Abstract
Today, the growth of the cosmetic industry and dramatic technological advances have led to the creation of functional cosmetical products that enhance beauty and health. Such products can be defined as topical cosmetic drugs to improve health and beauty functions or benefits. Implementing nanotechnology and advanced engineering in these products has enabled innovative product formulations and solutions. The search included organic molecules used as cosmeceuticals and nanoparticles (NPs) used in that field. As a result, this document analyses the use of organic and inorganic particles, metals, metal-oxides, and carbon-based particles. Additionally, this document includes lipid and nanoparticles solid lipid systems. In conclusion, using NPs as vehicles of active substances is a potential tool for transporting active ingredients. Finally, this review includes the nanoparticles used in cosmeceuticals while presenting the progress made and highlighting the hidden challenges associated with nanocosmeceuticals.
1 Introduction
As humans, it is natural to pursue beauty. The use of products to improve skin health has been consistent throughout history. Previously, natural ingredients such as milk, citric fruits, and clay were used [1]. Since 1986, when Christian Dior´s company developed the first cosmetic using a nanocarrier technology through a liposome system, a nanotechnology market emerged [2], and it is expected to exceed US$ 125 billion by 2024 [3].
Cosmeceuticals are products aiming to improve beauty using ingredients that offer health benefits. The concept was coined by Kligman, combining cosmetics and pharmaceuticals, in 1984 [4]. They are multifunctional products that deliver active pharmaceutical ingredients to repair or improve the skin [5]. They are applied to the skin as cosmetics but contain ingredients that contribute to the biological process of the skin [6]. New advances and growth in the cosmetic industry have created beauty-enhancing products and a functional pharmaceutical role.
There are many properties that cosmeceuticals can offer to obtain healthy skin, such as anti-aging and anti-wrinkle, among others [7]. However, retaining the unique functional characteristics of active compounds, particularly those that help promote frequent dermal absorption, is a question that requires better formulation. The implementation of nanoparticles (NPs) in these products has enabled innovative product formulations and solutions [5].
The International Cooperation on Cosmetic Regulation considers a cosmetics nanomaterial as an insoluble ingredient with one or more dimensions less than 100 nm [8]. Using micro- and nanostructured vehicles such as NPs can maximize the penetration and tolerability properties of the skin and optimize the aesthetic appeal of cosmeceutical formulations and their organoleptic characteristics [9]. Additionally, they protect the active ingredient, decreasing its degradation and increasing its stability. Each type of vehicle has different features that allow it to be used in various formulations [10].
With so many claims about the properties of NPs concerning cosmeceuticals, a series of questions arise that need answers. What is the role of NPs in the development of cosmeceutical skin products? What are the properties of nanometric structures? Is there any potential health problem related to these nanocosmetics? Therefore, this review will attempt to answer these questions, reviewing the NPs used in cosmeceuticals while presenting the progress made and highlighting the hidden challenges associated with nanocosmeceuticals.
This research was conducted based on a bibliographic analysis through extensive review. The articles analyzed and selected for this review were written in English and no older than 2008. The search included organic molecules used as cosmeceuticals and NPs used in that field. The study was developed using the following search words “cosmeceutical,” “cosmetic,” “nanoparticle skin,” “liposome cosmeceutical,” “solid lipid skin,” “nanocrystals skin,” and “Nanostructured Lipid Carriers” in the title, abstract, or keywords. The research was developed in the Science Direct, Springer Link, Ebsco, and Google Scholar databases. The search was developed in English. On the whole, 208 articles, books, or book chapters were analyzed
2 Cosmetics and skin
The Food and Drug Administration [11] considers cosmetics as particles intended to be applied to the human body to clean, improve beauty, or change appearance. Cosmetics include several products, depending on the surface of the skin. According to Effiong et al. [12], these can be classified as follows:
skincare cosmetics such as moisturizing agents or cleaning agents;
hair care products such as dyes, styling substances, or shampoos;
facial beauty products such as lip gloss powders or face bases;
manicure products such as enamel and nail removers;
fragrance products such as perfumes, lotions, deodorants, and colonies;
UV light protective preparations such as sunscreens.
Most cosmetological products possess both cosmetic and health objectives. For example, when shampoo is used to clean hair, its purpose is cosmetical. However, if anti-dandruff is used, it is considered a drug to treat the disease. Other examples of cosmetics and medications are moisturizers with sun protection and makeup deodorants with antiperspirants.
What sets cosmetics aside from drugs is their intended use. On the one hand, the function of drugs is to detect, treat, or prevent a health disorder or affect the body’s operation. On the other hand, when their use is solely for beautifying, cleaning, and promoting attractiveness, it is cosmetic and does not need approval by the FDA [13]. Nevertheless, when a product is a cosmetic drug, it must comply with the legislation corresponding to its use as a drug [11].
The term cosmeceutical is the fusion of two terms: cosmetic and pharmaceutical, and it is used to define a topic that involves medical and cosmetical characteristics [13]. Cosmeceuticals represent a new multifunctional product category that relies on science and technology to provide the skin with clinically proven active ingredients [5].
In skincare, this term describes a product with a quantifiable biological action on the skin as a drug. It is controlled as a cosmetic because it pretends to improve its appearance [14]. Kaul et al. [15] define cosmeceutical products as a substance that incorporates a bioactive ingredient with medicinal benefits on the applied surface and improves appearance. They can be naturally derived or chemically synthesized. Ideally, the ingredients should be safe, effective, novel, stable, factory-efficient, and metabolized within the skin [1].
Cosmeceuticals possess multiple advantages over standard cosmetics. They contain high entrapment capacity and improved sensory properties. They are more stable than conventional cosmetics, so they are interested in applying and researching natural ingredients [15]. There are many properties that cosmeceuticals can offer to obtain healthy skin, such as anti-aging signs, acne problems, and inflammatory problems. Besides, they have excellent properties to fight wrinkles and help skin regeneration. Likewise, cosmeceuticals have multiple protective effects on cells to rebuild healthy skin at the cellular level [16].
Despite the advantages of their active compounds, retaining their unique functional characteristics is often a question that requires a new pharmacological composition. Since 1959, it has been a health concern that the skin can easily absorb materials on the nanoscale [5,14,15].
Incorporating nanotechnology into cosmeceuticals aims to formulate more valuable and practical products. The use of NPs in cosmeceuticals has some advantages. For instance, they can improve the stability of the ingredients by encapsulating key components like vitamins and antioxidants. Similarly, applying the bioactive ingredient in the desired place is feasible and generates a controlled delivery for a prolonged effect [17].
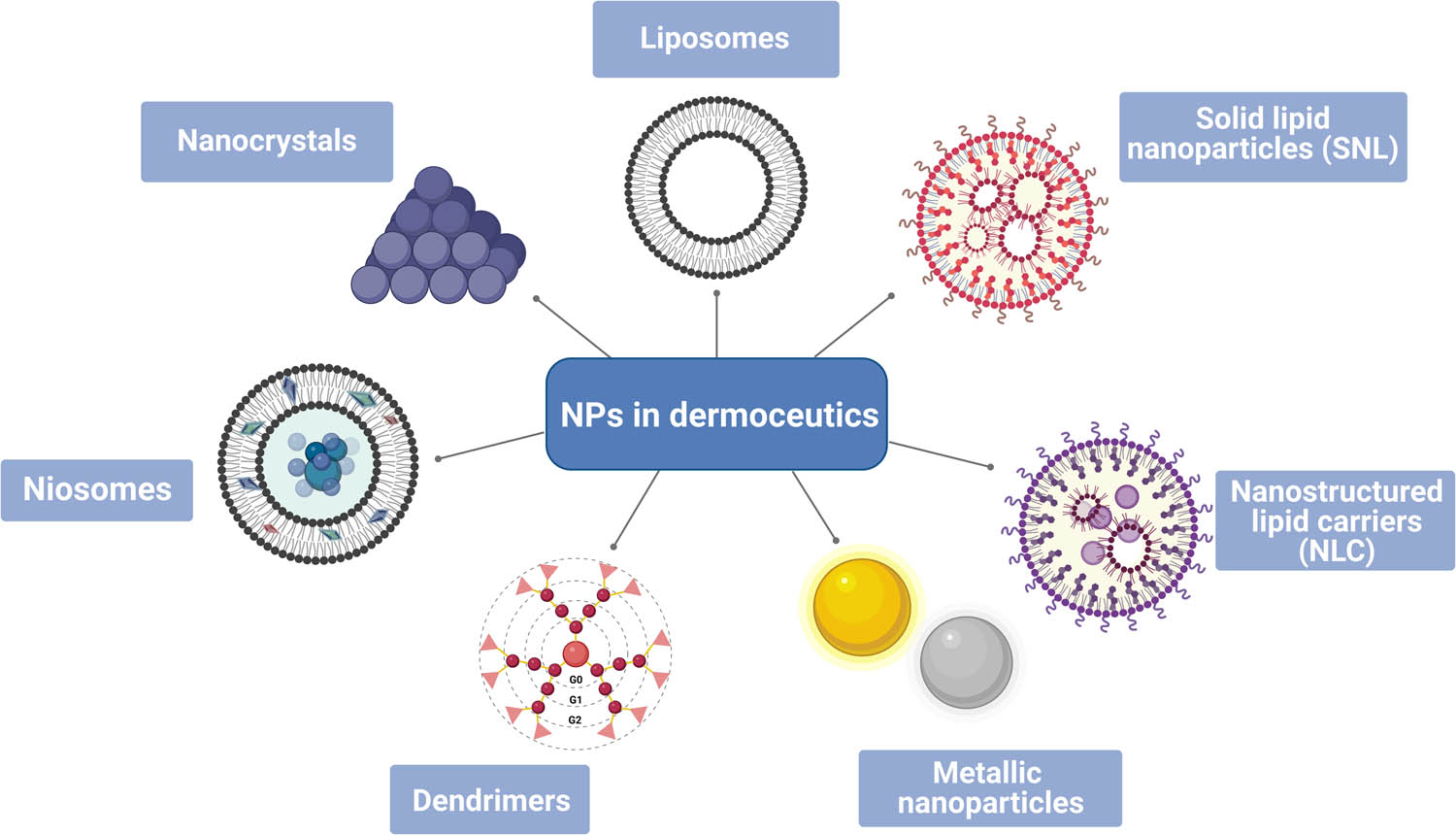
NPs can be used in products like shampoos and conditioners, creams, lotions, cosmetics, anti-aging creams, shower gels, soap, toothpaste, deodorants, fragrances, shaving cream, firming body oil, tanners, exfoliating, and gel for styling [18]. Nanocosmeceuticals have a wide application and are assimilated into the care of nails, hair, lips, and skin. The main classes of nanocosmeceuticals are presented in Figure 1.
![Figure 1
Main nanodermoceutics (created by BioRender.com [15]).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_001.jpg)
Main nanodermoceutics (created by BioRender.com [15]).
The skin is a complex organ that protects the body externally, with an area of 1.5–2 m2 in adults. Its thickness varies depending on the body’s location. Thick skin is on the hands and soles, and thin skin is on the rest of the body [19].
Healthy skin is a barrier against mechanical, chemical, toxic, heat, cold, UV radiation, and pathogenic microorganisms and protects internal organs, bones, muscles, and other soft structures. Besides, it is essential in vitamin D synthesis, sensation, and body temperature regulation. The skin prevents water loss, maintains thermal equilibrium, and transmits external information that accesses the body through the senses, temperature, and pain receptors [19,20].
The skin is divided into the epidermis and dermis [19]. As illustrated in Figure 2, the epidermis is the most superficial part of the skin that separates the body from the environment; it acts as a barrier against external factors and is the layer of the skin with the most significant number of cells and with an extensive replacement dynamics [21,22]. Commonly, it is divided into four layers: the basal cell layer or germination stratum, the squamous cell layer or spiny stratum, granular cells or grainy stratum, and the cornified cell layer or corneal stratum [23]. The epidermis is continuously renewed through the flaking process that involves keratinocytes. The process takes about four weeks [1].
![Figure 2
Human skin representation [27] (created with BioRender.com).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_002.jpg)
Human skin representation [27] (created with BioRender.com).
The dermis provides structural support to the skin. It protects the body from mechanical injuries [24]. It is located underlying the basal membrane of the epidermis. Its structure is like a sponge with numerous fibers associated with an intracellular matrix and few cells (fibroblasts, dendritic cells, and mast cells). The dermis provides structural support to the epidermis. In addition, it offers an adequate vascular contribution for metabolic exchange and abundant nervous innervation [25].
Nerves are derived from the neural crest, but the rest of the dermis components have a mesodermal origin. Collagen is one of its main constituents. Collagen fibers are the most numerous, and their thickness and arrangement vary according to the level at which they are found. The dermis contains the papillary and the deeper reticular layer. The papillary dermis is the outer and thinnest part of the dermis, constitutes approximately 10% of the dermis, and contains a relatively small and loose distribution of elastic fibers and collagen within a significant amount of the fundamental substance. On the other hand, the reticular dermis is a connective tissue containing collagen and thick elastic fibers. The reticular dermis provides skin strength, extensibility, and elasticity [26].
Protecting and preserving the skin is essential; environmental elements, ultraviolet radiation, air pollution, and natural aging can alter its barrier properties. During the aging process, the skin will experience significant structural modifications in the components of the epidermis and dermis. The appearance of wrinkles, pigmentation alterations, and skin atrophy are the main changes seen in senile skin [28].
The epidermis is the outer coat of the skin and therefore needs to regenerate continuously. The stratum corneum can achieve this process, the outer layer of the epidermis that prevents continuous detachment of keratinocytes, provides mechanical protection, and avoids water loss and invasion of external substances [29]. The stratum corneum cells of healthy skin are composed of free fatty acids, ceramides, triglycerides, cholesterol, water, and cholesterol sulfate [30]. This constitutes the lipid barrier of the skin. Damage to this barrier will result in dry, flaky, and rough skin that is easily irritated. Damage to the living epidermis will affect the function of the skin barrier. Likewise, dermal damage will result in wrinkles, stretch marks, or thin, sagging skin [1].
3 Nanomaterials
The chemical substances and materials used in nanotechnology are called nanomaterials. They differ depending on their provenance, size, shape, and chemical nature [31]. Although there are multiple categorizations, they are generally classified according to their origin. Nanomaterials can be obtained from a natural resource, like a volcano or a byproduct of an industrial process. They can be generated synthetically in a lab [32].
Manufactured nanomaterials can be presented as nano-objects. The nanomaterials can be characterized by having one to three external dimensions on the nanoscale. The nanostructured materials have a nanoscale internal or nanosurface structure [33]. These nanostructures form building blocks that, depending on whether they have one, two, or three external dimensions, are called nanolayers, nanofibers, and NPs [32]. Nanostructured materials can be presented in powder, composites, solid foam, porous materials, and fluid dispersion [34].
Materials pretended to be used in an organism are known as biomaterials [35]. The term is designated to those materials used to manufacture devices that interact with biological systems and apply to various medical specialties [36]. They are used in biomedical applications to assist, augment, or replace injured tissue or physical function [37]. They can be natural or synthetic, temporary or permanent in the body, and they aim to restore the existing defect and, in some cases, get tissue regeneration.
A biomaterial must be biocompatible. The body must accept compatible material; it should not alter living tissues while in direct contact. Likewise, it should not be toxic or carcinogenic. It must be chemically stable (no degradation over time) and inert unless it aims to achieve biodegradability. It must have adequate mechanical strength, density, and weight and be relatively economical, reproducible, and easy to manufacture and process for large-scale production [36].
There is a wide variety of biomaterials: metals, plastic, glass, and ceramics that can be cultured with cells. Alongside this structural diversity, there is another functional one. The biomaterials can be used in the prosthesis, orthosis, or scaffold. The scaffolds can be powders, films, foams, or fabrics with different microstructures [38].
4 NPs
NPs are structured less than 100 nm and can be synthesized from countless materials, including metals. Observing them requires high-resolution microscopes such as scanning electron microscopy or transmission electron microscopy [39]. Their small size and shape can incorporate substances that facilitate recognition by cells and tissues. In addition, they can act as a transport vehicle for antimicrobial agents. Evidence shows that NPs can promote tissue regeneration [40,41].
NPs are often considered simple molecules. However, they are complex mixtures, even in the most uncomplicated cases. These materials can be zero, one, two, or three dimensions [42]. Essentially, they consist of at least two layers, the surface and the nucleus. The surface can be functionalized with different functional groups, molecules, surfactants, metal ions, or antibodies; the shell layer properties are other than the nucleus. Finally, the core encloses the central part of the NP [43]. NPs can be analyzed according to their morphological characteristics and their physical and chemical properties as if they are based on metals, metal oxides, carbon, or composite materials.
4.1 Organic NPs
The most common organic NPs are liposomes, dendrimers, ferritin, and micelles. These are characterized by being nontoxic and biodegradable. Some micelles and liposomes have a vacant core sensitive to electromagnetic and thermal radiation [42]. Organic NPs have ideal characteristics for biomedical applications. One of its most significant benefits is that it can be injected containing some medication [44].
4.2 Metal-based and metal oxides NPs
On the other hand, inorganic NPs are considered all particles that are not carbon-based but are formed from metals and metal oxides. They are generally safe, biocompatible, and non-cytotoxic [45]. Metal-based NPs are elaborated using metals with nanometric sizes (10–100 nm) through destructive or constructive methods. Almost all metals can be used to elaborate NPs. The most commonly used are iron (Fe), zinc (Zn), aluminum (Al), gold (Au), silver (Ag), copper (Cu), cadmium (Cd), lead (Pb), and cobalt (Co). They are characterized by a high ratio of surface area to volume and their superficial load density. They have crystalline or amorphous structures. Furthermore, they mainly have spherical, cylindrical shapes (52) and anisotropic shapes like nanorods, nanotriangles, nanocubes, and nanostars [46]. They are reactive and sensitive to environmental factors such as sunlight, air, humidity, and thermal radiation [45].
Metal oxide-based NPs, on the other hand, are elaborated using metal precursors [47]. These particles have been prepared using electrochemical, sonochemical, microwave, sol–gel, solvothermal, and chemical vapor deposition methods [48]. Oxidation has been found to increase their reactiveness and efficiency. The synthesized are common copper oxide (CuO), silver oxide (Ag2O), zinc oxide (ZnO), titanium oxide (TiO2), oxidized aluminum (Al2O3), magnetite (Fe3O4), cerium oxide (CeO2), iron oxide (Fe2O3), and silicon dioxide (SiO2) [44,45,47]. The common applications of these particles include microbial activity, absorbents, semiconductors, gas sensors, ceramics, superconductors, and catalysts [47]. Moreover, some metal oxide NPs have anti-inflammatory properties. Titanium oxide has been used in pigments, cosmetics, skincare, etc. [48].
4.3 Carbon-based NPs
Another way to classify NPs is related to their origin, like carbon-based NPs. These, in turn, can be divided into carbon nanotubes (CNT), fullerenes, graphene, black carbon, nanofibers, and nano-sized activated charcoal [44].
Fullerenes and CNTs are the two main classes. Fullerenes are hollow spherical cages with distinctive physical properties. These particles can recover their original shape after being subjected to high pressures. Since shallow arrangements with dimensions are similar to various active biological molecules, they are helpful because different substances can be included in other applications [49]. While CNTs, given their excellent chemical and physical characteristics, are not only used in their original condition form but as nanocomposites. It has several industrial applications, such as absorbent for water contaminants and support material for heterogeneous catalysts [50].
4.4 Hybrid NPs
Hybrid NPs refer to particles composed of more than one NP sector, i.e., suppose the mixture of a polymeric NP and liposome is performed, resulting in a polymer–lipid hybrid system [45].
4.5 NPs in skincare cosmeceuticals
Recently, the cosmetic industry has developed products based on nanotechnology. In this regard, NPs have made it possible to improve outcomes using ingredients that interact with the skin tissue or enhance beauty [51].
Nanoencapsulation is any system capable of loading a substance to facilitate its penetration of active cosmetics due to its size from 20 to 1,000 nm. The characteristics of the carriers are essential for the permeation mechanism, such as hydrophobicity, stiffness, charge, and molecule size [52]. The transepidermal flow is influenced by the polarity, doses, nature, long-term stability of dissolved substances, solubility, and size of the particle [52,53]. Also, efficacy depends on the bioactive substance’s properties, such as size, charge, and solubility [54]. It has been documented that polymeric NPs can control their release mechanism and have high mechanical properties and non-deformability. Still, they have the limitation that they must avoid the immune system due to their size. Nanoemulsions have long-term stability and high solubilization capacity [55].
