Abstract
Aiming at the current situation that the existing acidizing corrosion inhibitors are difficult to apply in high temperature, high concentration acid, and other complex conditions, this article uses nano-SiO2 as the core and preferably functional monomers to synthesize nano-SiO2@octadecylbisimidazoline quaternary ammonium salt (nano-SiO2@OBQA). Analytical methods such as nuclear magnetic resonance, infrared, and scanning electron microscopy were used for characterization. The corrosion inhibition performance of the N80 steel sheet by nano-SiO2@OBQA in 20% concentrated hydrochloric acid was studied using the high-temperature corrosion testing machine and adsorption isotherm model combined with quantum chemistry calculations to explore its mechanism of action. The results show that nano-SiO2@OBQA has good high-temperature resistance. When the temperature is 180℃ and the dosage of nano-SiO2@OBQA is 4%, the corrosion inhibition rate is 61.42 g·m−2·h−1. Studies have shown that the adsorption of nano-SiO2@OBQA on the surface of the N80 steel sheet follows the Langmuir isotherm adsorption model, which is spontaneous chemical adsorption.
1 Introduction
With the development of drilling engineering, the number of deep wells and ultra-deep wells has increased, and the bottom hole environment is gradually becoming more complicated, which puts forward higher requirements for the production of stimulation techniques such as acid fracturing [1,2,3,4,5]. In the oilfield development process, the pickling liquid can effectively increase the output of crude oil, but its corrosion of tubing and other equipment also brings additional operating costs [6,7]. At present, adding acidizing corrosion inhibitor is the most commonly used method of anti-corrosion [8,9,10], but it has been studied more in low-temperature and low-concentration acid solutions, and less research in high-temperature and high-concentration acid solutions [11]. Therefore, acidizing corrosion inhibitors with high-temperature resistance and high concentration acid resistance have become a problem that needs to be solved urgently.
There are three main methods of anti-corrosion. First, the corrosion can be controlled from the source, that is the development of high-corrosion-resistant metals or the addition of corrosion-resistant elements [12,13]. Second, a layer of anti-corrosion paint can be applied to the metal surface to isolate the metal from the corrosive environment [14,15]. In the third aspect, acidizing corrosion inhibitors can be added [16]. This method is the easiest to operate, cheapest, and effective, but it also has problems such as high toxicity and difficult degradation [17,18]. Based on this, the development of low-toxic and easily degradable acidizing corrosion inhibitors is particularly important [19]. Imidazoline acidizing corrosion inhibitors are a commonly used type in oilfields. They can form a film structure on the metal surface through chemical adsorption to prevent direct contact between acid and metal. At the same time, imidazoline acidizing corrosion inhibitors have the advantages of low toxicity, high efficiency, and excellent thermal stability [20,21,22]. Therefore, imidazoline acidizing corrosion inhibitors are widely used in oil and gas field development projects.
The application of nanomaterials has improved efficiency for oil and gas field development projects [23,24], especially the treatment agent with a “core–shell” structure obtained by combining inorganic and organic materials. This can be explained by the nanomaterials providing a rigid skeleton structure for the polymer. On the one hand, it can improve the temperature resistance of the polymer. On the other hand, it can enter the fracturing channel and play the role of supporting the channel. Therefore, the research and development of acidizing corrosion inhibitors with the framework of inorganic nanomaterials have great significance.
2 Materials and methods
2.1 Materials
Azelaic acid (CAS:123-99-9), analytical grade, was purchased from Shanghai Macleans Biochemical Technology Co., Ltd. N-(2-Hydroxypropyl)ethylenediamine (CAS:102-60-3), analytical grade, was bought from Jiangsu Aikang Biomedical R&D Co., Ltd. Xylene (CAS:1330-20-7), analytical grade, was from Shanghai Macleans Biochemical Technology Co., Ltd. Acetone, absolute ethanol, and Al2O3, analytically pure, were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. 4-Methoxy-3-methylbenzyl chloride, chemically pure, was purchased from Beijing Hanlongda Technology Development Co., Ltd. 4-Methoxy-3-methyl-1-chloromethane-cyclohexane, industrial grade, was from Weifang Qianjin Fine Chemical Co., Ltd.
2.2 Synthesis of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt
2.2.1 Surface coating of nano-SiO2 microspheres
Disperse 0.05 mol nano-SiO2 microspheres in 200 mL of deionized water solution, slowly add 0.2 mol azelaic acid dropwise, stir at 300 rpm at 20°C for 3 h, filter and freeze-dry to obtain nano-SiO2 microspheres coated with azelaic acid.
2.2.2 Synthesis of nano-SiO2@bisimidazoline intermediate
Put the product obtained in step (1) into a 500 mL three-necked flask equipped with an electric stirrer, a water separator, and a spherical condenser and then slowly add 10 mL of xylene at 5 mL·min−1, and add 0.01 mol of the catalyst Al2O3, Stir for 20 min, then slowly add 0.5 mol of N-(2-hydroxypropyl)ethylenediamine into the system with a constant pressure drop funnel, and reflux at 170℃ for 6 h, then react at 190℃ for 10 h. Use a rotary evaporator to evaporate and remove toluene at 150°C to obtain a bisimidazoline quaternary ammonium salt intermediate (Figure 1).
