar
[40] Hildebrandt U, Kaldorf M, Bothe H. The zinc violet and its colonisation by arbuscular mycorrhizal fungi. J Plant Physiol. 1999;154(5–6):709–17.10.1016/S0176-1617(99)80249-1Suche in Google Scholar
[41] Hildebrandt U, Regvar M, Bothe H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry. 2007;68(1):139146.10.1016/j.phytochem.2006.09.023Suche in Google Scholar PubMed
[42] Buzas MA, Hayek LAC. SHE analysis for biofacies identification. J Foramin Res. 1998;28(3):233–9.Suche in Google Scholar
[43] Mana D. A test application of the SGE method as biostratigraphical parameter. Geo Alp. 2005;2:99–106.Suche in Google Scholar
[44] Mukherjee S, Banerjee S, Saha GK, Basu P, Aditya G. Butterfly diversity in Kolkata, India: an appraisal for conservation management. J Asia Pac Biodivers. 2015;8(3):210–21.10.1016/j.japb.2015.08.001Suche in Google Scholar
[45] Buzas MA, Hayek LAC. On richness and evenness within and between communities. Paleobiology. 2005;31(2):199–220.10.1666/0094-8373(2005)031[0199:ORAEWA]2.0.CO;2Suche in Google Scholar
[46] Barzegar AR, Yousefi A, Daryashenas A. The effect of addition of different amounts and types of organic materials on soil physical properties and yield of wheat. Plant Soil. 2002;247:295–301.10.1023/A:1021561628045Suche in Google Scholar
[47] Antolín MC, Pascual I, García C, Polo A, Sánchez-Díaz M. Growth: yield and solute content of barley in soils treated with sewage sludge under semiarid Mediterranean conditions. Field Crop Res. 2005;94(2–3):224–37.10.1016/j.fcr.2005.01.009Suche in Google Scholar
[48] Pascual I, Azcona I, Morales F, Aguirreolea J, Sánchez-Díaz M. Growth, yield and physiology of Verticillium-inoculated pepper plants treated with ATAD and composted sewage sludge. Plant Soil. 2009;319:291–306.10.1007/s11104-008-9870-3Suche in Google Scholar
[49] Bailey KL, Lazarovits G. Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res. 2003;72(2):169–80.10.1016/S0167-1987(03)00086-2Suche in Google Scholar
[50] Marschner P, Kandeler E, Marschner B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol Biochem. 2003;35(3):453–61.10.1016/S0038-0717(02)00297-3Suche in Google Scholar
[51] Del Val C, Barea JM, Azcón-Aguilar C. Diversity of arbuscular mycorrhizal fungus populations in heavy-metal-contaminated soils. Appl Environ Microbiol. 1999;65(2):718–23.10.1128/AEM.65.2.718-723.1999Suche in Google Scholar
[52] Macdonald CA, Singh BK, Peck JA, van Schaik AP, Hunter LC, Horswell J, et al. Long-term exposure to Zn-spiked sewage sludge alters soil community structure. Soil Biol Biochem. 2007;39(10):2576–86.10.1016/j.soilbio.2007.04.028Suche in Google Scholar
[53] Anderson IC, Parkin PI, Campbell CD. DNA- and RNA-derived assessments of fungal community composition in soil amended with sewage sludge rich in cadmium, copper and zinc. Soil Biol Biochem. 2008;40(9):2358–65.10.1016/j.soilbio.2008.05.015Suche in Google Scholar
[54] Gomes NCM, Landi L, Smalla K, Nannipieri P, Brookes PC, Renella G. Effects of Cd- and Zn-enriched sewage sludge on soil bacterial and fungal communities. Ecotoxicol Environ Saf. 2010;73(6):1255–63.10.1016/j.ecoenv.2010.07.027Suche in Google Scholar PubMed
[55] Macdonald CA, Clark IM, Zhao FJ, Hirsch PR, Singh BK, McGrath SP. Long-term impacts of zinc and copper enriched sewage sludge additions on bacterial, archaeal and fungal communities in arable and grassland soils. Soil Biol Biochem. 2011;43(5):932–41.10.1016/j.soilbio.2011.01.004Suche in Google Scholar