NPs have become an option to increase the efficiency of cosmeceutical products. Mainly, they increase the interaction of the ingredients with the area of interest in the skin, allowing more excellent stability and less toxicity. The particles are used to fulfill various functions in cosmetic products, such as active substances, nanocarriers, and modifiers of the appearance or viscosity of the product [56]. The morphology influences their properties. For instance, AgNP in colloidal form has higher antibacterial activity than disk, polygonal, or prism morphologies [57]. For example, in the case of nano cellulose for skin care, depending on its size, geometry ratio, porosity, and aspect ratio, it can be used as a moisturizer, formulation modifier, or additive [58]. Furthermore, the particles’ morphology can affect their surface reactivity [59]. Although lipid-based systems are widely used for their safety, efficacy, and high bioavailability, they have low drug loading capacity due to their crystalline structure, drug extrusion during storage, and particle size disadvantages [60]. On the contrary, the NPs protect substances against degradation and denaturation, reducing adverse effects. When the drug is covered with a polymer shell, it reaches better bioavailability and controlled release [61]. This protection allows them to be more convenient than lipid-based systems [55].
They have crystalline or amorphous structures. Furthermore, they mainly have spherical, cylindrical [52], and anisotropic shapes like nanorods, nanotriangles, nanocubes, nanostars, or other forms in nanosystems (Table 1) [46]. In the case of pickering emulsions, the morphology of particles is essential. Although NP morphology is crucial to define some aspects of its use in nanocosmetics, it is not the main criterion. Traditional emulsifiers are spherical, ellipsoid, cylinder, flake, dumbbell, or wire. The effect is related to the steric effect and from capillary forces in the interface [62]. On the contrary, the toxicity assessment of nanomaterials concludes that the safety of NPs is affected by size, morphology, shape, surface chemistry, and chemical composition [63]. For example, it has been evaluated the toxicity of nano-zinc oxide as a function of the particle size [64].
Advantages and disadvantages of different forms of nanosystems or NPs
| Systems geometry | Advantages | Disadvantages | Source |
|---|---|---|---|
| Liposomes | Easy preparation, improved solubility of active substances | Toxicity must be assessed. They cannot be sterilized with heat | [65,66,67] |
| Nanosomes | Composed of a row of H2O and a row of fatty tails. They are smaller than liposomes and can permeate into the skin | It cannot be employed in products containing chemical preservatives, sunscreen, fragrances, and colorants | [65] |
| Niosomes | They are biocompatible, nonimmunogenic, and biodegradable. They can incorporate lipophilic and hydrophilic compounds | Dry heat and steam sterilization are not adequate for them because heat destroys the lipidic membranes | [65,66] |
| Cubosomes | They have large internal surfaces and the potential to encapsulate hydrophobic, amphiphilic, and hydrophilic compounds | If the bulk cubic phase is divided into dispersed nanostructured particles, the diffusion rate is increased, which causes a complex controlled release system | [65,68] |
| Nanoemulsions | It can carry lipophilic molecules | They are unstable thermodynamically, which causes colloidal instabilities, and then leakage and degradation of encapsulated substances | [65,69] |
Figure 3 shows several novel nanocarriers for administering cosmeceuticals to the skin.
NPs used in developing dermoceutics. Created by BioRender.com.
NPs can enter the skin via transappendageal. For this purpose, they use sweat glands, hair follicles, or lipid matrices with keratinocytes [70].
5 Cubosomes
The transporter is expected to release the corresponding substance at a controlled rate and a specific concentration in a drug delivery system. The active substance will depend on the interaction between the vehicle and the drug [71]. Cubosomes are crystalline liquid NPs usually made of amphiphilic lipids. They have been considered a promising proposition for active drug loading, as they can be used in the medical industry’s oral, transdermal, ocular, or chemotherapy products (Table 2) [71]. Cubosomes are NPs that use surfactant and polymer systems. Unlike liposomes, cubosomes can include amphiphilic or lipid-, hydrophilic, and water-soluble active substances [72]. L’Oreal and Nivea use cubosomes in oil-in-water emulsion in cosmetics [73].
Applications of cubosome formulations
| Cubosome formulation | Application | Reference |
|---|---|---|
| Dexamethasone/Glyceryl monooleate, poloxamer 407, and oleic acid cubosomal | Treatment of vitiligo with 83.58% of drug release at the end of 12 h | [74] |
| Coenzyme Q10/poloxamer 407 and glyceryl monooleate cubosomal | The enzyme is an antioxidant that prevents lipid peroxidation. The formulation enhances the hepatoprotective activity of CoQ10 | [75] |
| Triclosan/Monoolein cubosomal | Triclosan cubosomes were evaluated for the dermal treatment of bacteria-related acne. The results showed that the cubosomes carrying triclosan could be safely used in anti-acne cosmetics and other antibacterial cosmetics | [76] |
| Alpha lipoic acid/poloxamer cubosomes | Anti-aging formulation with alpha lipoic acid that has antioxidant properties and anti-inflammatory effects | [77] |
| Cellulose polymers/hydroxypropyl methylcellulose acetate succinate cubosome | Cellulose polymers/Hydroxypropyl methylcellulose acetate succinate can act as a novel emulsifier of cubosomes with no internal structure modification | [78] |
| Monoolein cubosome containing hinokitiol | Cubosomes have higher skin permeation than hinokitiol dissolved in water | [79] |
6 Micro and nanoemulsions
The multilayered skin structure includes the stratum corneum, composed of dead keratinized cells and a matrix with fatty acids, cholesterol, cholesterol esters, and ceramides. There exists three pathways to penetrate the skin barrier, the inter and trans-cellular pathways, and hair follicles. Good penetration of the active substance in the skin is required to obtain benefits. An emulsion system in the nanoscale could improve the synergy between the base component of the formulation and the active compound, considering lipophilicity, molecular size, and degree of ionization [80]. It is used in the cosmetic market because it is an efficient vehicle for sustained active substance delivery and can reduce skin water loss [81,82]. The emulsion contains two immiscible vehicles, water, oil, and surfactant. Nanoemulsions are non-equilibrium systems, oil/water or water/oil emulsion, with droplets from 50 to 1,000 nm. Oil-in-water dispersions are metastable [80]. These systems are more stable against sedimentation than liposomes [83] but are more suitable for carrier lipophilic substances [84].
A nanoemulsion can transport high loads of lipophilic substances. Also, it protects bioactive substances from oxidation, hydrolysis, or enzymatic degradation. Furthermore, it can improve bioactivity and bioavailability [85].
Nanoemulsions have been used to treat dry skin in sunscreens, body lotions, wet wipes, skin creams [84], whitening, antiaging, or moisturizing agents (Table 3) [86]. Reducing ceramides causes dry skin, and emulsions improve skincare because nanodroplets penetrate the skin surface. They use bioactive compounds designed for transdermal hydrophobic drugs [87]. These systems have good sensory properties, such as merging textures and rapid penetration [80], and can be used when appropriate encapsulation for sensitive actives is required [88].
Applications of nanoemulsions
| System | Company | Description | Source |
|---|---|---|---|
| Bruma De Leite | Natura | Moisturizing solution | [89] |
| Skin Caviar | La Prairie | The formulation helps increase the skin’s tautness and suppleness | [89] |
| Bepanthol Facial Cream Ultra Protect | Bayer Healthcare | The nanoemulsión improves facial care for sensitive skin, moisturizes, and prevents aging of the skin | [89,90] |
| Coco Mademoiselle Fresh Moisture Mist | Chanel | A body mist with moisturizing properties | [89] |
| Nanovital VITANICS Crystal Moisture Cream | Vitacos Cosmetics | A nanoemulsion formulated with niacinamide, arbutin, vitamin c, oriental herbs and for antiaging effect of skin whitening due to the easy permeation of its active agents into the skin | [89] |
| Azelaic acid | It is a whitening agent composed of azelaic acid in a nanoemulsión with hyaluronic acid | [91] | |
| Nanocream | Sinerga | Emulsifier blend, which enables de production of oil/water nanoemulsions. It is a combination of vegetable-based substances lipoaminoacids and palm glycerides | [92] |
| Nano emulsion multi-peptide Moisturizer | Hanacure | The nanoemulsión affects the appearance of skin tone and texture. The nanoemulsión contains peptides, sodium hyaluronate, squalene, and mushroom extract. The skin gets long-lasting hydration | [89,93] |
| Nanoemulsion of citral, deionized water, and cosolvent | Health Sciences and Management/China University of Science and Technology/Providence University/Da-Yeh University/National Taiwan University | Citral can penetrate the lipid structure of bacteria destroying the cell membrane and causing cell lysis and death | [94] |
7 Lipid-based nanosystems
These systems have proven to play their part as dermal vehicles. They have biocompatibility, stability, improvement in penetration, effective delivery of active ingredients to different therapeutic targets, and the possibility to be incorporated into various innovative forms of dosing [51,95].
These nanosystems include lipid NPs and liposomes. Lipid NPs can be stable NPs (SLNs) or nanostructured lipid carriers (NLCs) [96].
Liposomes are spherical capsules of colloidal dimensions; their size usually varies between 20 nm and a few hundred micrometers and is generally made up of cholesterol and phospholipids in an aqueous environment in the proportion of lipids to water [12]. Depending on the preparation method, these vesicles may consist of a bilayer (ULV unilaminar vesicles) or more layers (MLV multilaminar vesicles) of phosphatidylcholine. They consist of two sets of amphiphilic phospholipids with nonpolar hydrophobic tails pointing directly toward the interior and groups of polar hydrophilic heads indicating outwardly laminar structures, as shown in Figure 4 [14,17].
![Figure 4
Liposome showing the phospholipid bilayer (created with BioRender.com [98]).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_004.jpg)
Liposome showing the phospholipid bilayer (created with BioRender.com [98]).
This structure allows the hydrophilic drug to be included in the nucleus. In contrast, lipophilic substances can be encapsulated in the vesicles’ walls. Due to their bilayer structure, liposomes can quickly enter the stratum corneum’s lipid coat. Therefore, they can enhance the dermal administration of medications and reduce systemic absorption [97].
Liposomes are very useful in cosmeceuticals because they are biodegradable, nontoxic, biocompatible, and adaptable vesicles that mimic cell membranes and can easily encapsulate active ingredients to protect them from the surroundings. Additionally, they increase the compositions’ durability and chemical stability [14,99]. Liposomes act as drug transporters and locators. They can act as drug deposits on the skin and surroundings, resulting in a dermal release of medicine compounds, thus improving the benefits at the target site and preventing systemic absorption [17].
As mentioned earlier, its composition and structure are similar to the epidermis, allowing it to penetrate epidermal barriers more efficiently. Therefore, the moisturizing and restorative action of lipids reduces the roughness of the skin. It is due to interaction with corneocytes [14].
In addition to their unique benefits, liposomes show some disadvantages, such as high manufacturing costs and low solubility. They can degrade by hydrolysis or oxidation, having a short life span during storage. Sometimes, drug molecules can leak from liposomes, depending on their composition and drug. Some gel bilayers containing cholesterol slowly lose the associated medication. Moreover, liquid bilayers are more likely to lose the drug [96,97,99]. The benefits and disadvantages of the lipid-based NPs used in cosmetics are summarized in Table 4.
Benefits and disadvantages of liposomes [15]
| Benefits | Disadvantages |
|---|---|
| Non-toxic, biodegradable, non-immunogenic, and biocompatible | It is possible an unintentional release of the encapsulated substance |
| They are naturally attracted to the skin. | Low solubility |
| It keeps the skin hydrated | Possibility of breakdown in contact with the skin and therefore cannot penetrate the skin |
| It can improve the effectiveness of medicine at the specific site | Short half-life |
| Load stability can be improved by encapsulation | High cost of production |
| It may decrease contact of sensitive tissues with toxic loads |
7.1 Application of liposomes as drug carriers in cosmeceuticals
Liposome applications include their use as fragrance carriers in deodorants, antiperspirants, and lipgloss [51]. Liposomes are widely exploited in the case of moisturizers due to their hydration properties, creating a phospholipid coat on the skin and resulting in a blockage effect. Even unoccupied liposomes have the property to keep and improve tissue moisture and, thus, restore skin barrier function [100].
Glycolic acid is one of the most incorporated active ingredients in liposomal systems. This active ingredient is used in many cosmetic products, such as exfoliating and moisturizing. Nevertheless, this has been linked to irritation and burns as side effects of the substance. It has been found that the lysosomal dosage, which contains glycolic acid, represents an adequate administration system to regulate drug delivery, which provides the best conditions to administer its side effects [99].
7.2 Sunscreens
Another application of liposomes is in the manufacture of sunscreens. In theory, an ideal sun protection product must show a high skin buildup with minimal flow to the systemic circulation; it should remain on the tissue surface and pass minimally through it [99]. Lipid release systems have demonstrated UV protection that depends on lipid structure and particle size; that is, the activity of the solar protector is inversely proportional to the particle size [97].
Among the pigments encapsulated in solid lipid nanoparticles (SLNs) for sunscreen is octyl methoxycinnamate [101]. Other lipid systems have been developed containing phosphatidylcholine to improve softening properties of skin care creams [102].
Liposomes help remediate pigmentation in the face and neck. These problems are usually unwanted in women due to hormones, cosmetics, perfumes, and sun radiation [103].
An example of this has been their incorporation into 4-n-butylresorcinol creams. This resorcinol derivative inhibits melanin production and the activity of both tyrosinase and protein 1. This formulation is more than 60% more efficient in treating melasma than the vehicle alone [99]. Liposome affiliation as a vehicle for linoleic acid in hydrogels has improved the incorporation into melanocytes. The permeation rate was higher, suggesting a longer retention time of acid in the skin, ideal for topical treatment of hyperpigmentation disorders [97].
7.3 Anti-acne agents
Acne is a chronic skin disease commonly present during adolescence. It is an inflammation of the sebaceous glands caused by Propionibacterium acnes and Staphylococcus epidermidis [104]. Liposomes were also shown to be an interesting vehicle of tretinoin for skin diseases. Negatively charged liposomes had better hydration in newborn pigskin and better retention of tretinoin in the skin [97,99].
On the other hand, cationic liposomes consist of a double-chain cationic surfactant. Experiments with phosphatidylcholine suggest cationic liposomes can be used to administer drugs intradermally, such as retinoic acid [105].
Similarly, salicylic acid, a drug used to treat acne, with a controlled delivery during a prolonged period using liposome vehicles, provides the opportunity to prepare a less irritating form of acid dosage and less frequent application [97].
7.4 Anti-aging treatments
Skin aging is related to biological, environmental, and hormonal mechanisms. Internal and external aging has been associated with the free radical activity. The cellular metabolism produces these radicals. In contrast, the aging process is increased externally by UV rays and habits such as smoking and drinking alcohol [106].
Antioxidants diminish the free radical unwanted effects by uptake, thus avoiding cell damage. However, the formulation stability and diminished permeability limit their use at the target site on the skin. Liposomes may be appropriate for delivering antioxidants, as they contain several ingredients for skin rejuvenation, such as phospholipids. They can encapsulate active antiaging components and bring them into tissue. Therefore, the liposome can be an effective carrier that absorbs quickly and enter the epidermis and dermis, mixing perfectly with membranes to prevent the initial signs of aging, as already mentioned [107].
Another helpful liposomal formulation in developing these products is sodium ascorbyl phosphate. This substance can attract free radicals and block UV light [108]. Similarly, liposomes loaded with curcumin-loaded soybean phosphatidylcholine have improved stability dispersed with ascorbyl palmitate [109]. In addition, it has been found that liposomes containing aloe vera improve cell proliferation, and collagen production increases antiaging and skin regeneration [110].
7.5 Anticellulite agents
Cellulite is an unwanted issue common in the lower extremities and pelvic region in more than 85% of adult women. This condition causes the skin to look like “orange peel.” While this is not a pathological condition, it remains one of the most recurrent cosmetic problems [111].
The role that liposomes have acquired in the face condition is critical. It has been found that the liposomal system can penetrate the subcutaneous tissue and then reach and decompose the lipid cells. Like other applications, liposomes help the drug get into fat cells affected by cellulite without noticeable waste [99].
One of the most active ingredients is caffeine, mainly used for its slimming effect. This can be applied topically to stimulate lipolysis in the epidermis. To optimize these formulations, they must reach the adipocytes. Liposomes have been used as vehicles to get to the active site fast [112].
Other natural medicines used for cellulite treatment are liposomes containing green tea extract, ginkgo biloba, kukui nut oil, and silicone resin. These substances act on adipose tissue metabolism, promoting the reduction of cells and the width of the subcutaneous layer. Consequently, softer skin is obtained due to the increase of moisture in the deeper layers of the skin [99].
Other liposomes include ethosomes and transferosomes. These vesicles are produced using ethanol or softening surfactants. Their main characteristic is ultra-deformability, which improves skin permeability [89]. Ethosomes are lipid-based vesicular systems with high ethanol concentrations to prolong their physical stability, resulting in easy penetration of medication into deeper skin layers [113,114].
A summary of all liposome applications is summarized in Figure 5.
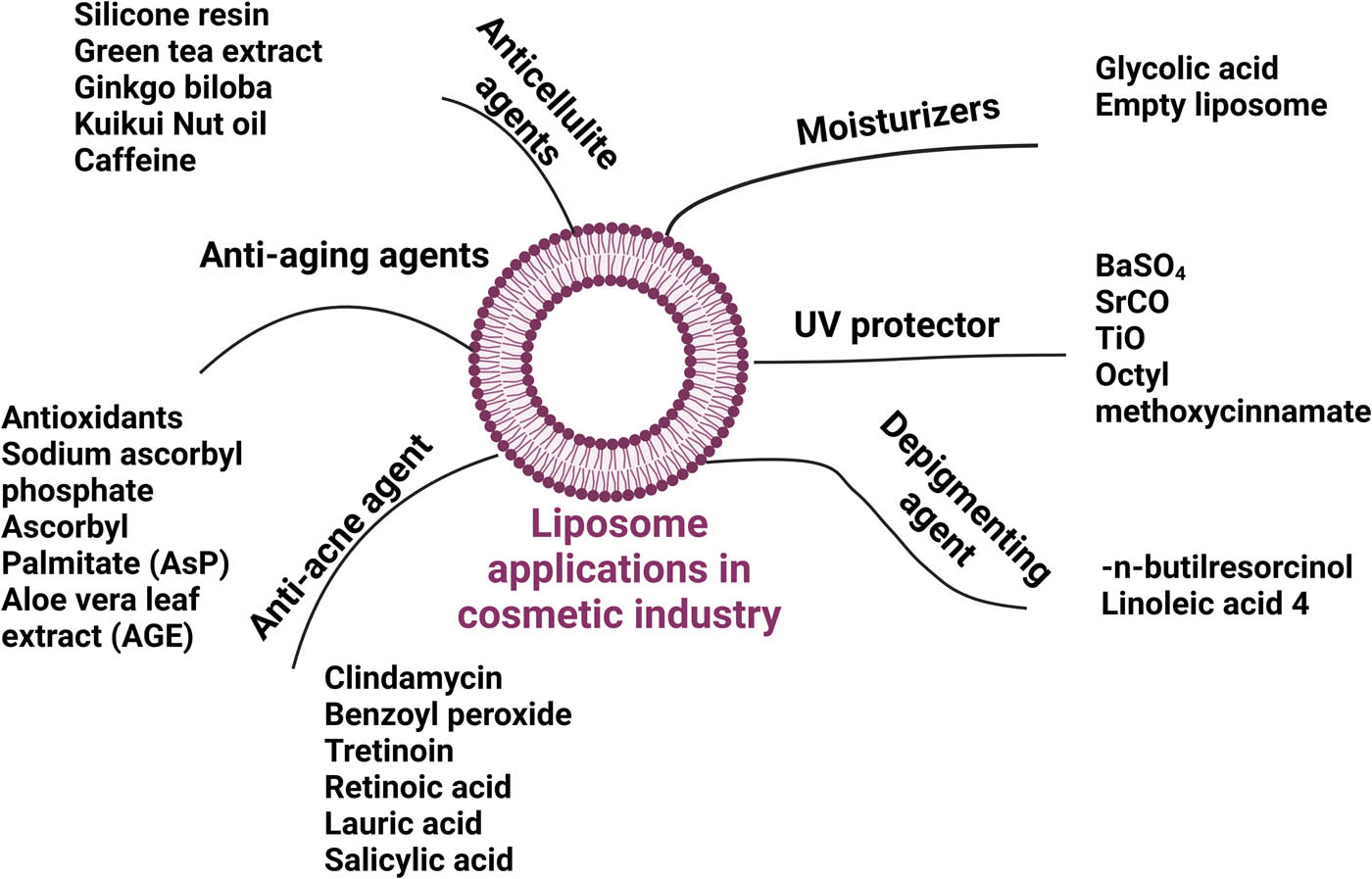
Liposome applications in the cosmeceutical industry. Created with BioRender.com.