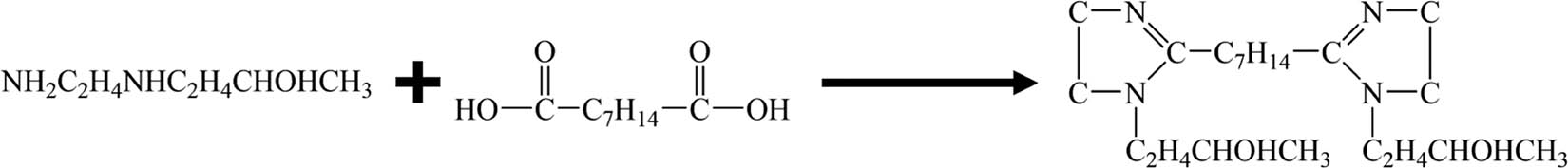
Synthesis of nano-SiO2@bisimidazoline intermediate.
2.2.3 Quaternization reaction
Disperse 0.2 mol of bisimidazoline quaternary ammonium salt intermediate in 250 mL of absolute ethanol to fully dissolve it, and add 0.5 mol of 4-methoxy-3-methyl-benzyl chloride at 40–50℃ for quaternization. After reacting for 2–4 h, the absolute ethanol is distilled out under reduced pressure, and then recrystallized three times with a mixed solution of ethanol and acetone in an ice-water bath, and dried in a vacuum drying oven to obtain the final product nano-SiO2@octadecylbisimidazoline quaternary ammonium salt (SiO2@OBQA) (Figure 2).
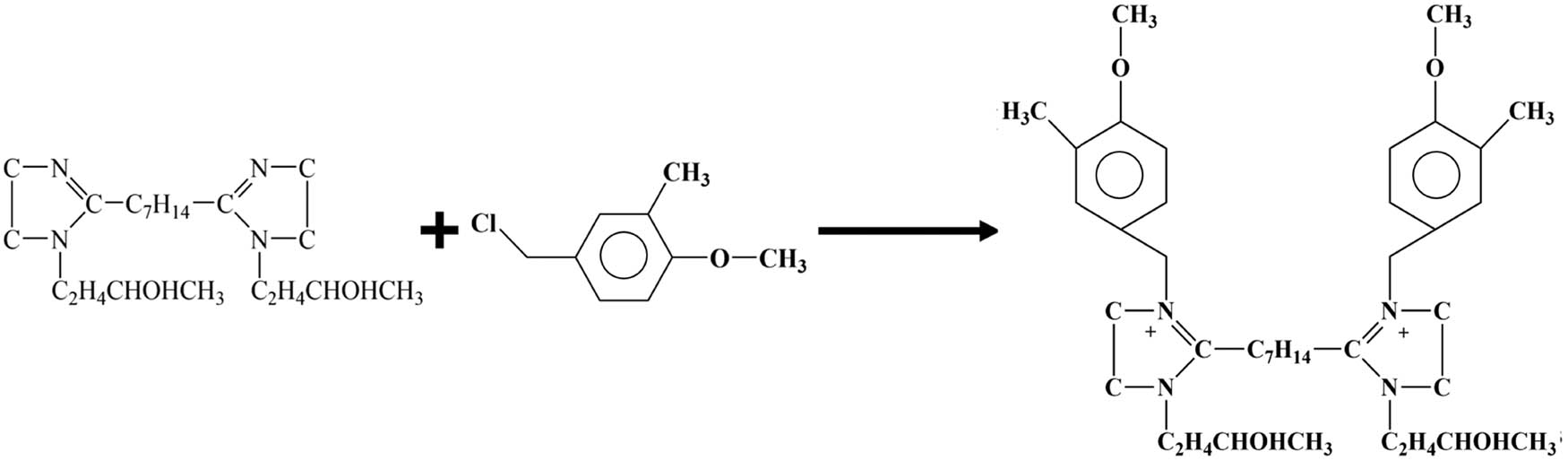
Quaternization reaction.
2.3 Characterization of physical and chemical properties
The nano-SiO2@OBQA was analyzed by infrared spectroscopy (resolution 4 cm−1, wavenumber range 4,000–400 cm−1, and scanning number 32) using American Thermo Fisher Nicolet IS5 FT-IR spectrometer. 1H-NMR analysis was performed on nano-SiO2@OBQA with a German Bruker AVANCE III 500 MHz nuclear magnetic resonance spectrometer (resolution ≤ 0.2 Hz, sensitivity 390:1 [5 mm 0.1% EB]). The thermal stability of nano-SiO2@OBQA was evaluated using the TG/DTA7300 thermogravimetric dual analyzer of Japan HITACHI company (the test temperature range is 20–600℃, the heating rate is 10℃·min−1, and the test gas atmosphere is N2). The microstructure of nano-SiO2@OBQA was characterized by the S-4800 SEM analyzer of Hitachi.
2.4 Evaluation of corrosion inhibition performance
Taking the N80 steel sheet as the corrosion test piece, the influence of temperature and nano-SiO2@OBQA concentration on corrosion inhibition performance was explored. The experiment uses two sets of parallel experiments to eliminate accidental errors and calculates the corrosion inhibition rate according to formula (1).
where η – corrosion inhibition rate, %. v – corrosion rate of steel sheet after adding acidizing corrosion inhibitor, g·m−2·h−1, v 0 – corrosion rate of steel sheet in acid, g·m−2·h−1.
2.5 Quantum chemistry research
Using the Chem3D Ultra program and the Huckel molecular orbital theory, the molecular structure optimization and quantum chemical calculations of the nano-SiO2@OBQA molecule are carried out.