[56] Krishnamoorthy R, Venkatramanan V, Senthilkumar M, Anandham R, Kumutha K, Sa T. Management of heavy metal polluted soils: perspective of arbuscular mycorrhizal fungi. In: Shah S, Venkatramanan V, Prasad R, eds., Sustainable green technologies for environmental management. Singapore: Springer; 2019. p. 67–85.10.1007/978-981-13-2772-8_4Suche in Google Scholar
[57] Kumar S, Saxena S. Arbuscular mycorrhizal fungi (AMF) from heavy metal-contaminated soils: molecular approach and application in phytoremediation. In: Giri B, Prasad R, Wu QS, Varma A, eds., Biofertilizers for sustainable agriculture and environment. Cham, Switzerland: Springer; 2019. p. 489–500.10.1007/978-3-030-18933-4_22Suche in Google Scholar
[58] Lin Y, Ye Y, Hu Y, Shi H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol Environ Saf. 2019;180:557–64.10.1016/j.ecoenv.2019.05.057Suche in Google Scholar PubMed
[59] Lloret E, Pascual JA, Brodie EL, Bouskill NJ, Insam H, Juárez MFD, et al. Sewage sludge addition modifies soil microbial communities and plant performance depending on the sludge stabilization process. Appl Soil Ecol. 2016;101:37–46.10.1016/j.apsoil.2016.01.002Suche in Google Scholar
[60] Sharma A, Verma RK. Root–microbe interactions: understanding and exploitation of microbiome. In: Giri B, Prasad R, Varma A, eds., Root biology. Cham, Switzerland: Springer; 2018. p. 323–39.10.1007/978-3-319-75910-4_13Suche in Google Scholar
[61] Jones DL, Hodge A, Kuzyakov Y. Plant and mycorrhizal regulation of rhizodeposition. N Phytol. 2004;163(3):459–80.10.1111/j.1469-8137.2004.01130.xSuche in Google Scholar PubMed
[62] Pasqualone A, Summo C, Centomani I, Lacolla G, Caranfa G, Cucci G. Effect of composted sewage sludge on morpho-physiological growth parameters, grain yield and selected functional compounds of barley. J Sci Food Agric. 2016;97(5):1502–8.10.1002/jsfa.7892Suche in Google Scholar PubMed
[63] Arduini I, Cardelli R, Pampana S. Biosolids affect the growth, nitrogen accumulation and nitrogen leaching of barley. Plant Soil Environ. 2018;64(3):95–101.10.17221/745/2017-PSESuche in Google Scholar
[64] Kępka W, Antonkiewicz J, Jasiewicz C, Gambuś F, Witkowicz R. The effect of municipal sewage sludge on the chemical composition of spring barley. Soil Sci Annu. 2016;67(3):124–30.10.1515/ssa-2016-0015Suche in Google Scholar
[65] Fernández JM, Plaza C, García-Gil JC, Polo A. Biochemical properties and barley yield in a semiarid Mediterranean soil amended with two kinds of sewage sludge. Appl Soil Ecol. 2009;42(1):18–24.10.1016/j.apsoil.2009.01.006Suche in Google Scholar
[66] Eid EM, Alamri SA, Shaltout KH, Galal TM, Ahmed MT, Brima EI, et al. A sustainable food security approach: controlled land application of sewage sludge recirculates nutrients to agricultural soils and enhances crop productivity. Food Energy Secur. 2020;9(2):e197.10.1002/fes3.197Suche in Google Scholar
[67] Kasim WA, Abokassem EM, Ragab GA, Sewelam N. Alleviation of lead stress toxicity in Vigna unguiculata by salicylic acid. Egypt J Exp Biol (Bot). 2014;10(1):37–49.Suche in Google Scholar
[68] Sewalem N, Elfeky S, El-Shintinawy F. Phytoremediation of lead and cadmium contaminated soils using sunflower plant. J Stress Physiol Biochem. 2014;10(1):122–34.Suche in Google Scholar
[69] Ekmekci Y, Tanyolac D, Ayhan B. A crop tolerating oxidative stress induced by excess lead: maize. Acta Physiol Plant. 2009;31:319–30.10.1007/s11738-008-0238-3Suche in Google Scholar
© 2021 Katarína Ondreičková et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Artikel in diesem Heft
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