8 NPs of solid lipids and carriers of nanostructured lipids
SLNs consist primarily of solid lipids such as waxes, triglycerides, and glycerides. These particles are active carriers to deliver drugs for different treatments at slow rates. The amount of active surface species is an important parameter to define their functionalities and physical stability [51,115]. The systems are used when solubility and bioavailability have to be enhanced in poorly water-soluble actives and to shield actives from degradation [116]. The particles are around 50–1,000 nm and are composed of a single layer with a lipid nucleus [14,17]. SLNs are common in the pharmaceutical industry because they are lipids with low toxicity and are biodegradable. Besides, they can enter through the skin to the stratum corneum. Furthermore, it has UV resistance properties and acts as physical sunscreen to enhance protection with diminished side effects (Figure 6) [117].
![Figure 6
(a) SLN and (b) NLC (created with BioRender.com [120]).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_006.jpg)
(a) SLN and (b) NLC (created with BioRender.com [120]).
The attributes that must be controlled in SLNs are the particle’s polymorphism, size, shape, long-term stability of the lipid particle, and surface properties for Pickering stabilization [116]. Lipid nanocarriers are often used due to their high loading capacity, appropriate kinetic release pattern, and good stability [118].
NLCs consist of solid and liquid lipids with a drug enclosed in their core [96]. They are considered the second generation of lipid NPs. NLCs are mainly three types whose base develops the structure according to their composition (Figure 6). They are spheres around 10–1,000 nm and have recently caught attention due to their lower toxicity and reaction effects [15]. When the nanocarrier is designed, it is essential to define if they have to overcome the epithelial barrier that limits their cellular entry. Then, the carrier systems’ size, surface hydrophobicity, and surface charge must be modified. A negative and neutral charge can permeate the mucus layer while the positive nanocarriers are entrapped [119].
NLCs have a modulated drug release profile (Figure 7), i.e., a two-phase drug delivery system; the drug is initially released with an outburst and after at a constant rate. Furthermore, it has greater skin hydration due to the barrier properties. Their nanometric dimension improves the exposure to the stratum corneum, causing more drugs to enter the skin. An advantage of this nanosystem is a UV-blocking system with diminished side effects [15].
![Figure 7
Effect of lipid NPs on the skin (created with BioRender.com [15]).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_007.jpg)
Effect of lipid NPs on the skin (created with BioRender.com [15]).
9 SLN vs NLC in cosmeceutical development
As already mentioned, lipid NPs (SLN and NLC) are essential in improving cosmetics’ usefulness. Due to their excellent safety, they have many applications in the treatment of skin tissue. These spheres can be added to existing medication friendly due to their structural stability and affinity to lipophilic, hydrophilic, and soluble active ingredients [15].
They are colloidal administration systems whose formulation results in better UV protection effects and skin moisture. These systems prevent the degradation of the drug inside the core, improve penetration and absorption, and control delivery at the target site [121].
While storing SLNs, they tend to generate reordering of the crystalline structure to obtain a more orderly and stable shape. The imperfections of the matrix provide a space to pack inside the drug preventing its loss [51]. Thus, NLCs have a higher load capacity than SLNs and low water in the suspension due to the amorphous structure that creates more space. Besides, NLCs can minimize vigorous load ejection during storage [122]. The benefits and detriments of the cosmetic application of lipid-based NPs are included in Table 5. Also, Table 6 shows the cosmetical application of NPs.
Benefits and detriments of SLNs [15]
| Lipid nanoparticle | Benefits | Detriments |
|---|---|---|
| Solid nanoparticle (SNL) | Controlled delivery of drugs and augmented | Ejecting cargo during storage |
| Bioavailability of trapped medicine | ||
| Increased stability of substances | ||
| Biocompatible and biodegradable | ||
| Moisturizing effect on the skin and good penetration of the drug | ||
| They act as physical sunscreens | High water content in nano lipid suspensions | |
| Suitable for transporting lipophilic loads as hydrophilic | ||
| Easy to scale and sterilize | ||
| Less expensive compared to polymeric/surfactant carriers | ||
| More comfortable with validating and getting regulatory approval | ||
| NLCs | It can produce a thin coat on the epidermis to prevent the chemical decomposition of the drug | Cytotoxic effects related to concentration |
| Simple preparation and enlargement | ||
| They have controlled the particle size | The irritating effect of some surfactants | |
| Good dispersibility in an aqueous environment | ||
| They have extended lead release | ||
| Close contact with the corneum stratum due to its small size |
Cosmeceutical application of lipid NPs
| Application | Active ingredient | Source |
|---|---|---|
| Chemical stabilizer | Vitamin E | [17,129] |
| Ascorbic palmitate | ||
| Retinol and retinoids | ||
| Carotenes | ||
| Lipoic acid | ||
| Moisturizers/occlusive | Argan oil | [17,129] |
| Empty lipid NPs | ||
| TiO2 | ||
| Kukui nut oil | ||
| Milk proteins | ||
| Coconut extract | ||
| Omega 3 and 6 | ||
| Sunflower oil | ||
| Avocado oil | ||
| Urea | ||
| Ginseng extract | ||
| Glycoproteins | ||
| UV Blocking | BaSO4 SrCO3 TiO2 Benzophenone 3 Orxibenzona Molecular sunscreens | |
| Insect repellents | DEET OMC |
9.1 Application of SLN and CNCs as drug carriers in cosmeceuticals
The essential features of lipid NPs are improving the hydrolysis, oxidation, and chemical stability of active optical sites, which benefits some active ingredients like Vitamin E, ascorbic palmitate, retinol, retinoids, carotenes, lipoic acid, lutein and some sunscreens [123].
Due to their occlusion effect, these NPs are widely used as moisturizers and wrinkle softeners. They prevent water loss from the tissue, consequently increasing the hydration of the skin increases [124]. An increment in moisture is noticed with a diminished particle size, which offers better adhesion and quick coat production. Dolatabadi et al. [125] analyzed the barrier effects of SLN and NLC particles. They concluded that SLNs and nanomicellar systems were better than NLCs as vesicles for curcuminoids.
Consequently, the occlusive properties of NLCs improve the penetration of medication into the epidermis. Moreover, it improves skin moisture and smoothes wrinkles [126].
Moreover, it was noticed that SLN presented the maximum barrier effects with a low melting point, lipids, high crystallinity, and small particle size [127]. On the other hand, nanometric SLN and NLC systems were analyzed In further research, showing occlusion factors around 36% and 39%, respectively. However, the score (red Nile) showed that NLCs penetrate deeper into the SC than SLNs [128].
Argan oil has been used for its medicinal properties, such as anti-aging, protection, and hydration in cosmeceutical products. Tichota et al. [129] developed an NLC argan oil composite to improve skin moisture. The composite was evaluated using in vivo human models. This analyzed drug improved skin moisture in the study participants.
Lipid NPs are widely used in the development of sunscreens. The SLNs have shown excellent properties in blocking UV light. The SLN matrix produced better UV absorption than the nanoemulsion containing oil in water (o/w). Also, titanium dioxide can block UV rays at the molecular level. Nevertheless, it could be photoallergic and phototoxic. When SLNs were combined with sun protection creams., the amount of titanium dioxide could be reduced, reducing the side effects associated with these sun protection molecules [17].
Dietyltoluamide (DEET) is a popular ingredient used to repel insects. However, its utilization is often affected because it can be absorbed in a massive systemic way. Puglia et al. [130] analyzed the encapsulation consequences of DEET and OMC on SLN, demonstrating that the particles could reduce the skin permeability of both compounds compared to an O/W emulsion.
10 Niosomes
Niosomes are vesicles composed of bilayers formed from organized nonionic surfactants hydrated with or without incorporating cholesterol or lipids. Its structure varies from 100 nm to 2 mm in diameter [4]. Its characteristics are comparable to liposome properties, except for improved stability, higher price, and adaptability [51]. The first commercial product elaborated with these vesicles was Niosomes® by Lancôme [89].
The niosomes can be simply laminar or multilaminar, depending on the preparation method. Due to their unique geometry, niosomes can enclose substances with different water affinities in their structure. The entrapment in niosomes can occur in the water domain or adsorb on the surface of the bilayer. In contrast, hydrophobic substances enter the bilayer structure dividing it (Figure 8) [131]. These vehicles are biodegradable, non-toxic, and more stable than liposomes [132].
![Figure 8
The bilayer structure of the niosome exhibits the entrapment of the hydrophilic and hydrophobic substances [131] (created by BioRender.com).](/document/doi/10.1515/rams-2022-0282/asset/graphic/j_rams-2022-0282_fig_008.jpg)
The bilayer structure of the niosome exhibits the entrapment of the hydrophilic and hydrophobic substances [131] (created by BioRender.com).
Niosomes are elaborated by some methods such as thin film hydration [133], microfluidics [134,135,136], sonication [137], multiple membrane extrusion [138], and reverse phase evaporation technique [138].
Niosomes offer excellent chemical stability than liposomes, a longer lifespan, and osmotic activity. Likewise, its surface can be easily modified due to a functional group in the hydrophilic face. Besides, they are more compatible and degradable by biological systems. Almost all routes of administration can administer niosomes. In particular, their size and encapsulation can be modified by changing additives, proportions, or combinations [131]. Also, they are known due to their capacity to be injected by ocular o transdermal routes, which are not common pathways [139]. Also, the niosomes are cheaper to fabricate than liposomes [140,141].
However, although the niosome delivery system has several advantages, it can be hydrolyzed in aqueous suspension. The issue of drug outflow from the encapsulation site and agglomeration of niosome formation and formulation techniques can be time-consuming [15,142]. Moreover, some challenges must be considered, such as consistent and reproducible construction of niosomes [134]. The strengths and weaknesses of the cosmetic uses of lipid-based NPs are summarized in Table 7.
Strengths and weaknesses of the niosomes [15]
| Strengths | Weaknesses |
|---|---|
| Non-toxic, biodegradable, Non-immunogenic and biocompatible | You may have fusion, leaching, or hydrolysis of the trapped drugs, limiting the lifespan |
| Controlled and targeted drug delivery | Requires specialized equipment for manufacturing |
| They can be administered in almost every way | Insufficient drug load capacity |
| Improves the therapeutic performance of the drug | Trapped drug leak |
| Better chemical stability | Physically unstable |
| Osmotic activity and longer lifespan | Time-consuming techniques for formulation |
Niosomes are utilized in cosmetic and skin treatments because they can reduce and repair the permeability of the cornea layer (Figure 9). There is more excellent protection of trapped drugs and an enhancement in the bioavailability of substances. The formation of niosomes is affected by surfactants, encapsulated substances, membrane properties, and hydration temperature. Also, they have problems with physical stability, including aggregation, fusion, leakage in storage, and sedimentation [143]. An alternative is the proniosomes. They are liquid crystalline compact niosome hybrids [144,145,146] and nonionic surfactant vesicles used to improve the administration of drugs because they can produce an aqueous dispersion of niosomes [15,142], using dry and free-flowing product storage and can be hydrated immediately before use to form a liposomal dispersion [143]. This system increases bioavailability and reduces side effects [124], especially on skin penetrating the barrier and enhancing the drug’s penetration due to its amphiphilic nature [147]. They can be used to entrap hydrophilic and lipophilic drugs [148].
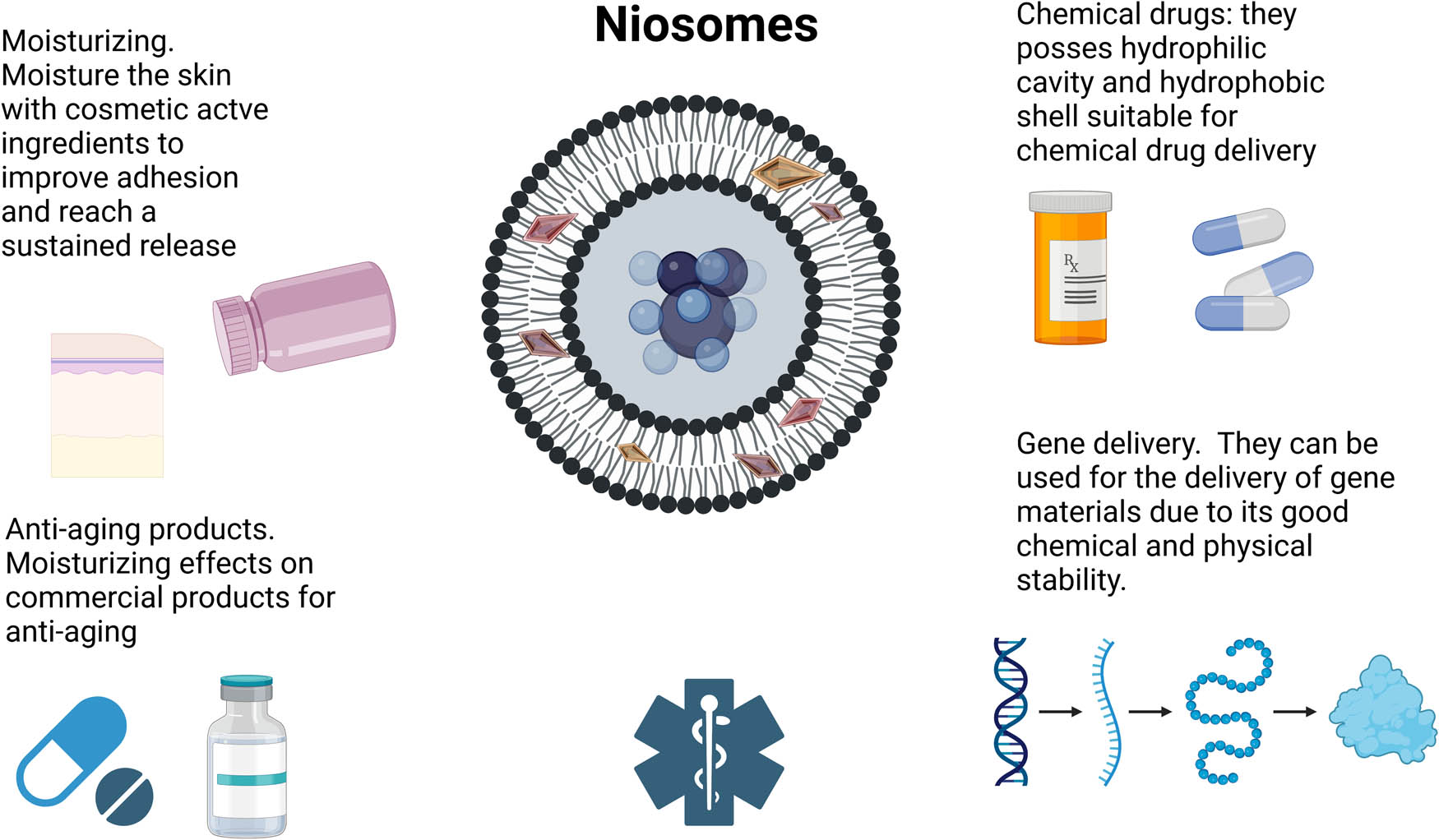
Niosomes applications. Created with BioRender.com.
Nevertheless, niosomes can be used in the pharmaceutical industry, oral, ocular, topical, pulmonary, parental, and transmucosal drug delivery and cosmetic applications [141]. Niosomes have been used to encapsulate essential oils characterized by low stability and biocompatibility because they cannot be used for direct topical administration [149], such as Lippia citriadora essential oil [150]. Also, niosomes can encapsulate poorly soluble drugs like melatonin [151]. Other substances include some for anti-aging treatments [152], like gallic acid for anti-skin aging [153,154], rice (Oryza sativa L.) bran for antiaging activity [155], deer antler velvet extract to stimulate the growth of skin and hair cells [156].
11 Metal, metal oxides, and inorganic NPs nanosystems
Metals, metal oxides, and inorganic NPs have appeared in the cosmetical industry, mainly skin formulations, providing an original approach to delivering entrapment drugs. These NPs are based on herbal medicine, cosmetics, sunscreens, and makeup [51]. The inorganics NPS include metal or metal oxides and carbon-based NPs with controlled physical properties (Table 8). In dermal products, it is common to use gold NPs, silver NPs, titanium oxide, zinc oxide NPs, silica NPs, cooper NPs, aluminum oxide NPs, nanotubes, fullerenes, and nanodiamonds [89]. Titanium dioxide and zinc oxide are common in sunscreen formulations. These substances protect from UVA and UVB light [157].
Applications of inorganic NPs in the cosmetic industry
| NP | Applications | Reference |
|---|---|---|
| Gold | Antiaging, colorant, carrier, preservative | [89,168,169] |
| Used in deodorant, anti-aging creams, and face packs. It Is commercialized Chantecaille with anti-inflammatory and antioxidant properties | ||
| Silver | Antifungal and antimicrobial | [14,89,96,168] |
| Effective against Trichophton leismaniasis, onychomycosis, and Staphylococcus aureus | ||
| Used in the face pack, antiaging cream Coil Whitening Mask by Natural Korea and deodorants Nano-In Hand by Nano-Infinity protected from UVA and UVB | ||
| Titanium oxide | Used commercially in products for sun tan effects like Mineral Fusion® | [89,158,170] |
| Zinc oxide | Protect from UVA and UVB. Used commercially in products like Neutrogen® or sunscreens and moisturizers by L’Oreal or Nivea | [14,89,158,169] |
| Silica | It improves the texture of cosmetic products | [14,89,160] |
| Used in Lancôme Microlift in Lancôme Renergie Microlift | ||
| Cooper | Biocidal and antiaging products | [89] |
| Fullerenes | Antioxidants and antiaging agents | [14,73,171] |
| It is used commercially in Zelens Fullerene C-60 by Zelens | ||
| CNT | Hair colorants and to improve permeabilization | [15,101,158] |
| Cerium oxide | Sunscreen preparation to block UV light | [172] |
| P. ginseng leaves-mediated gold NP | Cosmetic applications are due to their antioxidant, moisture retention, and whitening properties | [173] |
| Snail slime-based gold NPs | Particles synthesized by green chemistry for sunscreen products | [174] |
| Green gold NPs obtained through gold salt reduction by polyphenol contained in Hubertia ambavilla extracts | The NPs were evaluated by obtaining dermo-protective green gold NPs useful to block ultraviolet A | [175] |
| Gold NPs synthesized from P. ginseng berry | NPs exhibited moisture retention capacity that shows biocompatible and environmentally safe material for cosmetics | [176] |
| Silver NPs | Silver NPs were evaluated, and the results suggest they can be used as a preservative in cosmetics | [177] |
| Silver NPs synthesized with natural extracts | The particles were evaluated in hybrid sun-protective body milk showing a high degree of solar protection | [161] |
| Silver NPs synthesized with Phoenix sylvestris L. extract | The NPs have antimicrobial properties to treat acne-causing pathogens | [178] |
| ZnO and TiO2 | These NPs are commonly used as inorganic UV filters in sunscreens. They have high stability to light and good dispersive properties. TuO2 effectively blocks UV-B and ZnO to UV-A, eliminating sunscreens’ chalky white appearance. Furthermore, the substances are used in cosmetics and toothpaste | [166,179,180,181,182] |
| Collagen/SiO2 and ZnO composite materials | The composite was used in cosmetics creams for solar protection showing high antibacterial activity | [183] |
On the other hand, gold particles have several advantages, such as nontoxicity, high stability, chemically inert, and no photobleaching [15]. L’Oreal sells some products with gold nanoparticles to improve the elasticity and firmness of the skin [158]. Silica NPs have a hydrophilic surface and low cost. They are applied in the cosmetical industry because they can penetrate carrier drugs and release them into the skin. The particle size, surface charge, chemistry, chemical composition, and porosity enhance dermal penetration [159]. Fullerenes contain odd-numbered carbon rings, commonly used in anti-aging products [160]. On the other hand, CNT have been used in hair staining because they enhance the affinity of carbon black with gray or white hair [2]. Nevertheless, the main challenge of these systems is how to increase the penetration of the active ingredients through the skin barrier to achieve therapeutic goals [161].
They have been used for topical applications to enhance the solubility of apolar drugs, controlled release, higher stability, and capability to target specific areas to deliver active substances [161]. Their main applications of metal and metal oxide NPs include controlled release, sun-protected creams, and antimicrobial protection [51].
Silver and gold NPs have been used as novel cosmeceuticals [162]. They are used in toothpaste, soaps, deodorants, sunscreens, herbal products, hair cosmetics, wet wipes, body foams, lip, and makeup [102,163]. On the other hand, silica NPs are studied as the epidermal, dermal, and transdermal routes [164]. They are used in cosmetics, but their hazardous effects are recently evaluated on humans and the environment [165]. Zinc oxide is an inorganic compound insoluble in water. It has a hexagonal or cubic structural form. It is practical to absorb and scatter UV radiation [166]. The substance re-emits the absorbed radiation in less damaging UVA as fluorescence or heat [167].
12 Dendrimers
Dendrimers are macromolecules with a tree structure to encapsulate and deliver bioactive substances [184]. Dendrimers or fractal polymers are a class of highly branched three-dimensional dispersed mono-macromolecules with well-defined sizes and geometry that are precisely designed and contracted for their application. This is achieved by introducing desired functional groups at the ends of the surface branches that will react with the target [17]. In other words, they consist of a series of chemical layers built around a small nucleus molecule and have four main components: a nucleus, identically sized arms, linked or branched points, and final functional groups [14].