3 Results and discussion
3.1 Molecular structure characterization
3.1.1 FT-IR analysis
It can be seen from Figure 3 that 3401.13 cm−1 is the stretching vibration peak of –OH, 2924.04 and 2853.65 cm−1 are the stretching vibration peaks of –CH3 and –CH2, respectively, and 1614.13 cm−1 is the stretching vibration of C═N. Peaks 1,461 and 1374.62 cm−1 are the stretching vibration peaks of C–H, 1213.00 and 1086 cm−1 are the stretching vibration peaks of C–N, 1010.04 cm−1 is the stretching vibration peak of C–O, 879.86 and 754.03 cm−1 are the characteristic peaks of the mono-substituted benzene ring. Therefore, it can be explained that nano-SiO2@OBQA was successfully synthesized.
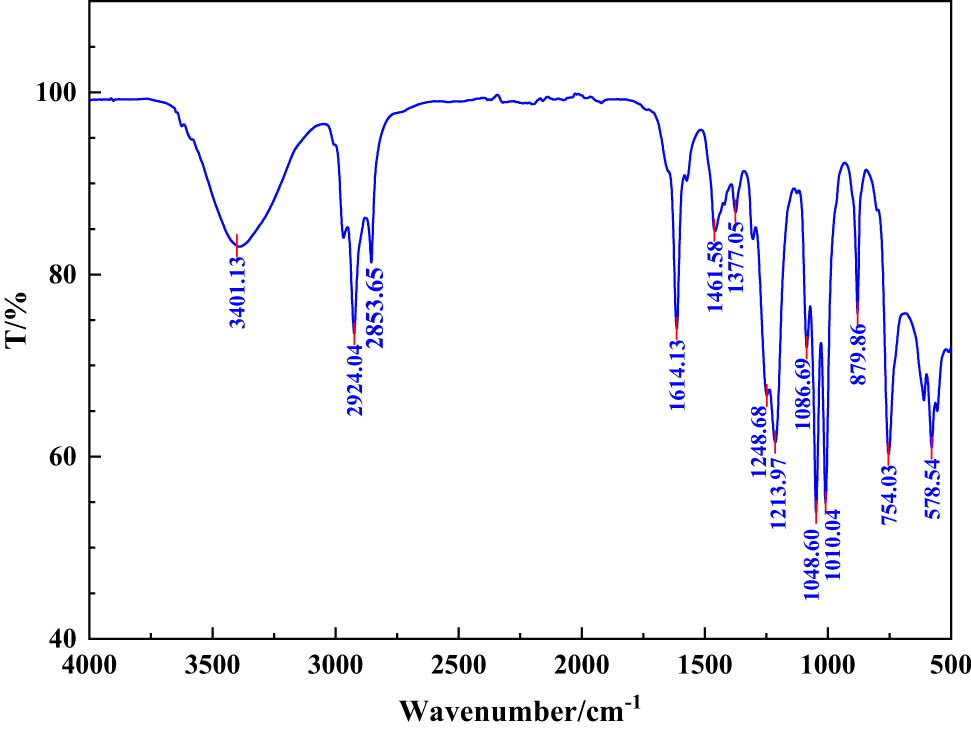
FT-IR analysis of emulsifier nano-SiO2@OBQA.
3.1.2 1H-NMR analysis
It can be seen from Figure 4 that nano-SiO2@OBQA mainly has nine kinds of absorption peaks, including nine kinds of hydrogen atoms. 0–2 ppm is alkane structure, 2–4 ppm is CH–NH2, hydroxyl structure, and 7.28 ppm is benzene ring structure. Among them, the absorption peak area with a chemical shift of 1.24 ppm is larger, indicating that the carbon chain in the alkane structure is longer, which can improve the temperature resistance of the imidazoline quaternary ammonium salt to a certain extent. The chemical shift of 2–4 ppm shows that an imidazoline ring is formed. At the same time, the addition ratio of azelaic acid and N-(2-hydroxypropyl)-ethylenediamine is 2:5, ensuring that azelaic acid can be combined with N-(2-hydroxypropyl)-ethylenediamine fully reacted to form a bisimidazole ring, which also shows that the imidazoline quaternary ammonium salt was successfully synthesized. The absorption peak with a chemical shift of 7.28 ppm indicates the presence of a benzene ring in the structure, which further improves the temperature resistance of the bisimidazoline quaternary ammonium salt.
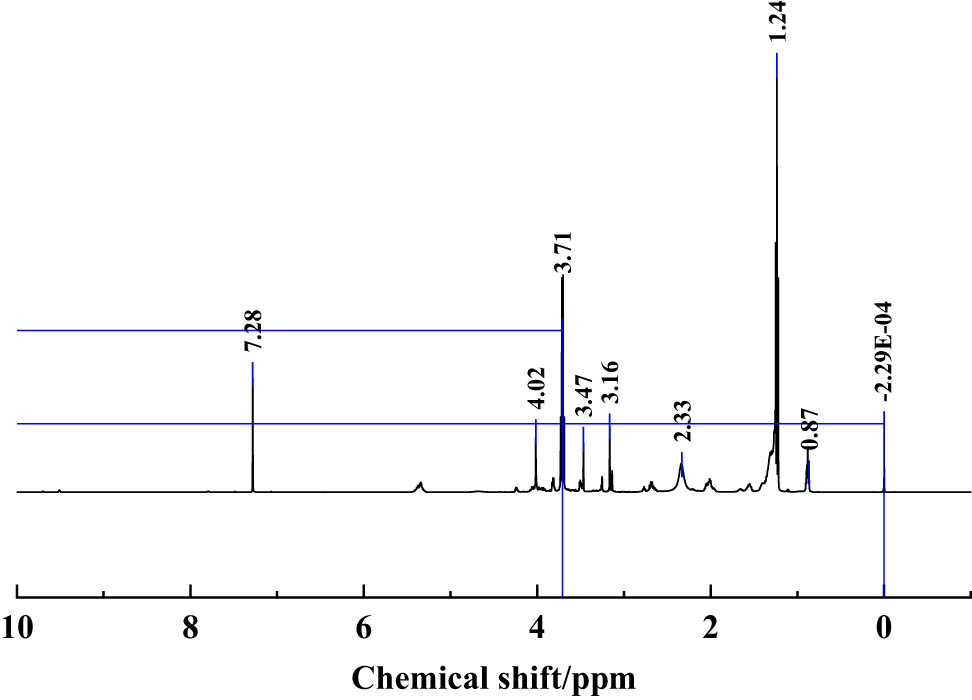
1H-NMR analysis of emulsifier nano-SiO2@OBQA.
3.1.3 Thermogravimetric analysis (TGA)
It can be seen from Figure 5 that the first mass loss of about 20% occurs in the range of room temperature to 100°C, which is mainly caused by the gradual evaporation of adsorbed water and bound water in the sample. At this time, the large mass loss rate is mainly due to the bisimidazoline intermediate produced by the reaction of azelaic acid and N-(2-hydroxypropyl)-ethylenediamine with benzyl chloride. Among them, the molar ratio of azelaic acid to N-(2-hydroxypropyl)-ethylenediamine is 2:5, and excess N-(2-hydroxypropyl)-ethylenediamine can ensure that two steps of dehydration will occur before the final formation of bisimidazoline. When the temperature is 100–300℃, the methoxy group and the methyl group on the molecular branch of the emulsifier will be broken, and the second mass loss rate is about 8.0%. When the temperature is 300–500℃, the mass loss rate is about 50%. The mass loss is mainly caused by the rupture of the imidazoline ring and main chain in the molecular structure of the emulsifier. The maximum mass loss occurs at 330°C. The results show that the bisimidazoline quaternary ammonium salt emulsifier nano-SiO2@OBQA has better thermal stability.
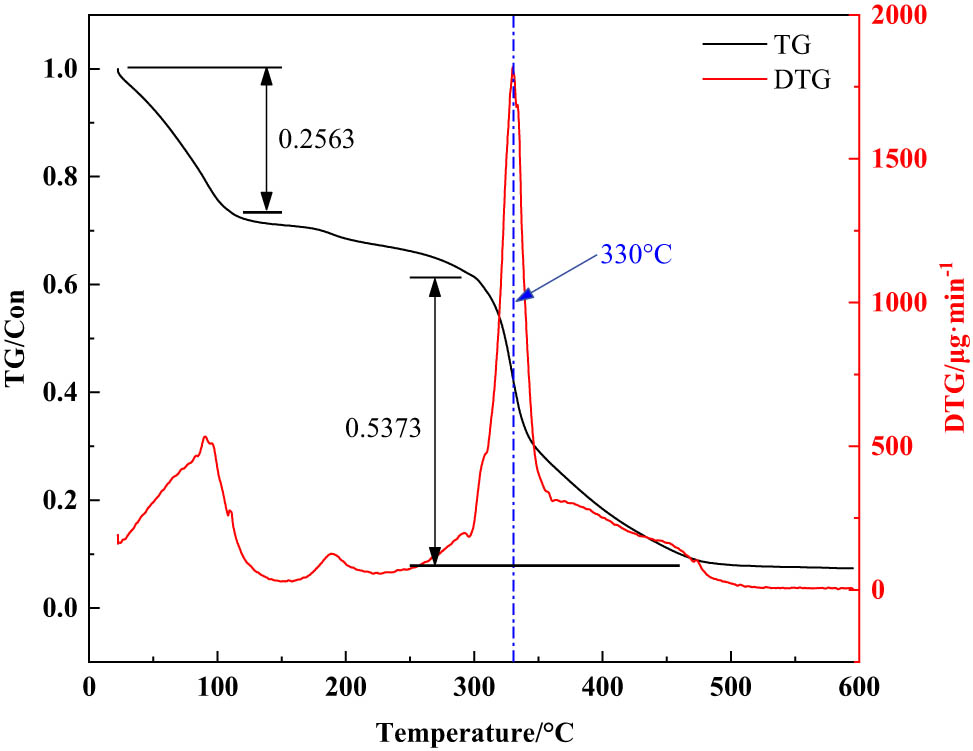
TG and DTG analysis of nano-SiO2@OBQA.
3.1.4 Microstructure characterization
The result is shown in Figure 6. The particle size of nano-SiO2@OBQA is 2–5 μm, the particle size distribution is uniform, the sphericity is excellent, and there is adhesion between a small number of particles as shown in Figure 6(b). This may be due to the synthesis process of bisimidazoline, the SiO2 microspheres adhere to each other by adsorption. This can indicate that the octadecylbisimidazoline quaternary ammonium salt successfully coated SiO2.