Dendrimers are nanoscopic, with diameters ranging from 2 to 10 nm. They are synthesized by controlling their chemical composition from a reactive polyfunctional nucleus that acts as a molecular center of proliferation, achieving an orderly configuration of monomers. The layers around the core are called dendrons [185]. They have uniform size, water solubility, high degree of branching, internal cavities available, and polyvalency [186]. Mainly lipophilic molecules are encapsulated in dendrimers via Van der Waals or apolar forces [184].
The dendrimersome is a self-assembly dendrimer used to encapsulate and deliver cosmetics, drugs, and diagnostic materials [187]. This nanocarrier was developed in Finland and the USA. It is biocompatible and has stability, tenability, monodispersity, and versatility. It is possible to produce them with uniform size and easy chemical functionalization [188].
Polyamidomin dendrimers (PAMAMs) are the most used in cosmetics development. The interior of this dendrimer contains empty spaces suitable for encapsulating host molecules. Simultaneously, the outer surface includes some potentially reactive sites adapted to alter the solubility of the NPs, making them a more efficient carrier polymer. Other dendrimers are DABs or PPIs (polypropylene imine dendrimers), both of which have 16 amino groups on the periphery of their configuration. These are used to increase the penetration of certain substances into the skin, like PMMH (phosphorus dendrimer) and bis-MPA (2.2-bis acid dendrimers (propionic methylol) [25].
It is essential to mention that something that characterizes dendrimers is their intrinsic viscosity. Dendrimers of lower molecular weight have a better viscosity, which is quite beneficial for the formulation of cosmetics [189]. Some L’Oreal patents describe how using dendrimers helps avoid disadvantages with high molecular weight polymers. Dendrimers’ low-viscosity suspensions have improved performance, including satisfactory sensory properties [25]. This company has used PAMAM dendrimers in deodorant and self-tanning compositions [25]. Moreover, the company uses dendrimers to fabricate mascara or nail polish [190].
On the other hand, Revlon has been used amidoamine dendrimers in personal care and cosmetic products. The company has encapsulated salicylic acid using the PAMAM-salicylic acid complex [191] for anti-acne compositions [25]. Unilever has used dendrimers in gels, lotions, and sprays (Table 9) [173,192,193,194,195].
Commercial uses of dendrimers
| Dendrimer | System | Source |
|---|---|---|
| Unilever | Hydroxyl functionalized dendrimers made from polyester units for gels, sprays, and lotions | [90,197] |
| Hair: composition with dendrimer macromolecule | ||
| Dow Corning Toray Co | Carbosiloxane dendrimer structure for use as a cosmetic raw material | [198] |
| L’Oréal | Skin: The company uses hydroxyl functionalized polyesters dendrimers for skin applications | [199,200] |
| Deodorant: Contains amine function dendrimers for human applications | ||
| Revlon | Cosmetics that have keratolytic and anti-acne activity | [201,202] |
| Nails, hair, and eyelash treatments: Keratin treatments nu hydrolyzed or partially hydrolyzed keratin |
On the other hand, dendrimers functionalized with ferulic, caffeic, and gallic acids have been used as antibacterial substances [196].
Dendrimers improve the skin permeability of the drug by interacting with lipid skin bilayers [51] and have applications in skin treatment, hair care, bath products, and fragrances [17].
13 Nanocrystals
Nanocrystals are drug particles stabilized by surfactants without any lipid or polymeric matrix [203]. They are aggregated of hundreds to thousands of atoms combined into a group and are in a size range of 10–400 nm. Generally, they are applied to improve the dermal penetration of poorly soluble cosmetic substances [204,205,206] and creams or lotions [207].
Nanocrystals are assembled from several hundred thousand atoms to form a polycrystalline or straightforward arrangement to release scarcely soluble active ingredients [73]. They have unique properties such as increased solubility of insoluble assets, higher dissolution rate, good adhesion, higher concentration gradient, and improved bioavailability [205].
Nanocrystals are used in cosmetic actives like lutein, flavonoids, coenzyme Q10, and flavonoids (Table 10) [203]. It was found that the dermal absorption and activity of the herbal compound can be improved using nanocrystals. One of their uses is flavonoids. Flavonoids of plants are part of the secondary metabolites. They have antioxidant and anti-inflammatory properties that decrease vascular permeability and protect blood vessels from treating rosacea. However, they are mainly used because of their UV protection properties [205]. The bioactivity of flavonoids was increased using a nanocrystal-based routine.
Uses of nanocrystals
| Nanocrystal | Description | Source |
|---|---|---|
| Nanocrystals of cosmetic actives | Increase bioactivity of the molecules in the skin | [216] |
| Nanocrystal cellulose | It is a sunscreen cream cosmetic composition of cellulose and nano-titanium dioxide | [217] |
| Rapamune | It was the first pharmaceutical product in the market | [214] |
| Juvedical | Renewing serum and skin optimizer fluid | [214] |
| Platinum Rare by La Prairie | Hesperidin crystals are one of the most expensive cosmetics in the world | [206,218] |
Moreover, similar behaviors have been seen in the case of the plant antioxidant hesperidin [208]. Also, nanocrystals of bioflavonoids have been evaluated for therapeutic efficacy, but more studies related to safety are necessary for commercial purposes [208]. Moreover, curcumin nanocrystals elaborated through smartCrystals® for use in dermal formulations provide a new mechanism for the nanocrystals’ concentration [209]. Research shows that Cinnamate-functionalized cellulose nanocrystals are photostable and with an enhanced sun protection factor [210].
It was reported a nanomaterial based on diethyl sinapate-grafted cellulose nanocrystals. The material has colloidal stability and exhibits antioxidant and anti-UV properties in moisturizing cream with glycerol [211]. Also, it was prepared a delivery system for Symurban as nanocrystals for the treatment of skin pollution [212].
Some companies have used nanocrystals in cosmetic products, such as Renewing Serum and Juvedical®, Juvedical®, DNA Skin Optimizer Fluid, and Cream SPF 20 by Juvena [213,214], and Capture produced by Dior [214] and Petersen for blocking UV radiation with enhancing SPF [215].
14 Discussion
Incorporating liposomes in cosmeceutical products has advantages, such as biodegradability and excellent biocompatibility with the skin and its derivatives. Also, they have similar epidermis permeability properties, allowing more penetration than other treatments [158]. It should be noted that liposomes can administer drugs locally in the skin layer and improve moisture in skin conditions due to the affinity between liposome components and skin lipids [17]. Therefore, liposomes can be used as vehicles of cosmeceutical material or as active agents. Additionally, they can interact not only with the skin but also with proteins and carbohydrates to repair or reach a normal state when the skin is damaged because of moisture deficiency or eczema. It has even been found that they can increase the concentration of tretinoin in the epidermis and dermis, which protects it from photodegradation and minimizes its irritation compared to conventional creams or gels [41].
The production of liposomes is simple since it does not use massive amounts of raw material due to its small size resulting in financial products [219]. However, the number of liposomal systems markets is well below expectations. The cause can be attributed to the inconvenient production methods proposed in the literature: low cell absorption, difficulty controlling the particle size distribution, low encapsulation efficiency, and continuous design. Among these, liposomes’ low entrapment efficiency and particle magnitude are challenging to maintain. They are responsible for the waste of vast percentages of the number of molecules trapped, and as a result, the cost of producing them increases considerably [220].
The main advantage of SNL compared to other systems is its high biocompatibility and reliable physical properties, as well as the ability to control the delivery of substances. Nevertheless, some issues have been found with the total payload of the drug, which becomes insufficient due to the limited miscibility of some substances in solid lipids [123].
Consequently, new systems such as NLC have been explored. These second-generation NPs consisting of solid and liquid lipids offer the convenience of drug-carrying capability and improved release properties. In that sense, NLCs are more effective for topical, dermal, and transdermal administration [221].
They are more productive systems, facilitate the load for the accommodation of drugs, and contain different C chains in the crystal structure. Thus, medicines such as ascorbic palmitate, clotrimazole, ketoconazole, and other antifungal agents show a controlled release behavior [222]. However, they have some limitations related to cytotoxic effects due to the nature of the polymer and its concentration. Furthermore, some surfactants can irritate and have specific application problems when using protein and peptide drugs [221].
Due to problems related to toxicity, research has shifted its interest to systems such as nyosomes, which are non-cytotoxic particles that contain nonionic surfactants and cholesterol. These substances produce a rigid structure and keep the charged molecule stable, both hydrophilic and hydrophobic [131].
They have been extensively investigated as a carrier system of cosmetic bioactive substances and the usefulness of niosomes in traditional formulations such as emulsions, which showed lower cytotoxicity. Some metabolites extracted from plant materials are relevant in cosmetic research; these compounds possess beneficial effects such as antioxidants and antiaging. Many natural bioactive compounds using niosomes to enhance their impact on the epidermis have even been investigated [223].
Bartelds et al. [224] characterized and optimized some formulations of niosomes and compared the properties of these compositions with those of liposomes elaborated from cholesterol and phosphatidylcholine lipids/phosphatidylethanolamine. Niosomes were stable and even more permeable than liposomes, as analyzed by ion calcein liberation; ion permeability (KCl) was higher than the liposomes. In addition, they found that the magnitude of niosomes diminishes substantially after freezing in liquid nitrogen and subsequent thawing. Similarly, they discovered that the antimicrobial peptides melittin and alamethicin behave similarly in these systems composed of unsaturated components and were not affected when saturated amphiphile drugs were used. Lastly, in permeability and stability for drug-sized molecules, niosomes are similar to liposomes and may offer a reasonable and economical option for administration purposes.
The main drawback of this kind of system is that the loaded drug can be hydrolyzed in aqueous suspensions. It can leak the drug from the site of entrapment or the added formation of a niosome [131].
NLC and lysosomes are economical and easy to produce based on the collected information. They present lower toxicological and leakage risks since they are more stable. However, if you are looking for aqueous suspensions, the best option is to use NCL. On the other hand, inorganic NPs, metal oxides, and metallic oxides appear in cosmetics, particularly skin formulations, to deliver encapsulated ingredients. They may be present in different compositions, such as formulations based on hair, cosmetics, sunscreens, and botanical products [51,225]. Metal NPs (TiO2 and ZnO), gold (Au), and silver (Ag) have attracted attention for their practical antibacterial effect [63], leading to the development of several methods to synthesize AuNPs and AgNPs. Generally, chemical processes are the most beneficial as they reduce production costs [226]. Their relevance is due to their tunable optics, small size, stability, chemical and physical surface, and absence of cytotoxicity. They can be functionalized with biomolecules such as DNA, amino acids, carboxylic acids, and DNA. They provide an excellent drug delivery system. Furthermore, AgNPs inhibit proliferation and microbial infection much more than AuNPs [227].
While these NPs are similar in application, some research says AgNPs tend to be more unstable than AuNPs. Pulit et al. [228] evaluated the risks of using cosmetic preparations with AgNPs and AuNPs, for which stable cosmetic formulations (creams) were made with AgNPs and AuNPs at different concentrations. Both showed that they had good fungicidal properties. The standard cream’s estimated yield limits were obtained based on the viscosity curves. The creams with NPs improved the consistency and distribution of the skin. However, the best ratings obtained in texture, absorption, lubrication, color, and odor were assigned to AuNPs emulsion.
The worst uniformity, color, and smell ratings were obtained for emulsion with AgNPs. The most noteworthy aspect of this study was evaluating the permeability of metallic NPs through artificial dermal membranes. Higher permeabilities were confirmed for creams with metal NPs, which is a cause for concern as the cytotoxic properties of metal NPs have not been accurately evaluated.
The properties of AuNPs are frequently affected by interactions between particles and their assembling. NPs can easily penetrate the skin and accumulate, stimulating actin filaments and abnormal matrix construction. They can decrease intracellular protein synthesis and reduce cell proliferation, adhesion, and motility [229]. The toxic effect of AgNPs is related to silver ions’ delivery and size. Particles of 10 nm are more harmful than higher ones. Based on the information collected, these particles have potential applicability. Nevertheless, they present some toxicological risks when applied in high doses or with minimal size [230].
Dendrimers are artificial polymers characterized by repeated branched units that emanate from a focal point and have many neutral, cationic, or anionic terminal groups on their surface. Their properties are different compared to conventional polymers. They are helpful as a transport system for drugs and genes, including some with medicinal uses, due to their antibacterial, antifungal, and cytotoxic properties [231]. In addition, many substances cannot be exploited due to their low toxicity, solubility, or stability issues, and dendrimers can solve these problems, improving their clinical applications [187].
There are a few drawbacks to them, but they should not be taken lightly; they have toxicity, location, biodistribution, and high cost of production. Its efficiency in the skin depends mainly on its size and surface load; the easy-to-control features are shape, size, liposome occlusion dendrimeric structure, surface functionality, length of branches, and synthesis of specific dendritic frameworks [232].
Vaidya et al. [233] mention that the toxicity of dendrimers can be reduced by changing their shell with biocompatible substances such as polyethylene glycol, fatty acids, carbohydrates, amino acids, and peptides. Another strategy is synthesizing and designing biodegradable and biocompatible dendrimers using reagents. They are biocompatible and easily assimilated by biological systems. Dendrimers of amino acids, carbohydrates, peptides, and triazines are examples of such biodegradable dendrimers. In addition, the PEGilation of PAMAM dendrimers significantly reduces toxicity due to the reduction of cationic charge by the PEGilation of the dendrimer surface.
On the other hand, nanocrystals are typically produced from poorly miscible substances, class II drugs classified by the biopharmaceutical system (BCS). They have been successful in the market since 2000 [234], and the first nanocrystal product emerged for cosmetics in Switzerland in 2007. Nevertheless, the nanocrystals were manufactured from poor miscible, traditional, and active ingredients. Recently, it has been proposed as a novel concept in dermatology for drugs of medium solubilities, such as caffeine [235].
Unlike dendrimers, this type of nanodrug has a high proportion of stabilizing medicines that leads to approximately 100% of the load capacity of the drug. Nanocrystals are suitable for drug delivery to produce an adequate concentration in the desired area, and they are much simpler and more viable for industrial production [234]. Moreover, nanocrystals have low toxicity, and their solubility permits the skin to release poorly miscible actives to increase the substance’s saturation solubility, which can improve the drug’s release, dissolution rate, dermal bioavailability, and surface adhesion [203,236].
In such a way, nanocrystals can improve the solubility of poorly miscible substances in the water much better than dendrimers. It is believed that they could increase epidermal deposition and enhance skin permeability, improving the permeability of traditional topical formulations [236]. One of the most significant drawbacks of these compounds is the requirement for recurrent dose administration that can produce clusters and precipitation of nanocrystals, which can increase the possibility of systemic permeability and adverse reactions [237].
In this sense, nanocrystals are more attractive for the formulation of cosmetics. They can work perfectly without the most significant risk if used in the relevant doses. Still, the constant advance of technology could undoubtedly improve or even displace this type of NPs.
15 Challenges in nanocosmeceuticals
The use of NPs in cosmetics has some challenges. It is hard to scale up the technology developed due mainly to the complicated operation and low efficiency [52,238]. Also, the market approval of nanoformulation requires additional studies. It is essential to evaluate biodegradability and biocompatibility with organisms. These studies imply in vivo studies. Furthermore is required a sustainable process that includes exposition during manufacturing, use, and final disposition [239]. Although some marketed cosmetics and patents exist, toxicity issues must be discussed and evaluated. These studies have to include an analysis of metabolization, permeation, biodistribution, and elimination of subproducts [67,96,164].
On the other hand, nanomaterials used in cosmetics can damage DNA, membranes, and proteins and produce increased quantities of oxygen species [240]. The substances’ safety must be analyzed and regulated to include guidelines about their toxicity, safety, and efficacy [52,241]. The risk of exposure is currently poorly established [238]. One of the restrictions on the use of NPs is that they must not reach the bloodstream, which means that penetration is allowed, but permeation is not. Similarly, there are risks of NPs being inhaled in cosmetic powders [52]. They have required in vivo and ex vivo studies about the effects and mechanisms of permeation [164].
In order to evaluate the benefits and risks of the NPs used, it is crucial to establish the potential hazards associated with the use, exposure levels, and the effects once released in the environment, for instance, water and soil. Zhao et al. analyze the impact of NPs in species exposed to the aquatic environment and the neurological toxicity in humans [240]. It is essential to control the particles’ attributes, such as size and shape, because they affect the health concerned. For instance, the average size of metallic NPs defines their antibacterial and catalytic activity [241].
Some lipidic systems, such as liposomes, are unsuitable for conventional sterilization methods. Although some researchers consider that gamma radiation could be an option, the effect on the physical and chemical properties of niosomes must be studied in the future [66].
On the other hand, it is difficult to control the release of substances in cubosomes, so some researchers are looking for ways to change their properties to control the drug loading and release kinetics [68].
Although some reports refer to different forms or geometries of NPs (Oake et al., 2019; Rawtal et al.), it is necessary to establish the relationship between the morphology and the delivery kinetics of the molecule to be used in cosmetics.
Although some reports refer to different forms or geometries of NPs (Oake et al., 2019; Rawtal et al.), it is necessary to establish the relationship between the morphology and the delivery kinetics of the molecule to be used in cosmetics.
16 Conclusion
NPs can be composed of organic (flexible) and inorganic (rigid) materials. Nanometric-sized structures are helpful in controlled and directed transport, increasing efficiency and stability. They have the characteristic of presenting biocompatibility with the skin and provide a prolonged effect, so it helps improve the final appearance of cosmetics.
Their use in cosmetics can be associated with the manufacture of cleansing lotions, lipsticks, anti-wrinkle treatments, eye shadows, hair conditioners, and makeup, among others.
Some biosafety mechanisms still need to be regulated for their use. However, recent research has harnessed this toxicity to combat other problems to help control some pathogens. One of the main challenges currently facing it is to determine the “right” to obtain these benefits without running the risk of poisoning or to ensure that the nanostructures used in no way cause us harm.
Due to its wide use, we consider that its development and application present a wide field of study within cosmetology science.
Acknowledgments
The authors would like to thank Miss Andrea Sofía Rangel Martel for her language edition, all the editors, and the anonymous referees for their constructive comments and suggestions. All figures in this manuscript were created with Biorender.com.
-
Funding information: The authors write this manuscript with the institutional resources of Juarez City Autonomous University.
-
Author contributions: All authors performed a literature review and analysis and wrote the article. Specifically, the main activities of each author were: Martel-Estrada, Santos-Adriana: conceptualization, investigation, writing – review and editing, supervision; Morales-Cardona, Andrea-Isabel: investigation, writing – original draft; Vargas-Requena, Claudia-Lucía: conceptualization, writing, supervision; Rubio-Lara, Juan Antonio: writing – review & editing; Martínez-Pérez, Carlos-Alberto: investigation, writing; Jiménez-Vega, Florinda: investigation, writing.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Data availability statement: Readers can access data through any reference in this document.