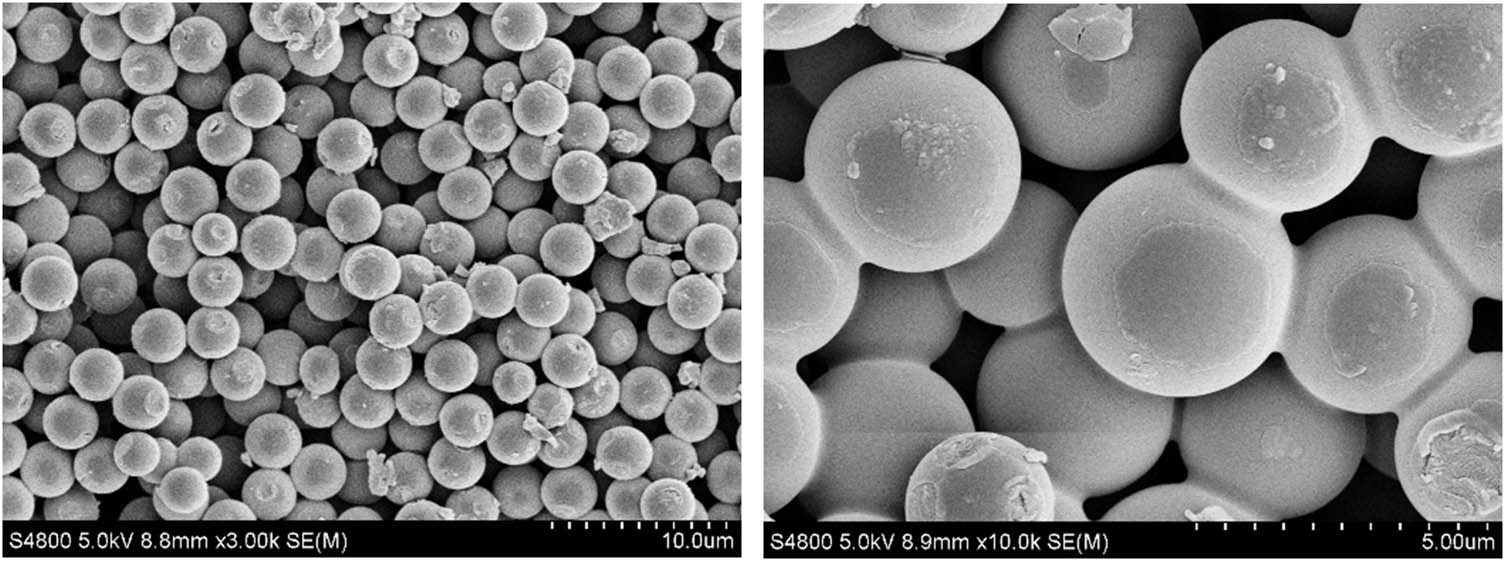
The scanning electron microscopy image of nano-SiO2@OBQA (left: 3,000×; right: 10,000×).
3.2 Quantum chemistry research
Chooto believes that during the reaction of molecules, molecular orbitals interact, and frontal orbitals have priority [25]. For acidizing corrosion inhibitors, it is generally believed that quantization parameters such as the highest occupied orbital energy E HOMO and the lowest occupied orbital energy E LUMO can characterize the interaction between acidizing corrosion inhibitor molecules and metal atoms. Among them, the HOMO orbital represents the ability to provide monads, the LUMO orbital represents the ability to accept electrons, and the difference between the energy of the HOMO orbital and the LUMO orbital is the energy gap, which is used to characterize the difficulty of chemical reactions [26].
Huckel analysis orbital theory is used to optimize the molecular structure and quantum chemical calculations of nano-SiO2@OBQA. As shown in Figure 7, the imidazoline and the vicinity of the benzene ring on the right side of the acidizing corrosion inhibitor have a higher ability to donate electrons, and the vicinity of the benzene ring on the left side has a higher ability to accept electrons, indicating that the acidizing corrosion inhibitor has the ability to acquire and lose electrons, and form chemical adsorption with atoms on the metal surface, reducing the corrosion of the metal by the acid solution. At the same time, the energy range (∆E) is 1.087 eV, indicating that the reaction is easier. As shown in Table 1, the value of (E LUMO,SiO2@OBQA – E HOMO,Fe) is greater than the value of (E LUMO,Fe – E HOMO,SiO2@OBQA), and the difference is greater than 3 eV, indicating that the tendency of the acidizing corrosion inhibitor nano-SiO2@OBQA molecule accepting electrons to interact with Fe is smaller than the tendency to donate electrons to interact with Fe [27].
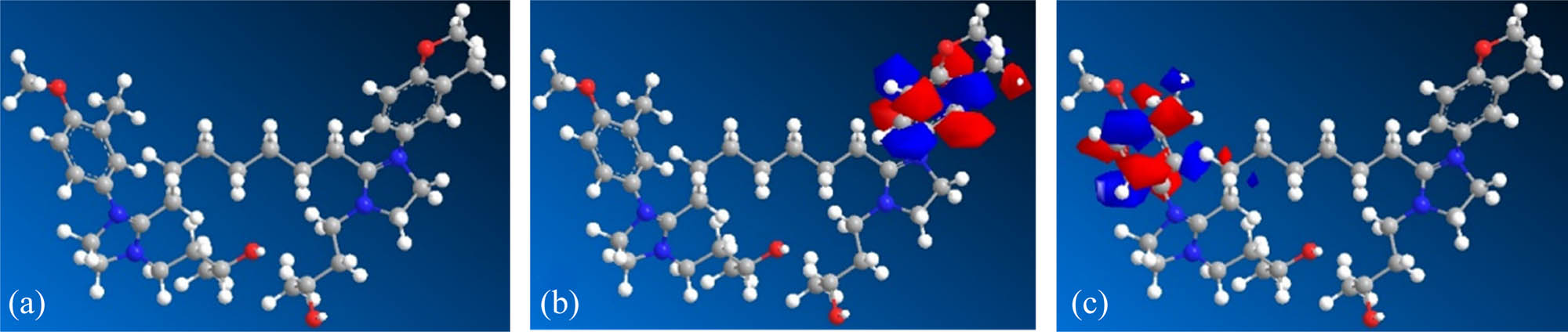
Optimized structure (a), HOMO (b), and LUMO (c) distributions of nano-SiO2@OBQA.
Frontier orbital energies of nano-SiO2@OBQA
| Molecular | E HOMO (eV) | E LUMO (eV) | E LUMO,SiO2@OBQA – E HOMO,Fe (eV) | E LUMO,Fe – E HOMO,SiO2@OBQA (eV) | ∆E (eV) |
|---|---|---|---|---|---|
| SiO2@OBQA | −0.631 | 0.456 | 8.266 | 0.381 | 1.087 |
| Fe | −7.81 | −0.25 | — | — | — |
3.3 Evaluation of corrosion inhibition performance
Different mass fractions of nano-SiO2@OBQA were added to 400 mL 20% hydrochloric acid solution. The corrosion of the N80 steel sheet was tested at different temperatures within 4 h, and its adsorption performance was characterized.
The corrosion rate and corrosion inhibition rate are shown in Figure 8. When the temperature is constant, the corrosion rate decreases as the concentration of nano-SiO2@OBQA increases. This is because nano-SiO2@OBQA can adhere to the surface of the N80 steel sheet to form a protective layer to inhibit acid corrosion. As the concentration of nano-SiO2@OBQA increases, the area attached to the N80 steel sheet also increases. When the concentration is constant, the surface coverage reaches the maximum. With the increase of the amount of the inhibitor, the corrosion rate first drops sharply and then tends to be stable. When the concentration of nano-SiO2@OBQA is constant, the corrosion rate increases as the temperature increases. This is caused by the thermal movement of molecules. Chooto and Manaboot believes that the increase in temperature helps to increase the absorption rate of inhibitor molecules [25], but the molecular movement of the acid will also increase with the increase in temperature. At the same time, Schmid found that metal surfaces included in organic molecules also undergo corrosion reactions, but the reaction rate is lower than that of bare metal surfaces [28]. Therefore, this can also explain the abovementioned experimental phenomenon.
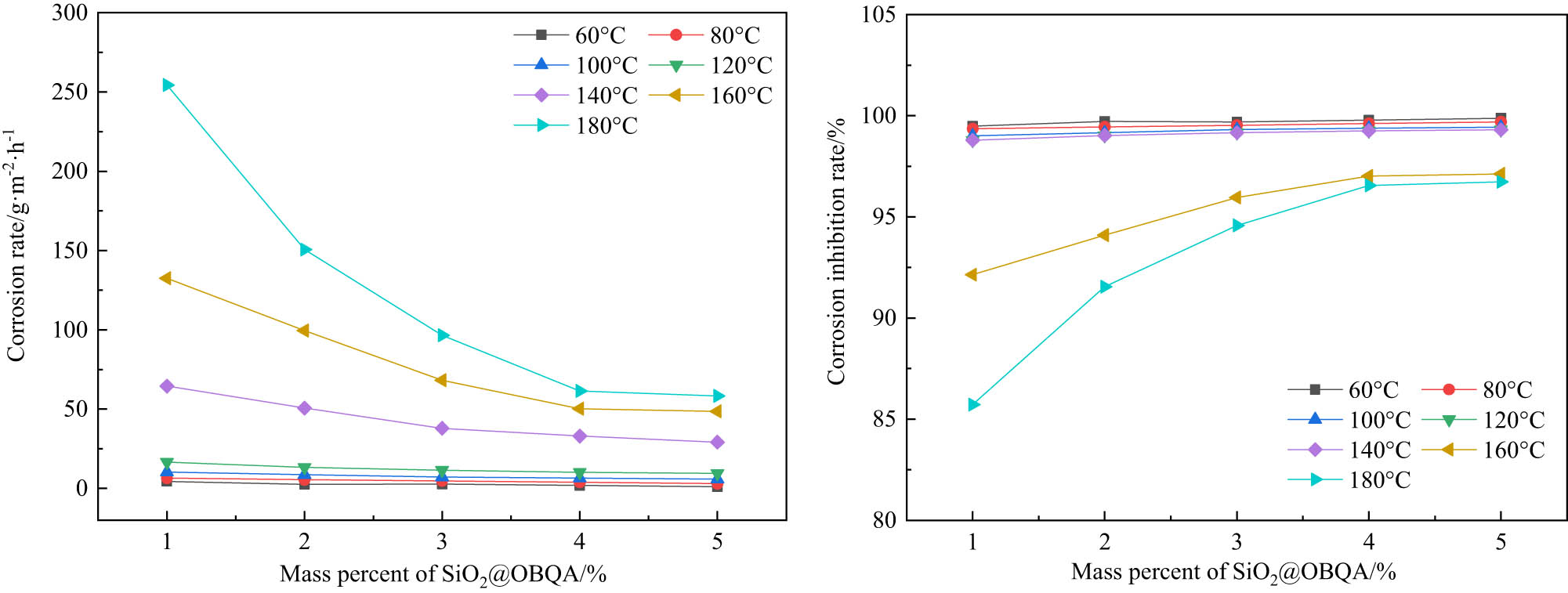
The influence of nano-SiO2@OBQA concentration on corrosion inhibition performance at different temperatures.