References
[1] Aziz, Z. A. A., H. Mohd-Nasir, A. Ahmad, S. H. Mohd Setapar, W. L. Peng, S. C. Chuo, et al. Role of nanotechnology for design and development of cosmeceutical: Application in makeup and skin care. Frontiers in Chemistry, Vol. 7, 2019, id. 739.10.3389/fchem.2019.00739Suche in Google Scholar PubMed PubMed Central
[2] Zhou, H., D. Luo, D. Chen, X. Tan, X. Bai, Z. Liu, et al. Current advances of nanocarrier technology-based active cosmetic ingredients for beauty applications. Clinical, Cosmetic and Investigational Dermatology, Vol. 14, 2021, pp. 867–887.10.2147/CCID.S313429Suche in Google Scholar PubMed PubMed Central
[3] Morganti, P. Nanocosmetics: An introduction. Nanocosmetics, Elsevier, Amsterdam, Netherlands, 2020, pp. 3–16.10.1016/B978-0-12-822286-7.00001-2Suche in Google Scholar
[4] Svarc, F. and L. Hermida. Transdermal and bioactive nanocarriers for skin care. Nanocosmetics, Elsevier, Amsterdam, Netherlands, 2020, 35–58.10.1016/B978-0-12-822286-7.00003-6Suche in Google Scholar
[5] Golubovic-Liakopoulos, N., S. R. Simon, and B. Shah. Nanotechnology use with cosmeceuticals. Seminars in Cutaneous Medicine and Surgery, Vol. 30, No. 3, 2011, pp. 176–180.10.1016/j.sder.2011.06.003Suche in Google Scholar PubMed
[6] Draelos, Z. D. Cosmeceuticals: What’s real, what’s not. Dermatologic Clinics, Vol. 37, No. 1, 2019, pp. 107–115.10.1016/j.det.2018.07.001Suche in Google Scholar PubMed
[7] Alves, A., E. Sousa, A. Kijjoa, and M. Pinto. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules, Vol. 25, No. 11, 2020.10.3390/molecules25112536Suche in Google Scholar PubMed PubMed Central
[8] Lu, P.-J., S.-C. Huang, Y.-P. Chen, L.-C. Chiueh, and D. Y.-C. Shih. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. Journal of Food and Drug Analysis, Vol. 23, No. 3, 2015, pp. 587–594.10.1016/j.jfda.2015.02.009Suche in Google Scholar PubMed PubMed Central
[9] Stobel Christy, E. J., A. Rajeswari, and A. Pius. Biopolymer applications in cosmeceutical industries. Biopolymers and their Industrial Applications, Elsevier, Amsterdam, Netherlands, 2021, pp. 219–243.10.1016/B978-0-12-819240-5.00009-2Suche in Google Scholar
[10] Fytianos, G., A. Rahdar, and G. Z. Kyzas. Nanomaterials in cosmetics: Recent updates. Nanomaterials, Vol. 10, No. 5, 2020.10.3390/nano10050979Suche in Google Scholar PubMed PubMed Central
[11] FDA. Food and Drug Administration Cosmetic Labeling Guide, FDA, United States, Accessed March 8th, 2021 2021. https://www.fda.gov/cosmetics/cosmetics-labeling-regulations/cosmetics-labeling-guide.Suche in Google Scholar
[12] Effiong, D., T. Uwah, E. Jumbo, and A. Akpabio. Nanotechnology in cosmetics: Basics, current trends and safety concerns—a review. Advances in nanoparticles, Vol. 9, No. 1, 2020, pp. 1–22.Suche in Google Scholar
[13] Eaglstein, W. H. What are cosmetics and cosmeceuticals. The FDA for doctors, Springer, Cham, 2014, pp. 29–31.10.1007/978-3-319-08362-9_7Suche in Google Scholar
[14] Lohani, A., A. Verma, H. Joshi, N. Yadav, and N. Karki. Nanotechnology-based cosmeceuticals. ISRN Dermatol, Vol. 2014, 2014, id. 843687.10.1155/2014/843687Suche in Google Scholar PubMed PubMed Central
[15] Kaul, S., N. Gulati, D. Verma, S. Mukherjee, and U. Nagaich. Role of nanotechnology in cosmeceuticals: A review of recent advances. Journal of Pharmaceutics, Vol. 2018, 2018, id. 3420204.10.1155/2018/3420204Suche in Google Scholar PubMed PubMed Central
[16] Morganti, P. and M.-B. Coltelli. A new carrier for advanced cosmeceuticals. Cosmetics, Vol. 6, No. 1, 2019.10.3390/cosmetics6010010Suche in Google Scholar
[17] Kaur, I. P., G. Sharma, M. Singh, M. Ramzan, J. Singh, S. K. Sandhu, et al. Solid lipid nanoparticles in dermaceuticals. Nanomaterials for Clinical Applications, Vol. 1, 2020, pp. 1–27.10.1016/B978-0-12-816705-2.00001-1Suche in Google Scholar
[18] Ahmad, I. Z., A. Ahmad, H. Tabassum, and M. Kuddus. A cosmeceutical perspective of engineered nanoparticles. Handbook of nanomaterials for manufacturing applications, Micro and Nano Technologies, Elsevier, 2020, pp. 191–223.10.1016/B978-0-12-821381-0.00008-9Suche in Google Scholar
[19] Dwivedi, A., N. Agarwal, L. Ray, and A. Tripathi. Skin aging & cancer, Springer Nature, Singpore, 2019.10.1007/978-981-13-2541-0Suche in Google Scholar
[20] Chouhan, D., N. Dey, N. Bhardwaj, and B. B. Mandal. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials, Vol. 216, 2019, id. 119267.10.1016/j.biomaterials.2019.119267Suche in Google Scholar PubMed
[21] Tarassoli, S. P., Z. M. Jessop, A. Al-Sabah, N. Gao, S. Whitaker, S. Doak, et al. Skin tissue engineering using 3D bioprinting: An evolving research field. Journal of Plastic, Reconstructive & Aesthetic Surgery, Vol. 71, No. 5, 2018, pp. 615–623.10.1016/j.bjps.2017.12.006Suche in Google Scholar PubMed
[22] Madni, A., R. Kousar, N. Naeem, and F. Wahid. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. Journal of Bioresources and Bioproducts, Vol. 6, 2021, pp. 11–25.10.1016/j.jobab.2021.01.002Suche in Google Scholar
[23] Fenner, J. and R. A. F. Clark. Anatomy, physiology, histology, and immunohistochemistry of human skin. Skin tissue engineering and regenerative medicine, Academic Press, Boston, 2016, pp. 1–17.10.1016/B978-0-12-801654-1.00001-2Suche in Google Scholar
[24] Sanchez-Adams, J. and K. A. Athanasiou. Dermis isolated adult stem cells for cartilage tissue engineering. Biomaterials, Vol. 33, No. 1, 2012, pp. 109–119.10.1016/j.biomaterials.2011.09.038Suche in Google Scholar PubMed
[25] Ammala, A. Biodegradable polymers as encapsulation materials for cosmetics and personal care markets. International Journal of Cosmetic Science, Vol. 35, No. 2, 2013, pp. 113–124.10.1111/ics.12017Suche in Google Scholar PubMed
[26] Zeng, Q., L. K. Macri, A. Prasad, R. A. F. Clark, D. I. Zeugolis, C. Hanley, et al. 6.20 Skin tissue engineering☆. Comprehensive biomaterials II, Elsevier, Oxford, 2017, pp. 334–382.10.1016/B978-0-12-803581-8.10157-2Suche in Google Scholar
[27] Ng, K. W. and W. M. Lau. Skin deep: The basics of human skin structure and drug penetration. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement, Springer-Verlag, 2015, pp. 3–11.10.1007/978-3-662-45013-0_1Suche in Google Scholar
[28] Russell-Goldman, E. and G. F. Murphy. The pathobiology of skin aging: New insights into an old dilemma. The American Journal of Pathology, Vol. 190, No. 7, 2020, pp. 1356–1369.10.1016/j.ajpath.2020.03.007Suche in Google Scholar PubMed PubMed Central
[29] Mohrin, M. and H. Jasper. Exploring human skin aging at the single-cell level. Developmental Cell, Vol. 56, No. 3, 2021, pp. 253–254.10.1016/j.devcel.2021.01.006Suche in Google Scholar PubMed
[30] Lei, L., J. Su, J. Chen, W. Chen, X. Chen, and C. Peng. The role of lysophosphatidic acid in the physiology and pathology of the skin. Life Sciences, Vol. 220, 2019, pp. 194–200.10.1016/j.lfs.2018.12.040Suche in Google Scholar PubMed
[31] Xu, Y., M. Zhao, D. Zhou, T. Zheng, and H. Zhang. The application of multifunctional nanomaterials in Alzheimer’s disease: A potential theranostics strategy. Biomedicine & Pharmacotherapy, Vol. 137, 2021, id. 111360.10.1016/j.biopha.2021.111360Suche in Google Scholar PubMed
[32] ur Rahman, M. Sources of nanomaterials. Nanomaterials: synthesis, characterization, hazards and safety, Elsevier, Amsterdam, Netherlands, 2021, pp. 15–29.10.1016/B978-0-12-823823-3.00007-0Suche in Google Scholar
[33] Kuehr, S., R. Kaegi, D. Maletzki, and C. Schlechtriem. Testing the bioaccumulation potential of manufactured nanomaterials in the freshwater amphipod Hyalella azteca. Chemosphere, Vol. 263, 2021, id. 127961.10.1016/j.chemosphere.2020.127961Suche in Google Scholar PubMed
[34] Clifford, C. A., M. Stinz, V.-D. Hodoroaba, W. E. S. Unger, and T. Fujimoto. International standards in nanotechnologies. Characterization of nanoparticles, Elsevier, 2020, pp. 511–525.10.1016/B978-0-12-814182-3.00026-2Suche in Google Scholar
[35] Hudecki, A., G. Kiryczyński, and M. J. Łos. Biomaterials, definition, overview. Stem cells and biomaterials for regenerative medicine, Academic Press, Amsterdam, Netherlands, 2019, pp. 85–98.10.1016/B978-0-12-812258-7.00007-1Suche in Google Scholar
[36] Ghasemi-Mobarakeh, L., D. Kolahreez, S. Ramakrishna, and D. Williams. Key terminology in biomaterials and biocompatibility. Current Opinion in Biomedical Engineering, Vol. 10, 2019, pp. 45–50.10.1016/j.cobme.2019.02.004Suche in Google Scholar
[37] Tanzi, M. C., S. Farè, and G. Candiani. Biomaterials and applications. Foundations of biomaterials engineering, Academic Press, Amsterdam, Netherlands, 2019, pp. 199–287.10.1016/B978-0-08-101034-1.00004-9Suche in Google Scholar
[38] Yaszemski, M. J., F. J. Schoen, and J. E. Lemons. 2.5.1 - Introduction to applications of biomaterials. Biomaterials science, 4th edn, Academic Press, Amsterdam, Netherlands, 2020, pp. 995–997.10.1016/B978-0-12-816137-1.00066-0Suche in Google Scholar
[39] Pavitra, E., B. Dariya, G. Srivani, S.-M. Kang, A. Alam, P.-R. Sudhir, et al. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Seminars in Cancer Biology, Vol. 69, 2019, pp. 293–306.10.1016/j.semcancer.2019.06.017Suche in Google Scholar PubMed
[40] Eivazzadeh-Keihan, R., A. Maleki, M. de la Guardia, M. S. Bani, K. K. Chenab, P. Pashazadeh-Panahi, et al. Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review. Journal of Advanced Research, Vol. 18, 2019, pp. 185–201.10.1016/j.jare.2019.03.011Suche in Google Scholar PubMed PubMed Central
[41] He, H., L. A. Pham-Huy, P. Dramou, D. Xiao, P. Zuo, and C. Pham-Huy. Carbon nanotubes: Applications in pharmacy and medicine. BioMed Research International, Vol. 2013, 2013, id. 578290.10.1155/2013/578290Suche in Google Scholar PubMed PubMed Central
[42] Tiwari, J. N., R. N. Tiwari, and K. S. Kim. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Progress in Materials Science, Vol. 57, No. 4, 2012, pp. 724–803.10.1016/j.pmatsci.2011.08.003Suche in Google Scholar
[43] Shin, W. K., J. Cho, A. G. Kannan, Y. S. Lee, and D. W. Kim. Cross-linked Composite Gel Polymer Electrolyte using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Scientific Reports, Vol. 6, 2016, id. 26332.10.1038/srep26332Suche in Google Scholar PubMed PubMed Central
[44] Anu Mary Ealia, S. and M. P. Saravanakumar. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conference Series: Materials Science and Engineering, Vol. 263, 2017, pp. 1–15.10.1088/1757-899X/263/3/032019Suche in Google Scholar
[45] Youssef, F. S., H. A. El-Banna, H. Y. Elzorba, and A. M. Galal. Application of some nanoparticles in the field of veterinary medicine. International Journal of Veterinary Science and Medicine, Vol. 7, No. 1, 2019, pp. 78–93.10.1080/23144599.2019.1691379Suche in Google Scholar PubMed PubMed Central
[46] Reguera, J., J. Langer, D. Jiménez de Aberasturi, and L. M. Liz-Marzán. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chemical Society Reviews, Vol. 4613, 2017, pp. 3866–3885.10.1201/9780429295188-24Suche in Google Scholar
[47] Naseem, T. and T. Durrani. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environmental Chemistry and Ecotoxicology, Vol. 3, 2021, pp. 59–75.10.1016/j.enceco.2020.12.001Suche in Google Scholar
[48] Devikala, S., J. M. Abisharani, and M. Bharath. Biosynthesis of TiO2 nanoparticles from Caesalpinia pulcherrima flower extracts. Materials Today: Proceedings, Vol. 6, 2020, pp. S185–S188.10.1016/j.matpr.2020.08.578Suche in Google Scholar
[49] Pal, S., U. Jana, P. K. Manna, G. P. Mohanta, and R. Manavalan. Nanoparticle: An overview of preparation and characterization. Journal of Applied Pharmaceutical Science, Vol. 1, No. 6, 2011, pp. 228–234.Suche in Google Scholar
[50] Khan, I., K. Saeed, and I. Khan. Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry, Vol. 12, No. 7, Amsterdam, Netherlands, 2019, pp. 908–931.10.1016/j.arabjc.2017.05.011Suche in Google Scholar
[51] Santos, A. C., F. Morais, A. Simões, I. Pereira, J. A. D. Sequeira, M. Pereira-Silva, et al. Nanotechnology for the development of new cosmetic formulations. Expert Opinion on Drug Delivery, Vol. 16, No. 4, 2019, pp. 313–330.10.1080/17425247.2019.1585426Suche in Google Scholar PubMed
[52] Chiari-Andréo, B. G., M. G. J. D Almeida-Cincotto, J. A. Oshiro, C. Y. Y. Taniguchi, L. A. Chiavacci, and V. L. B. Isaac. Nanoparticles for cosmetic use and its application. Nanoparticles in pharmacotherapy, William Andrew Publishing, Amsterdam, Netherlands, 2019, pp. 113–146.10.1016/B978-0-12-816504-1.00013-2Suche in Google Scholar
[53] Wokovich, A. M., S. Prodduturi, W. H. Doub, A. S. Hussain, and L. F. Buhse. Transdermal drug delivery system (TDDS) adhesion as a critical safety, efficacy and quality attribute. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 64, No. 1, 2006, pp. 1–8.10.1016/j.ejpb.2006.03.009Suche in Google Scholar PubMed
[54] Paul, S., E. B. L. Hmar, H. Pathak, and H. K. Sharma. 6 - An overview on nanocarriers. Nanocarriers for drug-targeting brain tumors, Elsevier, Amsterdam, Netherlands, 2022, pp. 145–204.10.1016/B978-0-323-90773-6.00004-XSuche in Google Scholar
[55] Jeong, W. Y., M. Kwon, H. E. Choi, and K. S. Kim. Recent advances in transdermal drug delivery systems: A review. Biomaterials Research, Vol. 25, No. 1, 2021, id. 24.10.1186/s40824-021-00226-6Suche in Google Scholar PubMed PubMed Central
[56] Vinod, T. P., and R. Jelinek. Inorganic nanoparticles in cosmetics. Nanocosmetics: from ideas to products, Springer International Publishing, Cham, 2019, pp. 29–46.10.1007/978-3-030-16573-4_3Suche in Google Scholar
[57] Carrouel, F., S. Viennot, L. Ottolenghi, C. Gaillard, and D. Bourgeois. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: A review of the current situation. Nanomaterials, Vol. 10, No. 1, 2020, id. 140.10.3390/nano10010140Suche in Google Scholar PubMed PubMed Central
[58] Meftahi, A., P. Samyn, S. A. Geravand, R. Khajavi, S. Alibkhshi, M. Bechelany, et al. Nanocelluloses as skin biocompatible materials for skincare, cosmetics, and healthcare: Formulations, regulations, and emerging applications. Carbohydrate Polymers, Vol. 278, 2022, id. 118956.10.1016/j.carbpol.2021.118956Suche in Google Scholar PubMed
[59] Huang, Y., S. C. Lenaghan, L. Xia, J. N. Burris, C. N. Stewart Jr, and M. Zhang. Characterization of physicochemical properties of ivy nanoparticles for cosmetic application. Journal of Nanobiotechnology, Vol. 11, No. 1, 2013, id. 310.1186/1477-3155-11-3Suche in Google Scholar PubMed PubMed Central
[60] Tapfumaneyi, P., M. Imran, Y. Mohammed, and M. S. Roberts. Recent advances and future prospective of topical and transdermal delivery systems. Frontiers in Drug Delivery, Vol. 2, 2022, pp. 1–8.10.3389/fddev.2022.957732Suche in Google Scholar
[61] Venditti, I. Morphologies and functionalities of polymeric nanocarriers as chemical tools for drug delivery: A review. Journal of King Saud University - Science, Vol. 31, No. 3, 2019, pp. 398–411.10.1016/j.jksus.2017.10.004Suche in Google Scholar
[62] Yang, Y., Z. Fang, X. Chen, W. Zhang, Y. Xie, Y. Chen, et al. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Frontiers in Pharmacology, Vol. 8, 2017, pp. 1–20.10.3389/fphar.2017.00287Suche in Google Scholar PubMed PubMed Central
[63] Cao, M., J. Li, J. Tang, C. Chen, and Y. Zhao. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses, Characterization, and Dermal Safety Assessment. Small, Vol. 1239, 2016, pp. 5488–5496.10.1002/smll.201601574Suche in Google Scholar PubMed
[64] Huang, J. H., H. J. Parab, R. S. Liu, T. C. Lai, M. Hsiao, C. H. Chen, et al. Characterization of Functional Nanomaterials in Cosmetics and its Cytotoxic Effects. 13th International Conference on Biomedical Engineering, Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 806–809.10.1007/978-3-540-92841-6_198Suche in Google Scholar
[65] Nafisi, S., and H. I. Maibach. Nanotechnology in Cosmetics. Cosmetic science and technology, Elsevier, Amsterdam, 2017, pp. 337–369.10.1016/B978-0-12-802005-0.00022-7Suche in Google Scholar
[66] Chen, S., S. Hanning, J. Falconer, M. Locke, and J. Wen. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 144, 2019, pp. 18–39.10.1016/j.ejpb.2019.08.015Suche in Google Scholar PubMed
[67] Lahkar, S., and M. K. Das. Smart lipid nanoparticles for cosmetic use. Nanocosmeceuticals, Academic Press, Amsterdam, Netherlands, 2022, pp. 307–317.10.1016/B978-0-323-91077-4.00003-XSuche in Google Scholar
[68] Karami, Z. and M. Hamidi. Cubosomes: Remarkable drug delivery potential. Drug Discovery Today, Vol. 21, No. 5, 2016, pp. 789–801.10.1016/j.drudis.2016.01.004Suche in Google Scholar PubMed
[69] Van Tran, V., T. Loi Nguyen, J.-Y. Moon, and Y.-C. Lee. Core-shell materials, lipid particles and nanoemulsions, for delivery of active anti-oxidants in cosmetics applications: Challenges and development strategies. Chemical Engineering Journal, Vol. 368, 2019, pp. 88–114.10.1016/j.cej.2019.02.168Suche in Google Scholar
[70] Gimeno-Benito, I., A. Giusti, S. Dekkers, A. Haase, and G. Janer. A review to support the derivation of a worst-case dermal penetration value for nanoparticles. Regulatory Toxicology and Pharmacology, Vol. 119, 2021, id. 104836.10.1016/j.yrtph.2020.104836Suche in Google Scholar PubMed
[71] Zakaria, F., S. E. Ashari, I. D. Mat Azmi, and M. B. Abdul Rahman. Recent advances in encapsulation of drug delivery (active substance) in cubosomes for skin diseases. Journal of Drug Delivery Science and Technology, Vol. 68, 2022, id. 103097.10.1016/j.jddst.2022.103097Suche in Google Scholar
[72] Varghese, R., S. Salvi, P. Sood, B. Kulkarni, and D. Kumar. Cubosomes in cancer drug delivery: A review. Colloid and Interface Science Communications, Vol. 46, 2022, id. 100561.10.1016/j.colcom.2021.100561Suche in Google Scholar
[73] Ray, L. and K. C. Gupta. Role of Nanotechnology in Skin Remedies. Photocarcinogenesis & Photoprotection, Vol. 1, 2018, pp. 141–157.10.1007/978-981-10-5493-8_13Suche in Google Scholar
[74] Sanjana, A., M. G. Ahmed, and J. Gowda Bh. Development and evaluation of dexamethasone loaded cubosomes for the treatment of vitiligo. Materials Today: Proceedings, Vol. 50, 2022, pp. 197–205.10.1016/j.matpr.2021.04.120Suche in Google Scholar
[75] Mohsen, A. M., M. M. Younis, A. Salama, and A. B. Darwish. Cubosomes as a potential oral drug delivery system for enhancing the hepatoprotective effect of coenzyme Q10. Journal of Pharmaceutical Sciences, Vol. 110, No. 7, 2021, pp. 2677–2686.10.1016/j.xphs.2021.02.007Suche in Google Scholar PubMed
[76] Kwon, T. K., S. K. Hong, and J.-C. Kim. In vitro skin permeation of cubosomes containing triclosan. Journal of Industrial and Engineering Chemistry, Vol. 18, No. 1, 2012, pp. 563–567.10.1016/j.jiec.2011.11.031Suche in Google Scholar
[77] El-Komy, M., S. Shalaby, R. Hegazy, R. Abdel Hay, S. Sherif, and E. Bendas. Assessment of cubosomal alpha lipoic acid gel efficacy for the aging face: A single-blinded, placebo-controlled, right-left comparative clinical study. Journal of Cosmetic Dermatology, Vol. 16, No. 3, 2017, pp. 358–363.10.1111/jocd.12298Suche in Google Scholar PubMed