3.4 Adsorption isotherm model
The isothermal adsorption model of nano-SiO2@OBQA was constructed, and the Gibbs free energy
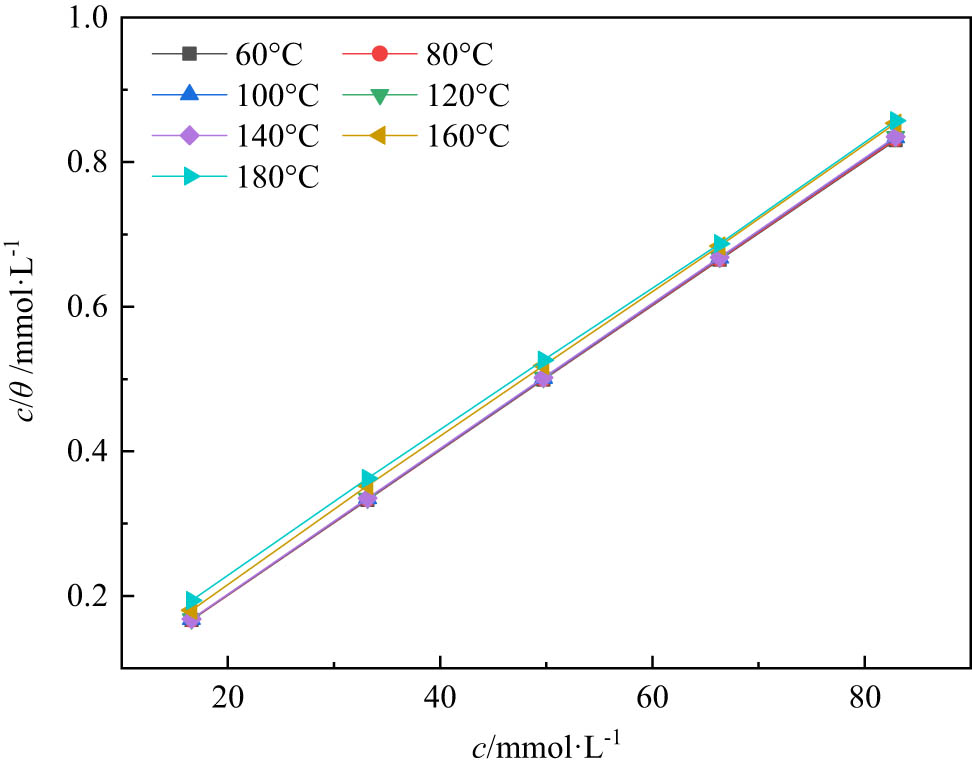
Curves fitting of the corrosion data for steel at different temperatures.
The isotherm fitting parameters
| T (K) | Intercept (mmol·L−1) | K ads (L·mmol−1) |
|
R 2 |
|---|---|---|---|---|
| 333.15 | 0.0009 | 1111.1111 | −30.21 | 1 |
| 353.15 | 0.001 | 1,000 | −31.71 | 1 |
| 373.15 | 0.0011 | 909.0909 | −33.22 | 1 |
| 393.15 | 0.0013 | 769.2308 | −34.45 | 1 |
| 413.15 | 0.0013 | 769.2308 | −36.20 | 1 |
| 433.15 | 0.014 | 71.4286 | −29.41 | 0.99 |
| 453.15 | 0.0295 | 33.8983 | −27.97 | 0.99 |
3.5 Mechanism discussion
According to the microscopic characterization and performance evaluation, the molecular mechanism of nano-SiO2@OBQA mainly includes two aspects: adsorption mechanism and temperature resistance mechanism as shown in Figure 10. Generally, the temperature resistance of the polymer can be improved by increasing the rigidity of the main chain and the side chain. The nano-SiO2@OBQA molecule synthesized has good thermal stability because of the long carbon chain and the benzene ring structure. Meanwhile, the introduction of inorganic nanomaterials will also improve the temperature resistance of molecules to a certain extent [33,34]. Thereby, corrosion inhibition needs to be closely adsorbed with the metal under different well conditions, generally divided into physical adsorption and chemical adsorption. On the one hand, the nano-SiO2@OBQA molecule contains a longer alkyl hydrophobic chain, which greatly enhances the hydrophobicity of the nano-SiO2@OBQA molecule and improves the density of the molecular film of the acidizing corrosion inhibitor on the metal surface. On the other hand, the π electron of the benzene ring, the unbound electron of the oxygen in the methoxy group, the C═N double bond on the bisimidazoline ring, and the N atom with a lone pair of electrons can provide electrons and then occur covalent effect on the metal surface. The combination forms π–d coordination bonds that enhances the activation energy of the metal surface and improves the adsorption capacity of the acidizing corrosion inhibitor molecules on the metal surface, thereby helping to form a dense adsorption film, reducing the direct contact between the corrosive medium and the metal.