[78] Uyama, M., M. Nakano, J. Yamashita, and T. Handa. Useful modified cellulose polymers as new emulsifiers of cubosomes. Langmuir, Vol. 25, No. 8, 2009, pp. 4336–4338.10.1021/la900386qSuche in Google Scholar PubMed
[79] Kwon, T. K. and J.-C. Kim. Preparation and In Vitro skin permeation of cubosomes containing hinokitiol. Journal of Dispersion Science and Technology, Vol. 31, No. 7, 2010, pp. 1004–1009.10.1080/01932690903224862Suche in Google Scholar
[80] Sonneville-Aubrun, O., J. T. Simonnet, and F. L’Alloret. Nanoemulsions: A new vehicle for skincare products. Advances in Colloid and Interface Science, Vol. 108–109, 2004, pp. 145–149.10.1016/j.cis.2003.10.026Suche in Google Scholar PubMed
[81] Chellapa, P., F. D. Ariffin, A. M. Eid, A. A. Almahgoubi, A. T. Mohamed, Y. S. Issa, et al. Nanoemulsion for cosmetic application. European Journal of Biomedical and Pharmaceutical Sciences, Vol. 3, No. 7, 2016, pp. 8–11.Suche in Google Scholar
[82] Tiwari, U., N. G. Ganesan, J. Junnarkar, and V. Rangarajan. Toward the formulation of bio-cosmetic nanoemulsions: From plant-derived to microbial-derived ingredients. Journal of Dispersion Science and Technology, Vol. 43, No. 7, 2022, pp. 1061–1078.10.1080/01932691.2020.1847664Suche in Google Scholar
[83] Yukuyama, M. N., D. D. M. Ghisleni, T. J. A. Pinto, and N. A. Bou-Chacra. Nanoemulsion: Process selection and application in cosmetics – a review. International journal of cosmetic science, Vol. 38, No. 1, 2016, pp. 13–24.10.1111/ics.12260Suche in Google Scholar PubMed
[84] Sharma, S. and K. Sarangdevot. Nanoemulsions for cosmetics. International Journal of Advanced Research in Pharmaceutical & Bio Sciences, Vol. 1, 2012, id. 408.Suche in Google Scholar
[85] de Alcantara Lemos, J., A. Oliveira, R. S. Araujo, D. M. Townsend, L. A. M. Ferreira, and A. L. B. de Barros. Recent progress in micro and nano-encapsulation of bioactive derivatives of the Brazilian genus Pterodon. Biomed Pharmacother, Vol. 143, 2021, id. 112137.10.1016/j.biopha.2021.112137Suche in Google Scholar PubMed PubMed Central
[86] Sonneville-Aubrun, O., M. N. Yukuyama, and A. Pizzino. Application of Nanoemulsions in Cosmetics. Nanoemulsions, Academic Press, Amsterdam, Netherlands, 2018, pp. 435–475.10.1016/B978-0-12-811838-2.00014-XSuche in Google Scholar
[87] A, N., L. Kovooru, A. K. Behera, K. P. P. Kumar, and P. Srivastava. A critical review of synthesis procedures, applications and future potential of nanoemulsions. Advances in Colloid and Interface Science, Vol. 287, 2021, id. 102318.10.1016/j.cis.2020.102318Suche in Google Scholar PubMed
[88] Alliod, O., J.-P. Valour, S. Urbaniak, H. Fessi, D. Dupin, and C. Charcosset. Preparation of oil-in-water nanoemulsions at large-scale using premix membrane emulsification and Shirasu Porous Glass (SPG) membranes. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 557, 2018, pp. 76–84.10.1016/j.colsurfa.2018.04.045Suche in Google Scholar
[89] Salvioni, L., L. Morelli, E. Ochoa, M. Labra, L. Fiandra, L. Palugan, et al. The emerging role of nanotechnology in skincare. Advances in Colloid and Interface Science, Vol. 293, 2021, id. 102437.10.1016/j.cis.2021.102437Suche in Google Scholar PubMed
[90] Cardoza, C., V. Nagtode, A. Pratap, and S. N. Mali. Emerging applications of nanotechnology in cosmeceutical health science: Latest updates. Health Sciences Review, Vol. 4, 2022, pp. 1–8.10.1016/j.hsr.2022.100051Suche in Google Scholar
[91] Jacobus Berlitz, S., D. De Villa, L. A. Maschmann Inácio, S. Davies, K. C. Zatta, S. S. Guterres, et al. Azelaic acid-loaded nanoemulsion with hyaluronic acid – a new strategy to treat hyperpigmentary skin disorders. Drug Development and Industrial Pharmacy, Vol. 45, No. 4, 2019, pp. 642–650.10.1080/03639045.2019.1569032Suche in Google Scholar PubMed
[92] Sinerga. Sinerga skin revolution, Sinergia, Italy, 2022. https://www.sinerga.it/files/materie-prime/nanocream/nanocream-flyer.pdfOctober 6th.Suche in Google Scholar
[93] Hanacure. Nano emulsion moisturizer, Hanacure, 2022. https://www.hanacure.com/products/multi-peptide-nano-emulsion.Suche in Google Scholar
[94] Lu, W. C., D. W. Huang, C. R. Wang, C. H. Yeh, J. C. Tsai, Y. T. Huang, et al. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. Journal of Food and Drug Analysis, Vol. 26, No. 1, 2018, pp. 82–89.10.1016/j.jfda.2016.12.018Suche in Google Scholar PubMed PubMed Central
[95] Roberts, M. S., Y. Mohammed, M. N. Pastore, S. Namjoshi, S. Yousef, A. Alinaghi, et al. Topical and cutaneous delivery using nanosystems. Journal of Controlled Release, Vol. 247, 2017, pp. 86–105.10.1016/j.jconrel.2016.12.022Suche in Google Scholar PubMed
[96] Khezri, K., M. Saeedi, and S. Maleki Dizaj. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomedicine & Pharmacotherapy, Vol. 106, 2018, pp. 1499–1505.10.1016/j.biopha.2018.07.084Suche in Google Scholar PubMed
[97] Rahimpour, Y. and H. Hamishehkar. Liposomes in cosmeceutics. Expert Opinion on Drug Delivery, Vol. 9, No. 4, 2012, pp. 443–455.10.1517/17425247.2012.666968Suche in Google Scholar PubMed
[98] Boixeda, P., F. Feltes, J. L. Santiago, and J. Paoli. Future prospects in dermatologic applications of lasers, nanotechnology, and other new technologies. Actas Dermo-Sifiliográficas, Vol. 106, No. 3, 2015, pp. 168–179.10.1016/j.adengl.2015.01.002Suche in Google Scholar
[99] Soni, V., S. Chandel, P. Jain, and S. Asati. Role of liposomal drug-delivery system in cosmetics. Nanobiomaterials in Galenic Formulations and Cosmetics, Vol. 10, 2016, pp. 93–120.10.1016/B978-0-323-42868-2.00005-XSuche in Google Scholar
[100] Li, P.-H., W.-C. Lu, Y.-J. Chan, W.-C. Ko, C.-C. Jung, D. T. Le Huynh, et al. Extraction and characterization of collagen from sea cucumber (Holothuria cinerascens) and its potential application in moisturizing cosmetics. Aquaculture, Vol. 515, 2020, id. 734590.10.1016/j.aquaculture.2019.734590Suche in Google Scholar
[101] Abbasi, B. H., H. Fazal, N. Ahmad, M. Ali, N. Giglioli-Guivarch, and C. Hano. Nanomaterials for cosmeceuticals: Nanomaterials-induced advancement in cosmetics, challenges, and opportunities. Nanocosmetics, Vol. 1, 2020, pp. 79–108.10.1016/B978-0-12-822286-7.00005-XSuche in Google Scholar
[102] Abu Hajleh, M. N., R. Abu-Huwaij, A. Al-Samydai, L. K. Al-Halaseh, and E. A. Al-Dujaili. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. Journal of Cosmetic Dermatology, Vol. 20, No. 12, 2021, pp. 3818–3828.10.1111/jocd.14441Suche in Google Scholar PubMed
[103] Atallah, C., C. Viennet, S. Robin, S. Ibazizen, H. Greige-Gerges, and C. Charcosset. Effect of cysteamine hydrochloride-loaded liposomes on skin depigmenting and penetration. European Journal of Pharmaceutical Sciences, Vol. 168, 2022, id. 106082.10.1016/j.ejps.2021.106082Suche in Google Scholar PubMed
[104] Patel, R. and P. Prabhu. Nanocarriers as versatile delivery systems for effective management of acne. International Journal of Pharmaceutics, Vol. 579, 2020, id. 119140.10.1016/j.ijpharm.2020.119140Suche in Google Scholar PubMed
[105] Cuomo, F., S. Ceglie, M. Miguel, B. Lindman, and F. Lopez. Oral delivery of all-trans retinoic acid mediated by liposome carriers. Colloids and Surfaces B: Biointerfaces, Vol. 201, 2021, id. 111655.10.1016/j.colsurfb.2021.111655Suche in Google Scholar PubMed
[106] Ho, C. Y. and O. Dreesen. Faces of cellular senescence in skin aging. Mechanisms of Ageing and Development, Vol. 198, 2021, id. 111525.10.1016/j.mad.2021.111525Suche in Google Scholar PubMed
[107] Risaliti, L., A. Kehagia, E. Daoultzi, D. Lazari, M. C. Bergonzi, S. Vergkizi-Nikolakaki, et al. Liposomes loaded with Salvia triloba and Rosmarinus officinalis essential oils: In vitro assessment of antioxidant, antiinflammatory and antibacterial activities. Journal of Drug Delivery Science and Technology, Vol. 51, 2019, pp. 493–498.10.1016/j.jddst.2019.03.034Suche in Google Scholar
[108] Dong, X., T. Zhang, H. Wei, and L. Dang. Stability of sodium ascorbyl phosphate in the water-glycerol system. Journal of Pharmaceutical and Biomedical Analysis, Vol. 181, 2020, id. 113103.10.1016/j.jpba.2020.113103Suche in Google Scholar PubMed
[109] Li, J., C. Chang, J. Zhai, Y. Yang, and H. Yu. Ascorbyl palmitate effects on the stability of curcumin-loaded soybean phosphatidylcholine liposomes. Food Bioscience, Vol. 41, 2021, id. 100923.10.1016/j.fbio.2021.100923Suche in Google Scholar
[110] Estanqueiro, M., J. Conceição, M. H. Amaral, and J. M. Sousa Lobo. The role of liposomes and lipid nanoparticles in the skin hydration. Nanobiomaterials in galenic formulations and cosmetics, William Andrew Publishing, 2016, pp. 297–326.10.1016/B978-0-323-42868-2.00012-7Suche in Google Scholar
[111] Abosabaa, S. A., M. G. Arafa, and A. N. ElMeshad. Drug delivery systems integrated with conventional and advanced treatment approaches toward cellulite reduction. Journal of Drug Delivery Science and Technology, Vol. 60, 2020, id. 102084.10.1016/j.jddst.2020.102084Suche in Google Scholar
[112] Seyedabadi, M. M., H. Rostami, S. M. Jafari, and M. Fathi. Development and characterization of chitosan-coated nanoliposomes for encapsulation of caffeine. Food Bioscience, Vol. 40, 2021, id. 100857.10.1016/j.fbio.2020.100857Suche in Google Scholar
[113] Pandey, N. Proniosomes and ethosomes: New prospect in transdermal and dermal drug delivery system. International Journal of Pharmaceutical Sciences and Research, Vol. 2, No. 8, 2011, id. 1988.Suche in Google Scholar
[114] Verma, P. and K. Pathak. Therapeutic and cosmeceutical potential of ethosomes: An overview. Journal of Advanced Pharmaceutical Technology & Research, Vol. 1, No. 3, 2010, pp. 274–282.10.4103/0110-5558.72415Suche in Google Scholar PubMed PubMed Central
[115] Sakellari, G. I., I. Zafeiri, H. Batchelor, and F. Spyropoulos. Solid lipid nanoparticles and nanostructured lipid carriers of dual functionality at emulsion interfaces. Part I: Pickering stabilisation functionality. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 654, 2022, id. 130135.10.1016/j.colsurfa.2022.130135Suche in Google Scholar
[116] Sakellari, G., J. Zafeiri, H. Batchelor, and F. Spyropoulos. Formulation design, production and characterisation of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the encapsulation of a model hydrophobic active. Food Hydrocolloids for Health, Vol. 1, 2021, id. 100024.10.1016/j.fhfh.2021.100024Suche in Google Scholar PubMed PubMed Central
[117] Arora, N., S. Agarwal, and R. Murthy. Latest technology advances in cosmaceuticals. International Journal of Pharmaceutical Sciences and Drug Research, Vol. 4, No. 3, 2012, pp. 168–182.Suche in Google Scholar
[118] Viegas, C., F. Seck, and P. Fonte. An insight on lipid nanoparticles for therapeutic proteins delivery. Journal of Drug Delivery Science and Technology, Vol. 77, 2022, id. 103839.10.1016/j.jddst.2022.103839Suche in Google Scholar
[119] Veider, F., Z. B. Akkuş-Dağdeviren, P. Knoll, and A. Bernkop-Schnürch. Design of nanostructured lipid carriers and solid lipid nanoparticles for enhanced cellular uptake. International Journal of Pharmaceutics, Vol. 624, 2022, id. 122014.10.1016/j.ijpharm.2022.122014Suche in Google Scholar PubMed
[120] Fang, C. L., I. A. Aljuffali, Y. C. Li, and J. Y. Fang. Delivery and targeting of nanoparticles into hair follicles. Therapeutic Delivery, Vol. 5, No. 9, 2014, pp. 991–1006.10.4155/tde.14.61Suche in Google Scholar PubMed
[121] Eftekhari, A., H. Ahmadian, M. A. Eghbal, and E. Ahmadian. The protective and antioxidant role of betanin against atrazine-induced toxicity in primary cultured rat hepatocytes. Journal of advanced chemical and pharmaceutical materials (JACPM), Vol. 1, No. 1, 2018, pp. 3–9.Suche in Google Scholar
[122] Akbarzadeh, A., R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, et al. Liposome: Classification, preparation, and applications. Nanoscale research letters, Vol. 8, No. 1, 2013, pp. 1–9.10.1186/1556-276X-8-102Suche in Google Scholar PubMed PubMed Central
[123] Puglia, C. and F. Bonina. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opinion on Drug Delivery, Vol. 9, No. 4, 2012, pp. 429–441.10.1517/17425247.2012.666967Suche in Google Scholar PubMed
[124] Byun, H. J., K. H. Cho, H. C. Eun, M.-J. Lee, Y. Lee, S. Lee, et al. Lipid ingredients in moisturizers can modulate skin responses to UV in barrier-disrupted human skin in vivo. Journal of Dermatological Science, Vol. 65, No. 2, 2012, pp. 110–117.10.1016/j.jdermsci.2011.12.005Suche in Google Scholar PubMed
[125] Dolatabadi, S., M. Karimi, S. Nasirizadeh, M. Hatamipour, S. Golmohammadzadeh, and M. R. Jaafari. Preparation, characterization and in vivo pharmacokinetic evaluation of curcuminoids-loaded solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs). Journal of Drug Delivery Science and Technology, Vol. 62, 2021, id. 102352.10.1016/j.jddst.2021.102352Suche in Google Scholar
[126] Salvi, V. R. and P. Pawar. Nanostructured lipid carriers (NLC) system: A novel drug targeting carrier. Journal of Drug Delivery Science and Technology, Vol. 51, 2019, pp. 255–267.10.1016/j.jddst.2019.02.017Suche in Google Scholar
[127] Amasya, G., O. Inal, and C. T. Sengel-Turk. SLN enriched hydrogels for dermal application: Full factorial design study to estimate the relationship between composition and mechanical properties. Chemistry and Physics of Lipids, Vol. 228, 2020, id. 104889.10.1016/j.chemphyslip.2020.104889Suche in Google Scholar PubMed
[128] López-García, R. and A. Ganem-Rondero. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. Journal of Cosmetics, Dermatological Sciences and Applications, Vol. 0502, 2015, pp. 62–72.10.4236/jcdsa.2015.52008Suche in Google Scholar
[129] Tichota, D. M., A. C. Silva, J. M. Sousa Lobo, and M. H. Amaral. Design, characterization, and clinical evaluation of argan oil nanostructured lipid carriers to improve skin hydration. International Journal of Nanomedicine, Vol. 9, 2014, pp. 3855–3864.10.2147/IJN.S64008Suche in Google Scholar PubMed PubMed Central
[130] Puglia, C., F. Bonina, L. Rizza, P. Blasi, A. Schoubben, R. Perrotta, et al. Lipid nanoparticles as carrier for octyl-methoxycinnamate: In vitro percutaneous absorption and photostability studies. Journal of Pharmaceutical Sciences, Vol. 101, No. 1, 2012, pp. 301–311.10.1002/jps.22741Suche in Google Scholar PubMed
[131] Bhardwaj, P., P. Tripathi, R. Gupta, and S. Pandey. Niosomes: A review on niosomal research in the last decade. Journal of Drug Delivery Science and Technology, Vol. 56, 2020, p. 101581.10.1016/j.jddst.2020.101581Suche in Google Scholar
[132] Kaur, D. and S. Kumar. Niosomes: Present scenario and future aspects. Journal of Drug Delivery and Therapeutics, Vol. 8, No. 5, 2018, pp. 35–43.10.22270/jddt.v8i5.1886Suche in Google Scholar
[133] Cheng, R., T. Xu, C. Wang, and C. Gan. The stabilization and antioxidant performances of coenzyme Q10-loaded niosomes coated by PEG and chitosan. Journal of Molecular Liquids, Vol. 325, 2021, id. 115194.10.1016/j.molliq.2020.115194Suche in Google Scholar
[134] Thabet, Y., M. Elsabahy, and N. G. Eissa. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods, Vol. 199, 2022, pp. 9–15.10.1016/j.ymeth.2021.05.004Suche in Google Scholar PubMed
[135] Machado, N. D., P. García-Manrique, M. A. Fernández, M. C. Blanco-López, M. Matos, and G. Gutiérrez. Cholesterol free niosome production by microfluidics: Comparative with other conventional methods. Chemical Engineering Research and Design, Vol. 162, 2020, pp. 162–171.10.1016/j.cherd.2020.08.002Suche in Google Scholar
[136] Lo, C. T., A. Jahn, L. E. Locascio, and W. N. Vreeland. Controlled Self-Assembly of Monodisperse Niosomes by Microfluidic Hydrodynamic Focusing. Langmuir, Vol. 2611, 2010, pp. 8559–8566.10.1021/la904616sSuche in Google Scholar PubMed
[137] Nasir, A., S. Harikumar, and K. Amanpreet. Niosomes: An excellent tool for drug delivery. International Journal of Research in Pharmacy and Chemistry, Vol. 2, No. 2, 2012, pp. 479–487.Suche in Google Scholar
[138] Arunachalam, A., S. Jeganath, K. Yamini, and K. Tharangini. Niosomes: A novel drug delivery system. International Journal of Novel Trends in Pharmaceutical Sciences, Vol. 2, No. 1, 2012, pp. 25–31.Suche in Google Scholar
[139] Yasamineh, S., P. Yasamineh, H. Ghafouri Kalajahi, O. Gholizadeh, Z. Yekanipour, H. Afkhami, et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. International Journal of Pharmaceutics, Vol. 624, 2022, id. 121878.10.1016/j.ijpharm.2022.121878Suche in Google Scholar PubMed
[140] Masjedi, M. and T. Montahaei. An illustrated review on nonionic surfactant vesicles (niosomes) as an approach in modern drug delivery: Fabrication, characterization, pharmaceutical, and cosmetic applications. Journal of Drug Delivery Science and Technology, Vol. 61, 2021, id. 102234.10.1016/j.jddst.2020.102234Suche in Google Scholar
[141] Chen, S., S. Hanning, J. Falconer, M. Locke, and J. Wen. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 144, 2019, pp. 18–39.10.1016/j.ejpb.2019.08.015Suche in Google Scholar PubMed
[142] Sudheer, P. and K. Kaushik. Review on niosomes. A novel approach for drug targeting. Journal of Pharmaceutical Research, Vol. 14, No. 1, 2015, pp. 20–25.10.18579/jpcrkc/2015/14/1/78376Suche in Google Scholar
[143] Kakar, R., R. Rao, A. Goswami, S. Nanda, and K. Saroha. Proniosomes: An emerging vesicular system in drug delivery and cosmetics. Der Pharmacia Lettre, Vol. 2, No. 4, 2010, pp. 227–239.Suche in Google Scholar
[144] Yasam, V. R., S. L. Jakki, J. Natarajan, and G. Kuppusamy. A review on novel vesicular drug delivery: Proniosomes. Drug Delivery, Vol. 21, No. 4, 2014, pp. 243–249.10.3109/10717544.2013.841783Suche in Google Scholar PubMed
[145] Kumari, R., K. Verma, A. Verma, G. K. Yadav, and S. D. Maurya. Proniosomes: A key to improved drug delivery. Journal of Drug Delivery and Therapeutics, Vol. 1, 2014, pp. 56–65.10.22270/jddt.v0i0.875Suche in Google Scholar
[146] Bachhav, A. A. Proniosome: A novel non-ionic provesicules as potential drug carrier. Asian Journal of Pharmaceutics (AJP), Vol. 10, No. 3, 2016, pp. 1–13.10.22377/ajp.v10i03.757Suche in Google Scholar
[147] Singla, S., S. HariKumar, and G. Aggarwal. Proniosomes for penetration enhancement in transdermal system. International Journal of Drug Development and Research, Vol. 4, No. 2, 2012.Suche in Google Scholar
[148] Patel, N. N., K. R. Vikran, A. Gupta, and A. Gupta. Proniosomes for improved transdermal drug delivery–A review. The Pharma, a Journal of Pharmacy Research, Vol. 4, 2013, pp. 42–48.Suche in Google Scholar
[149] Purohit, S. J., M. Tharmavaram, D. Rawtani, P. Prajapati, H. Pandya, and A. Dey. Niosomes as cutting edge nanocarrier for controlled and targeted delivery of essential oils and biomolecules. Journal of Drug Delivery Science and Technology, Vol. 73, 2022, id. 103438.10.1016/j.jddst.2022.103438Suche in Google Scholar
[150] Saleh, A., M. Pirouzifard, and M. Alizadeh. khaledabad, and H. Almasi. Optimization and Characterization of Lippia citriodora Essential Oil Loaded Niosomes: A Novel Plant-based Food Nano Preservative. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 650, 2022, id. 129480.10.1016/j.colsurfa.2022.129480Suche in Google Scholar
[151] Temprom, L., S. Krongsuk, S. Thapphasaraphong, A. Priperm, and S. Namuangruk. A novel preparation and characterization of melatonin loaded niosomes based on using a ball milling method. Materials Today Communications, Vol. 31, 2022, id. 103340.10.1016/j.mtcomm.2022.103340Suche in Google Scholar
[152] Singh, P., H. Ansari, and S. Dabre. Niosomes-a novel tool for anti-ageing cosmeceuticals. Journal of Pharmaceutical Research, Vol. 610, 2016, pp. 6691–6703.Suche in Google Scholar
[153] Chaikul, P., N. Khat-udomkiri, K. Iangthanarat, J. Manosroi, and A. Manosroi. Characteristics and in vitro anti-skin aging activity of gallic acid loaded in cationic CTAB niosome. European Journal of Pharmaceutical Sciences, Vol. 131, 2019, pp. 39–49.10.1016/j.ejps.2019.02.008Suche in Google Scholar PubMed
[154] Manosroi, A., P. Jantrawut, H. Akazawa, T. Akihisa, W. Manosroi, and J. Manosroi. Transdermal absorption enhancement of gel containing elastic niosomes loaded with gallic acid from Terminalia chebula galls. Pharmaceutical Biology, Vol. 49, No. 6, 2011, pp. 553–562.10.3109/13880209.2011.576347Suche in Google Scholar PubMed
[155] Manosroi, A., R. Chutoprapat, M. Abe, W. Manosroi, and J. Manosroi. Anti-aging efficacy of topical formulations containing niosomes entrapped with rice bran bioactive compounds. Pharmaceutical Biology, Vol. 50, No. 2, 2012, pp. 208–224.10.3109/13880209.2011.596206Suche in Google Scholar PubMed
[156] Tansathien, K., P. Chareanputtakhun, T. Ngawhirunpat, P. Opanasopit, and W. Rangsimawong. Hair growth promoting effect of bioactive extract from deer antler velvet-loaded niosomes and microspicules serum. International Journal of Pharmaceutics, Vol. 597, 2021, id. 120352.10.1016/j.ijpharm.2021.120352Suche in Google Scholar PubMed
[157] Lane, M. E. Nanoparticles and the skin–applications and limitations. Journal of Microencapsulation, Vol. 28, No. 8, 2011, pp. 709–716.10.3109/02652048.2011.599440Suche in Google Scholar PubMed
[158] Souto, E. B., A. R. Fernandes, C. Martins-Gomes, T. E. Coutinho, A. Durazzo, M. Lucarini, et al. Nanomaterials for skin delivery of cosmeceuticals and pharmaceuticals. Applied Sciences, Vol. 10, No. 5, 2020, pp. 1–23.10.3390/app10051594Suche in Google Scholar
[159] Nafisi, S., M. Schafer-Korting, and H. I. Maibach. Perspectives on percutaneous penetration: Silica nanoparticles. Nanotoxicology, Vol. 9, No. 5, 2015, pp. 643–657.10.3109/17435390.2014.958115Suche in Google Scholar PubMed
[160] Singh, T. G. and N. Sharma. Nanobiomaterials in cosmetics: Current status and future prospects. Nanobiomaterials in Galenic Formulations and Cosmetics, Vol. 10, 2016, pp. 149–174.10.1016/B978-0-323-42868-2.00007-3Suche in Google Scholar
[161] Paiva-Santos, A. C., A. M. Herdade, C. Guerra, D. Peixoto, M. Pereira-Silva, M. Zeinali, et al. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. International Journal of Pharmaceutics, Vol. 597, 2021, id. 120311.10.1016/j.ijpharm.2021.120311Suche in Google Scholar PubMed
[162] Pan, S., T. B. Goudoulas, J. Jeevanandam, K. X. Tan, S. Chowdhury, and M. K. Danquah. Therapeutic applications of metal and metal-oxide nanoparticles: Dermato-cosmetic perspectives. Frontiers in Bioengineering and Biotechnology, Vol. 9, 2021, id. 724499.10.3389/fbioe.2021.724499Suche in Google Scholar PubMed PubMed Central
[163] Gajbhiye, S. and S. Sakharwade. Silver nanoparticles in cosmetics. Journal of Cosmetics, Dermatological Sciences and Applications, Vol. 0601, 2016, pp. 48–53.10.4236/jcdsa.2016.61007Suche in Google Scholar
[164] Morais, R. P., S. Hochheim, C. C. de Oliveira, I. C. Riegel-Vidotti, and C. E. B. Marino. Skin interaction, permeation, and toxicity of silica nanoparticles: Challenges and recent therapeutic and cosmetic advances. International Journal of Pharmaceutics, Vol. 614, 2022, id. 121439.10.1016/j.ijpharm.2021.121439Suche in Google Scholar PubMed
[165] Mebert, A. M., C. J. Baglole, M. F. Desimone, and D. Maysinger. Nanoengineered silica: Properties, applications and toxicity. Food and Chemical Toxicology, Vol. 109, 2017, pp. 753–770.10.1016/j.fct.2017.05.054Suche in Google Scholar PubMed
[166] Subramaniam, V. D., S. V. Prasad, A. Banerjee, M. Gopinath, R. Murugesan, F. Marotta, et al. Health hazards of nanoparticles: Understanding the toxicity mechanism of nanosized ZnO in cosmetic products. Drug and Chemical Toxicology, Vol. 42, No. 1, 2019, pp. 84–93.10.1080/01480545.2018.1491987Suche in Google Scholar PubMed
[167] Nipane, D., S. Thakare, and N. Khati. ZnO nanoparticle by sol–gel and its UV application in cosmetics formulation. International Journal of Knowledge Engineering, Vol. 3, No. 1, 2012, pp. 168–169.Suche in Google Scholar
[168] Nath, R., R. Chakraborty, R. Roy, D. Mukherjee, S. Nag, and A. Bhattacharya. Nanotechnology based cosmeceuticals. International Journal of Scientific Research in Science and Technology, Vol. 8, 2021, pp. 94–106.10.32628/IJSRST218421Suche in Google Scholar
[169] Nanda, S., A. Nanda, S. Lohan, R. Kaur, and B. Singh. Nanocosmetics: Performance enhancement and safety assurance. Nanobiomaterials in Galenic Formulations and Cosmetics, Vol. 10, 2016, pp. 47–67.10.1016/B978-0-323-42868-2.00003-6Suche in Google Scholar
[170] Gupta, S., R. Bansal, S. Gupta, N. Jindal, and A. Jindal. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatology Online Journal, Vol. 4, No. 4, 2013, pp. 267–272.10.4103/2229-5178.120635Suche in Google Scholar PubMed PubMed Central
[171] Ganesan, P. and D. K. Choi. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. International Journal of Nanomedicine, Vol. 11, 2016, pp. 1987–2007.10.2147/IJN.S104701Suche in Google Scholar PubMed PubMed Central
[172] Bilal, M. and H. M. N. Iqbal. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics, Vol. 7, No. 2, 2020, pp. 1–16.10.3390/cosmetics7020024Suche in Google Scholar
[173] Jiménez-Pérez, Z. E., P. Singh, Y.-J. Kim, R. Mathiyalagan, D.-H. Kim, M. H. Lee, et al. Applications of Panax ginseng leaves-mediated gold nanoparticles in cosmetics relation to antioxidant, moisture retention, and whitening effect on B16BL6 cells. Journal of Ginseng Research, Vol. 42, No. 3, 2018, pp. 327–333.10.1016/j.jgr.2017.04.003Suche in Google Scholar PubMed PubMed Central
[174] Rizzi, V., J. Gubitosa, P. Fini, S. Nuzzo, A. Agostiano, and P. Cosma. Snail slime-based gold nanoparticles: An interesting potential ingredient in cosmetics as an antioxidant, sunscreen, and tyrosinase inhibitor. Journal of Photochemistry and Photobiology B: Biology, Vol. 224, 2021, id. 112309.10.1016/j.jphotobiol.2021.112309Suche in Google Scholar PubMed
[175] Ben Haddada, M., E. Gerometta, R. Chawech, J. Sorres, A. Bialecki, S. Pesnel, et al. Morel. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids and Surfaces B: Biointerfaces, Vol. 189, 2020, id. 110855.10.1016/j.colsurfb.2020.110855Suche in Google Scholar PubMed
[176] Jiménez, Z., Y.-J. Kim, R. Mathiyalagan, K.-H. Seo, P. Mohanan, J.-C. Ahn, et al. Assessment of radical scavenging, whitening and moisture retention activities of Panax ginseng berry mediated gold nanoparticles as safe and efficient novel cosmetic material. Artificial Cells, Nanomedicine, and Biotechnology, Vol. 46, No. 2, 2018, pp. 333–340.10.1080/21691401.2017.1307216Suche in Google Scholar PubMed
[177] Kokura, S., O. Handa, T. Takagi, T. Ishikawa, Y. Naito, and T. Yoshikawa. Silver nanoparticles as a safe preservative for use in cosmetics. Nanomedicine, Vol. 6, No. 4, 2010, pp. 570–574.10.1016/j.nano.2009.12.002Suche in Google Scholar PubMed
[178] Qidwai, A., R. Kumar, and A. Dikshit. Green synthesis of silver nanoparticles by seed of Phoenix sylvestris L. and their role in the management of cosmetics embarrassment. Green Chemistry Letters and Reviews, Vol. 11, No. 2, 2018, pp. 176–188.10.1080/17518253.2018.1445301Suche in Google Scholar
[179] Nohynek, G. J., E. K. Dufour, and M. S. Roberts. Nanotechnology, cosmetics and the skin: Is there a health risk? Skin Pharmacology and Physiology, Vol. 21, No. 3, 2008, pp. 136–149.10.1159/000131078Suche in Google Scholar PubMed
[180] Mu, L. and R. L. Sprando. Application of nanotechnology in cosmetics. Pharmaceutical Research, Vol. 27, No. 8, 2010, pp. 1746–1749.10.1007/s11095-010-0139-1Suche in Google Scholar PubMed
[181] Darvin, M., K. König, M. Kellner-Hoefer, H. Breunig, W. Werncke, M. Meinke, et al. Safety assessment by multiphoton fluorescence/second harmonic generation/hyper-Rayleigh scattering tomography of ZnO nanoparticles used in cosmetic products. Skin Pharmacology and Physiology, Vol. 25, No. 4, 2012, pp. 219–226.10.1159/000338976Suche in Google Scholar PubMed
[182] Yu, J. X. and T. H. Li. Distinct biological effects of different nanoparticles commonly used in cosmetics and medicine coatings. Cell & Bioscience, Vol. 1, No. 1, 2011, pp. 1–9.10.1186/2045-3701-1-19Suche in Google Scholar PubMed PubMed Central
[183] Spoiala, A., G. Albu, A. Ficai, E. Andronescu, G. Voicu, and C. Ungureanu. The SiO2/ZnO composite materials for cosmetic creams. Digest Journal of Nanomaterials and Biostructures, Vol. 9, No. 4, 2014, pp. 1729–1737.Suche in Google Scholar
[184] Yousefi, M., A. Narmani, and S. M. Jafari. Dendrimers as efficient nanocarriers for the protection and delivery of bioactive phytochemicals. Advances in Colloid and Interface Science, Vol. 278, 2020, id. 102125.10.1016/j.cis.2020.102125Suche in Google Scholar PubMed
[185] Abbasi, E., S. F. Aval, A. Akbarzadeh, M. Milani, H. T. Nasrabadi, S. W. Joo, et al. Dendrimers: Synthesis, applications, and properties. Nanoscale Research Letters, Vol. 9, No. 1, 2014, pp. 247–247.10.1186/1556-276X-9-247Suche in Google Scholar PubMed PubMed Central
[186] Sherje, A. P., M. Jadhav, B. R. Dravyakar, and D. Kadam. Dendrimers: A versatile nanocarrier for drug delivery and targeting. International Journal of Pharmaceutics, Vol. 548, No. 1, 2018, pp. 707–720.10.1016/j.ijpharm.2018.07.030Suche in Google Scholar PubMed
[187] Kalhapure, R. S., M. K. Kathiravan, K. G. Akamanchi, and T. Govender. Dendrimers – from organic synthesis to pharmaceutical applications: An update. Pharmaceutical Development and Technology, Vol. 20, No. 1, 2015, pp. 22–40.10.3109/10837450.2013.862264Suche in Google Scholar PubMed
[188] Bradley, D. Dendrimersome library: Characterization. Materials Today, Vol. 13, No. 7, 2010, id. 12.10.1016/S1369-7021(10)70117-6Suche in Google Scholar
[189] Tabarzad, M., and F. Ghorbani-Bidkorbeh. Dendrimers formulations to enhance skin drug delivery. Dendrimer-based nanotherapeutics, Academic Press, Amsterdam, Netherlands, 2021, pp. 399–416.10.1016/B978-0-12-821250-9.00011-1Suche in Google Scholar
[190] Kaushik, R., J. Singh, and M. Chawla. Dendritic polymers: An innovative step towards green future. International Journal of Pharmaceutics Research, Vol. 11, No. 1, 2019, pp. 272–278.10.31838/ijpr/2019.11.01.028Suche in Google Scholar
[191] Chauhan, A., C. Patil, P. Jain, and H. Kulhari. Dendrimer-based marketed formulations and miscellaneous applications in cosmetics, veterinary, and agriculture. Pharmaceutical Applications of Dendrimers, Vol. 1, 2020, pp. 325–334.10.1016/B978-0-12-814527-2.00014-7Suche in Google Scholar
[192] Kaur, M. Dendrimers: Synthesis, its dosage forms and advantage over linear polymers. Mintage Journal of Pharmaceutical & Medical Sciences, Vol. 3, No. 3, 2014, pp. 15–19.Suche in Google Scholar
[193] Parat, A. and D. Felder-Flesch. General introduction on dendrimers, classical versus accelerated syntheses and characterizations. Dendrimers in Nanomedicine, Vol. 1, 2016, pp. 1–22.10.1201/9781315364513-2Suche in Google Scholar
[194] Yadwade, R., S. Gharpure, and B. Ankamwar. Nanotechnology in cosmetics pros and cons. Nano Express, Vol. 2, No. 2, 2021.10.1088/2632-959X/abf46bSuche in Google Scholar
[195] Prakash, P., K. K. Kunjal, and A. Shabaraya. Dendrimer architecture: A comprehensive review, World Journal of Pharmaceutical Research, 2021.Suche in Google Scholar
[196] Sanz del Olmo, N., C. E. Peña González, J. D. Rojas, R. Gómez, P. Ortega, A. Escarpa, et al. Antioxidant and Antibacterial Properties of Carbosilane Dendrimers Functionalized with Polyphenolic Moieties. Pharmaceutics, Vol. 12, No. 8, 2020, id. 698.10.3390/pharmaceutics12080698Suche in Google Scholar PubMed PubMed Central
[197] Derici, L., J. Harcup, and E. Khoshdel. Cosmetic composition useful for straightening hair comprising a dendrimer or hyperbranched polymer. Google Patents, No. CA2575381A1, Canada, 2005.Suche in Google Scholar
[198] Limura, T. and H. Furukawa. Copolymer having carbosiloxane dendrimer structure, and composition and cosmetic containing the same. WIPO, No. WO2011078407A1, United States, 2010.Suche in Google Scholar
[199] Tournilhac, F., and P. Simon. Cosmetic or dermatological topical compositions comprising dendritic polyesters. US Patent 6287552 B1, 2001.Suche in Google Scholar
[200] Forestier, S., and I. Rollat-Corvol. Deodorant composition and use thereof. US 6001342 A, 1999.Suche in Google Scholar
[201] Wolf, B. A., and F. Snyder. Cosmetic compositions having keratolytic and anti-acne activity. Google Patents, CA 2152640 C, 1995.Suche in Google Scholar
[202] Valia, D., M. Kaiser, C. Conger, and S. Scheibert. Keratin treatments. WIPO, No. WO2016160796A1, United States, WO 2016160796 A1, 2016.Suche in Google Scholar
[203] Shegokar, R. What nanocrystals can offer to cosmetic and dermal formulations. Nanobiomaterials in Galenic Formulations and Cosmetics, Applications of Nanobiomaterials, Elsevier, Vol. 10, 2016, pp. 69–91.10.1016/B978-0-323-42868-2.00004-8Suche in Google Scholar
[204] Zhai, X., J. Lademann, C. M. Keck, and R. H. Müller. Nanocrystals of medium soluble actives—Novel concept for improved dermal delivery and production strategy. International Journal of Pharmaceutics, Vol. 470, No. 1, 2014, pp. 141–150.10.1016/j.ijpharm.2014.04.060Suche in Google Scholar PubMed
[205] Pelikh, O., S. F. Hartmann, A. M. Abraham, and C. M. Keck. Nanocrystals for Dermal Application. Nanocosmetics, Vol. 1, 2019, pp. 161–177.10.1007/978-3-030-16573-4_8Suche in Google Scholar
[206] Müller, R. H., S. Gohla, and C. M. Keck. State of the art of nanocrystals – Special features, production, nanotoxicology aspects and intracellular delivery. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 78, No. 1, 2011, pp. 1–9.10.1016/j.ejpb.2011.01.007Suche in Google Scholar PubMed
[207] Manikanika J., S. Kumar, and S. Jaswal. Role of nanotechnology in the world of cosmetology: A review. Materials Today: Proceedings, Elsevier, Netherlands, 2021.10.1016/j.matpr.2020.12.638Suche in Google Scholar
[208] Gujar, K. and S. Wairkar. Nanocrystal technology for improving therapeutic efficacy of flavonoids. Phytomedicine, Vol. 71, 2020, id. 153240.10.1016/j.phymed.2020.153240Suche in Google Scholar PubMed
[209] Vidlářová, L., G. B. Romero, J. Hanuš, F. Štěpánek, and R. H. Müller. Nanocrystals for dermal penetration enhancement – Effect of concentration and underlying mechanisms using curcumin as model. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 104, 2016, pp. 216–225.10.1016/j.ejpb.2016.05.004Suche in Google Scholar PubMed
[210] Zhang, Z., B. Zhang, N. Grishkewich, R. Berry, and K. C. Tam. Cinnamate-Functionalized Cellulose Nanocrystals as UV-Shielding Nanofillers in Sunscreen and Transparent Polymer Films. Advanced Sustainable Systems, Vol. 3, No. 4, 2019, id. 1800156.10.1002/adsu.201800156Suche in Google Scholar
[211] Mendoza, D. J., M. Maliha, V. S. Raghuwanshi, C. Browne, L. M. M. Mouterde, G. P. Simon, et al. Diethyl sinapate-grafted cellulose nanocrystals as nature-inspired UV filters in cosmetic formulations. Materials Today Bio, Vol. 12, 2021, id. 100126.10.1016/j.mtbio.2021.100126Suche in Google Scholar PubMed PubMed Central
[212] Köpke, D. and S. M. Pyo. Symurban nanocrystals for advanced anti-pollution skincare. Cosmetics, Vol. 7, No. 1, 2020, id. 17.10.3390/cosmetics7010017Suche in Google Scholar
[213] Lu, Y., J. Qi, X. Dong, W. Zhao, and W. Wu. The in vivo fate of nanocrystals. Drug Discovery Today, Vol. 22, No. 4, 2017, pp. 744–750.10.1016/j.drudis.2017.01.003Suche in Google Scholar PubMed
[214] Müller, R. H., X. Zhai, G. B. Romero, and C. M. Keck. Nanocrystals for Passive Dermal Penetration Enhancement. Percutaneous penetration enhancers chemical methods in penetration enhancement: nanocarriers, Springer Berlin Heidelberg, Berlin, Heidelberg, 2016, pp. 283–295.10.1007/978-3-662-47862-2_18Suche in Google Scholar
[215] Bansal, S., M. Bansal, and R. Kumria. Nanocrystals: Current strategies and trends. International Journal of Research in Pharmaceutical and Biomedical Sciences, Vol. 4, 2012, id. 10.Suche in Google Scholar
[216] Petersen, R. Nanocrystals for use in topical cosmetic formulations and method of production thereof. US Patent, No. US9114077B2, United States, 2007.Suche in Google Scholar
[217] Zhuhai HongJing Development Co. Sunscreen cream cosmetic composition containing nano crystal cellulose. China Patents, No. CN103230355B, China, 2013.Suche in Google Scholar
[218] Keck, C., R. Chen, and R. Müller. SmartCrystals for consumer care & cosmetics: Enhanced dermal delivery of poorly soluble plant actives. Househ Pers Care Today, Vol. 8, No. 5, 2013, pp. 18–24.Suche in Google Scholar
[219] Ahmadi Ashtiani, H. R., P. Bishe, N.-A. Lashgari, and M. A. Zare. Liposomes in cosmetics. Journal of Skin and Stem Cell, Vol. 3, No. 3, 2016, pp. 1–6.10.5812/jssc.65815Suche in Google Scholar
[220] Trucillo, P., R. Campardelli, S. Iuorio, P. De Stefanis, and E. Reverchon. Economic analysis of a new business for liposome manufacturing using a high-pressure system. Processes, Vol. 8, No. 12, 2020, pp. 1–13.10.3390/pr8121604Suche in Google Scholar
[221] Mrudula, B., K. Sanjay, M. Husain, M. Kailas, I. Vaibhav, S. Kiran, et al. Nanostructured lipid carriers based drug delivery system: A review. Indo American Journal of Pharmaceutical Research, Vol. 703, 2017, pp. 8045–8058.Suche in Google Scholar
[222] Karnati, V. C., G. N. Vishal, and K. Sandeep. Nanostructured lipid carriers: The frontiers in drug delivery. Asian Journal of Pharmaceutical and Clinical Research, 2019, pp. 8–12.10.22159/ajpcr.2019.v12i7.33595Suche in Google Scholar
[223] Chen, S., H. Huang, and G. Huang. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. International Journal of Biological Macromolecules, Vol. 140, 2019, pp. 1047–1053.10.1016/j.ijbiomac.2019.08.203Suche in Google Scholar PubMed
[224] Bartelds, R., M. H. Nematollahi, T. Pols, M. C. A. Stuart, A. Pardakhty, G. Asadikaram, et al. Niosomes, an alternative for liposomal delivery. PLoS One, Vol. 13, No. 4, 2018, id. e0194179.10.1371/journal.pone.0194179Suche in Google Scholar PubMed PubMed Central
[225] Borowska, S. and M. M. Brzoska. Metals in cosmetics: Implications for human health. Journal of Applied Toxicology, Vol. 35, No. 6, 2015, pp. 551–572.10.1002/jat.3129Suche in Google Scholar PubMed
[226] Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci, Vol. 15, No. 1, 2020, pp. 819–839.10.1515/biol-2020-0094Suche in Google Scholar PubMed PubMed Central
[227] Alaqad, K. and T. A. Saleh. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. Journal of Environmental & Analytical Toxicology, Vol. 6, No. 4, 2016, pp. 2–10.10.4172/2161-0525.1000384Suche in Google Scholar
[228] Pulit-Prociak, J., A. Grabowska, J. Chwastowski, T. M. Majka, and M. Banach. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloids and Surfaces B: Biointerfaces, Vol. 183, 2019, id. 110416.10.1016/j.colsurfb.2019.110416Suche in Google Scholar PubMed
[229] Niska, K., E. Zielinska, M. W. Radomski, and I. Inkielewicz-Stepniak. Metal nanoparticles in dermatology and cosmetology: Interactions with human skin cells. Chemico-Biological Interactions, Vol. 295, 2018, pp. 38–51.10.1016/j.cbi.2017.06.018Suche in Google Scholar PubMed
[230] Ong, W. T. J. and K. L. Nyam. Evaluation of silver nanoparticles in cosmeceutical and potential biosafety complications. Saudi Journal of Biological Sciences, Vol. 29, 2022, pp. 2085–2094.10.1016/j.sjbs.2022.01.035Suche in Google Scholar PubMed PubMed Central
[231] Chis, A. A., C. Dobrea, C. Morgovan, A. M. Arseniu, L. L. Rus, A. Butuca, et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules, Vol. 25, No. 17, 2020, pp. 1–41.10.3390/molecules25173982Suche in Google Scholar PubMed PubMed Central
[232] Cojocaru, F. D., D. Botezat, I. Gardikiotis, C. M. Uritu, G. Dodi, L. Trandafir, et al. Nanomaterials Designed for Antiviral Drug Delivery Transport across Biological Barriers. Pharmaceutics, Vol. 12, No. 2, 2020, pp. 1–34.10.3390/pharmaceutics12020171Suche in Google Scholar PubMed PubMed Central
[233] Vaidya, A., S. Jain, K. Pathak, and D. Pathak. Dendrimers: Nanosized Multifunctional Platform for Drug Delivery. Drug Delivery Letters, Vol. 8, No. 1, 2018, pp. 3–19.10.2174/2210303107666171109112523Suche in Google Scholar
[234] Muller, R. H., S. Gohla, and C. M. Keck. State of the art of nanocrystals--special features, production, nanotoxicology aspects and intracellular delivery. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 78, No. 1, 2011, pp. 1–9.10.1016/j.ejpb.2011.01.007Suche in Google Scholar PubMed
[235] Zhai, X., J. Lademann, C. M. Keck, and R. H. Muller. Dermal nanocrystals from medium soluble actives – physical stability and stability affecting parameters. European Journal of Pharmaceutics and Biopharmaceutics, Vol. 88, No. 1, 2014, pp. 85–91.10.1016/j.ejpb.2014.07.002Suche in Google Scholar PubMed
[236] Ghasemiyeh, P. and S. Mohammadi-Samani. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Design, Development and Therapy, Vol. 14, 2020, pp. 3271–3289.10.2147/DDDT.S264648Suche in Google Scholar PubMed PubMed Central
[237] Doge, N., S. Honzke, F. Schumacher, B. Balzus, M. Colombo, S. Hadam, et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin - assessment by intradermal microdialysis and extraction from the different skin layers. Journal of Controlled Release, Vol. 242, 2016, pp. 25–34.10.1016/j.jconrel.2016.07.009Suche in Google Scholar PubMed
[238] Chauhan, A. and C. Chauhan. Emerging trends of nanotechnology in beauty solutions: A review. Materials Today: Proceedings, Vol. 1, 2021, pp. 1–8.10.1016/j.matpr.2021.04.378Suche in Google Scholar
[239] Pereira-Silva, M., A. M. Martins, I. Sousa-Oliveira, H. M. Ribeiro, F. Veiga, J. Marto, et al. Nanomaterials in hair care and treatment. Acta Biomaterialia, Vol. 142, 2022, pp. 14–35.10.1016/j.actbio.2022.02.025Suche in Google Scholar PubMed
[240] Sarma, A., T. Chakraborty, and M. K. Das. Nanocosmeceuticals: Current trends, market analysis, and future trends. Nanocosmeceuticals, Academic Press, Amsterdam, Netherlands, 2022, pp. 525–558.10.1016/B978-0-323-91077-4.00013-2Suche in Google Scholar
[241] Zhao, Y., Q. Yang, D. Liu, T. Liu, and L. Xing. Neurotoxicity of nanoparticles: Insight from studies in zebrafish. Ecotoxicology and Environmental Safety, Vol. 242, 2022, id. 113896.10.1016/j.ecoenv.2022.113896Suche in Google Scholar PubMed
[242] Nadaf, S. J., N. R. Jadhav, H. S. Naikwadi, P. L. Savekar, I. D. Sapkal, M. M. Kambli, et al. Green synthesis of gold and silver nanoparticles: Updates on research, patents, and future prospects. OpenNano, Vol. 8, 2022, id. 100076.10.1016/j.onano.2022.100076Suche in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Review Articles
- State of the art, challenges, and emerging trends: Geopolymer composite reinforced by dispersed steel fibers
- A review on the properties of concrete reinforced with recycled steel fiber from waste tires
- Copper ternary oxides as photocathodes for solar-driven CO2 reduction
- Properties of fresh and hardened self-compacting concrete incorporating rice husk ash: A review
- Basic mechanical and fatigue properties of rubber materials and components for railway vehicles: A literature survey
- Research progress on durability of marine concrete under the combined action of Cl− erosion, carbonation, and dry–wet cycles
- Delivery systems in nanocosmeceuticals
- Study on the preparation process and sintering performance of doped nano-silver paste
- Analysis of the interactions between nonoxide reinforcements and Al–Si–Cu–Mg matrices
- Research Articles
- Study on the influence of structural form and parameters on vibration characteristics of typical ship structures
- Deterioration characteristics of recycled aggregate concrete subjected to coupling effect with salt and frost
- Novel approach to improve shale stability using super-amphiphobic nanoscale materials in water-based drilling fluids and its field application
- Research on the low-frequency multiline spectrum vibration control of offshore platforms
- Multiple wide band gaps in a convex-like holey phononic crystal strip
- Response analysis and optimization of the air spring with epistemic uncertainties
- Molecular dynamics of C–S–H production in graphene oxide environment
- Residual stress relief mechanisms of 2219 Al–Cu alloy by thermal stress relief method
- Characteristics and microstructures of the GFRP waste powder/GGBS-based geopolymer paste and concrete
- Development and performance evaluation of a novel environmentally friendly adsorbent for waste water-based drilling fluids
- Determination of shear stresses in the measurement area of a modified wood sample
- Influence of ettringite on the crack self-repairing of cement-based materials in a hydraulic environment
- Multiple load recognition and fatigue assessment on longitudinal stop of railway freight car
- Synthesis and characterization of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt used as acidizing corrosion inhibitor
- Perforated steel for realizing extraordinary ductility under compression: Testing and finite element modeling
- The influence of oiled fiber, freeze-thawing cycle, and sulfate attack on strain hardening cement-based composites
- Perforated steel block of realizing large ductility under compression: Parametric study and stress–strain modeling
- Study on dynamic viscoelastic constitutive model of nonwater reacted polyurethane grouting materials based on DMA
- Mechanical behavior and mechanism investigation on the optimized and novel bio-inspired nonpneumatic composite tires
- Effect of cooling rate on the microstructure and thermal expansion properties of Al–Mn–Fe alloy
- Research on process optimization and rapid prediction method of thermal vibration stress relief for 2219 aluminum alloy rings
- Failure prevention of seafloor composite pipelines using enhanced strain-based design
- Deterioration of concrete under the coupling action of freeze–thaw cycles and salt solution erosion
- Creep rupture behavior of 2.25Cr1Mo0.25V steel and weld for hydrogenation reactors under different stress levels
- Statistical damage constitutive model for the two-component foaming polymer grouting material
- Nano-structural and nano-constraint behavior of mortar containing silica aggregates
- Influence of recycled clay brick aggregate on the mechanical properties of concrete
- Effect of LDH on the dissolution and adsorption behaviors of sulfate in Portland cement early hydration process
- Comparison of properties of colorless and transparent polyimide films using various diamine monomers
- Study in the parameter influence on underwater acoustic radiation characteristics of cylindrical shells
- Experimental study on basic mechanical properties of recycled steel fiber reinforced concrete
- Dynamic characteristic analysis of acoustic black hole in typical raft structure
- A semi-analytical method for dynamic analysis of a rectangular plate with general boundary conditions based on FSDT
- Research on modification of mechanical properties of recycled aggregate concrete by replacing sand with graphite tailings
- Dynamic response of Voronoi structures with gradient perpendicular to the impact direction
- Deposition mechanisms and characteristics of nano-modified multimodal Cr3C2–NiCr coatings sprayed by HVOF
- Effect of excitation type on vibration characteristics of typical ship grillage structure
- Study on the nanoscale mechanical properties of graphene oxide–enhanced shear resisting cement
- Experimental investigation on static compressive toughness of steel fiber rubber concrete
- Study on the stress field concentration at the tip of elliptical cracks
- Corrosion resistance of 6061-T6 aluminium alloy and its feasibility of near-surface reinforcements in concrete structure
- Effect of the synthesis method on the MnCo2O4 towards the photocatalytic production of H2
- Experimental study of the shear strength criterion of rock structural plane based on three-dimensional surface description
- Evaluation of wear and corrosion properties of FSWed aluminum alloy plates of AA2020-T4 with heat treatment under different aging periods
- Thermal–mechanical coupling deformation difference analysis for the flexspline of a harmonic drive
- Frost resistance of fiber-reinforced self-compacting recycled concrete
- High-temperature treated TiO2 modified with 3-aminopropyltriethoxysilane as photoactive nanomaterials
- Effect of nano Al2O3 particles on the mechanical and wear properties of Al/Al2O3 composites manufactured via ARB
- Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors
- Effect of freeze–thaw cycles on deformation properties of deep foundation pit supported by pile-anchor in Harbin
- Temperature-porosity-dependent elastic modulus model for metallic materials
- Effect of diffusion on interfacial properties of polyurethane-modified asphalt–aggregate using molecular dynamic simulation
- Experimental study on comprehensive improvement of shear strength and erosion resistance of yellow mud in Qiang Village
- A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism
- In situ bow reduction during sublimation growth of cubic silicon carbide
- Adhesion behaviour of 3D printed polyamide–carbon fibre composite filament
- An experimental investigation and machine learning-based prediction for seismic performance of steel tubular column filled with recycled aggregate concrete
- Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel
- Application research of acoustic black hole in floating raft vibration isolation system
- Multi-objective parametric optimization on the EDM machining of hybrid SiCp/Grp/aluminum nanocomposites using Non-dominating Sorting Genetic Algorithm (NSGA-II): Fabrication and microstructural characterizations
- Estimating of cutting force and surface roughness in turning of GFRP composites with different orientation angles using artificial neural network
- Displacement recovery and energy dissipation of crimped NiTi SMA fibers during cyclic pullout tests
Artikel in diesem Heft
- Review Articles
- State of the art, challenges, and emerging trends: Geopolymer composite reinforced by dispersed steel fibers
- A review on the properties of concrete reinforced with recycled steel fiber from waste tires
- Copper ternary oxides as photocathodes for solar-driven CO2 reduction
- Properties of fresh and hardened self-compacting concrete incorporating rice husk ash: A review
- Basic mechanical and fatigue properties of rubber materials and components for railway vehicles: A literature survey
- Research progress on durability of marine concrete under the combined action of Cl− erosion, carbonation, and dry–wet cycles
- Delivery systems in nanocosmeceuticals
- Study on the preparation process and sintering performance of doped nano-silver paste
- Analysis of the interactions between nonoxide reinforcements and Al–Si–Cu–Mg matrices
- Research Articles
- Study on the influence of structural form and parameters on vibration characteristics of typical ship structures
- Deterioration characteristics of recycled aggregate concrete subjected to coupling effect with salt and frost
- Novel approach to improve shale stability using super-amphiphobic nanoscale materials in water-based drilling fluids and its field application
- Research on the low-frequency multiline spectrum vibration control of offshore platforms
- Multiple wide band gaps in a convex-like holey phononic crystal strip
- Response analysis and optimization of the air spring with epistemic uncertainties
- Molecular dynamics of C–S–H production in graphene oxide environment
- Residual stress relief mechanisms of 2219 Al–Cu alloy by thermal stress relief method
- Characteristics and microstructures of the GFRP waste powder/GGBS-based geopolymer paste and concrete
- Development and performance evaluation of a novel environmentally friendly adsorbent for waste water-based drilling fluids
- Determination of shear stresses in the measurement area of a modified wood sample
- Influence of ettringite on the crack self-repairing of cement-based materials in a hydraulic environment
- Multiple load recognition and fatigue assessment on longitudinal stop of railway freight car
- Synthesis and characterization of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt used as acidizing corrosion inhibitor
- Perforated steel for realizing extraordinary ductility under compression: Testing and finite element modeling
- The influence of oiled fiber, freeze-thawing cycle, and sulfate attack on strain hardening cement-based composites
- Perforated steel block of realizing large ductility under compression: Parametric study and stress–strain modeling
- Study on dynamic viscoelastic constitutive model of nonwater reacted polyurethane grouting materials based on DMA
- Mechanical behavior and mechanism investigation on the optimized and novel bio-inspired nonpneumatic composite tires
- Effect of cooling rate on the microstructure and thermal expansion properties of Al–Mn–Fe alloy
- Research on process optimization and rapid prediction method of thermal vibration stress relief for 2219 aluminum alloy rings
- Failure prevention of seafloor composite pipelines using enhanced strain-based design
- Deterioration of concrete under the coupling action of freeze–thaw cycles and salt solution erosion
- Creep rupture behavior of 2.25Cr1Mo0.25V steel and weld for hydrogenation reactors under different stress levels
- Statistical damage constitutive model for the two-component foaming polymer grouting material
- Nano-structural and nano-constraint behavior of mortar containing silica aggregates
- Influence of recycled clay brick aggregate on the mechanical properties of concrete
- Effect of LDH on the dissolution and adsorption behaviors of sulfate in Portland cement early hydration process
- Comparison of properties of colorless and transparent polyimide films using various diamine monomers
- Study in the parameter influence on underwater acoustic radiation characteristics of cylindrical shells
- Experimental study on basic mechanical properties of recycled steel fiber reinforced concrete
- Dynamic characteristic analysis of acoustic black hole in typical raft structure
- A semi-analytical method for dynamic analysis of a rectangular plate with general boundary conditions based on FSDT
- Research on modification of mechanical properties of recycled aggregate concrete by replacing sand with graphite tailings
- Dynamic response of Voronoi structures with gradient perpendicular to the impact direction
- Deposition mechanisms and characteristics of nano-modified multimodal Cr3C2–NiCr coatings sprayed by HVOF
- Effect of excitation type on vibration characteristics of typical ship grillage structure
- Study on the nanoscale mechanical properties of graphene oxide–enhanced shear resisting cement
- Experimental investigation on static compressive toughness of steel fiber rubber concrete
- Study on the stress field concentration at the tip of elliptical cracks
- Corrosion resistance of 6061-T6 aluminium alloy and its feasibility of near-surface reinforcements in concrete structure
- Effect of the synthesis method on the MnCo2O4 towards the photocatalytic production of H2
- Experimental study of the shear strength criterion of rock structural plane based on three-dimensional surface description
- Evaluation of wear and corrosion properties of FSWed aluminum alloy plates of AA2020-T4 with heat treatment under different aging periods
- Thermal–mechanical coupling deformation difference analysis for the flexspline of a harmonic drive
- Frost resistance of fiber-reinforced self-compacting recycled concrete
- High-temperature treated TiO2 modified with 3-aminopropyltriethoxysilane as photoactive nanomaterials
- Effect of nano Al2O3 particles on the mechanical and wear properties of Al/Al2O3 composites manufactured via ARB
- Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors
- Effect of freeze–thaw cycles on deformation properties of deep foundation pit supported by pile-anchor in Harbin
- Temperature-porosity-dependent elastic modulus model for metallic materials
- Effect of diffusion on interfacial properties of polyurethane-modified asphalt–aggregate using molecular dynamic simulation
- Experimental study on comprehensive improvement of shear strength and erosion resistance of yellow mud in Qiang Village
- A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism
- In situ bow reduction during sublimation growth of cubic silicon carbide
- Adhesion behaviour of 3D printed polyamide–carbon fibre composite filament
- An experimental investigation and machine learning-based prediction for seismic performance of steel tubular column filled with recycled aggregate concrete
- Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel
- Application research of acoustic black hole in floating raft vibration isolation system
- Multi-objective parametric optimization on the EDM machining of hybrid SiCp/Grp/aluminum nanocomposites using Non-dominating Sorting Genetic Algorithm (NSGA-II): Fabrication and microstructural characterizations
- Estimating of cutting force and surface roughness in turning of GFRP composites with different orientation angles using artificial neural network
- Displacement recovery and energy dissipation of crimped NiTi SMA fibers during cyclic pullout tests