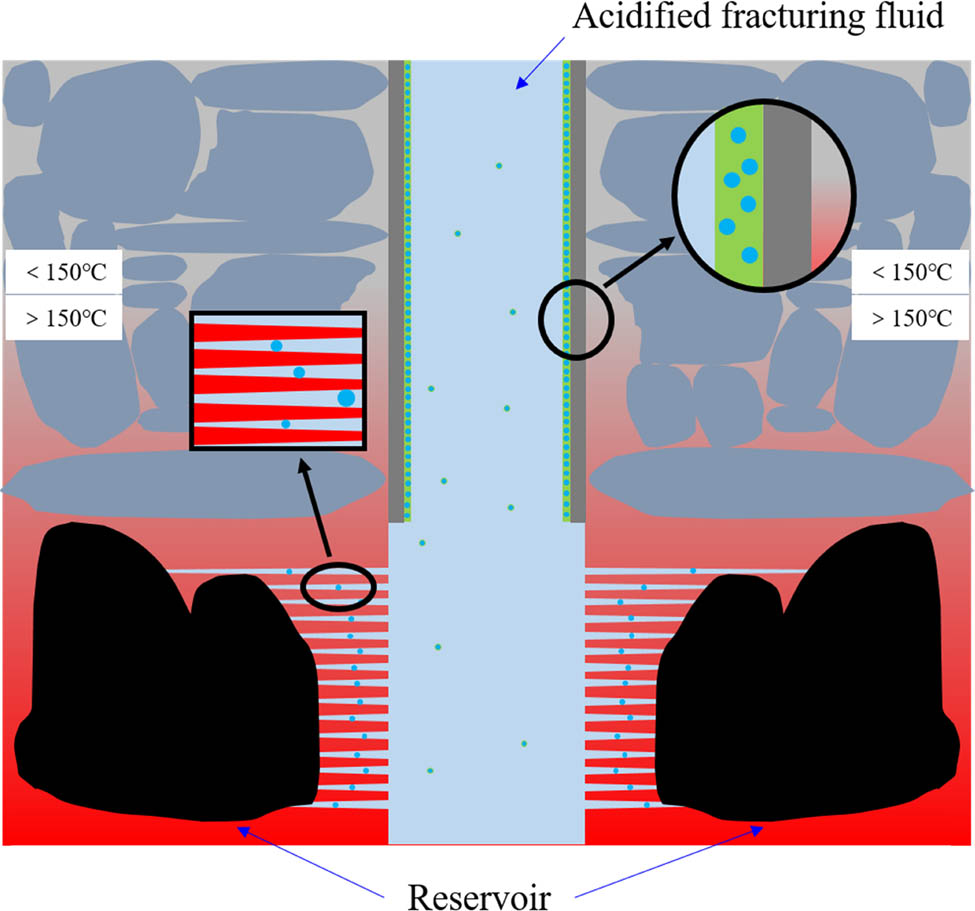
The mechanism of nano-SiO2@OBQA (the color of the formation changes from gray to red, indicating that the temperature of the formation increases with the increase in depth).
In the process of acid fracturing, it is generally necessary to add rigid materials to support the reservoir channels. Nano-SiO2@OBQA has a rigid core structure and is insoluble in hydrochloric acid. Therefore, it can be used as a proppant to enter the oil reservoir channel, reduce operating costs, and achieve the goal of reducing costs and increasing efficiency.
3.6 Innovation point
At present, there is no report claiming the introduction of inorganic nanomaterials in acidizing corrosion inhibitors. In this article, the innovative introduction of electrodeless nanomaterials as the “core” structure of acidizing corrosion inhibitors is presented. On the one hand, it can improve the molecular structure strength of the acidizing corrosion inhibitor and improve the thermal stability of the polymer molecules. On the other hand, the acidizing corrosion inhibitor molecules are injected into the formation in acid fracturing operations, so that they can also act as a support channel in the target formation, thereby improving the operating efficiency of acid fracturing.
4 Conclusion
The nano-SiO2@OBQA can effectively inhibit the corrosion of steel sheets in 20% HCl. At 180℃, when the addition of nano-SiO2@OBQA is 4%, the corrosion rate is 61.42 g·m−2·h−1, and the corrosion inhibition rate reaches more than 95%. The nano-SiO2@OBQA has excellent corrosion inhibition performance. Studies have shown that the adsorption of nano-SiO2@OBQA salt on the surface of the N80 steel sheet follows the Langmuir isotherm adsorption model, which is a spontaneous chemical adsorption. At the same time, nano-SiO2@OBQA can form stable adsorption with metals.
Acknowledgements
All the techniques who have contributed this research should be also expressed our most sincere thanks.
-
Funding information: This work was financially supported by the Research Project of Southwest Oil and Gas Field Company of PetroChina named “Popularization and application of 180℃ high temperature corrosion inhibitor and acid system” (No. 20210302-29) and “Research on autogenous acid at 180℃ and its mechanism” (No. 20210302-07).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Son, A. J., T. M. Ballard, and R. E. Loftin. Temperature-stable polymeric fluid-loss reducer tolerant to high electrolyte contamination. SPE Drilling Engineering, Vol. 2, No. 03, 1987, pp. 209–217.10.2118/13160-PASearch in Google Scholar
[2] Akpan, E. U., G. C. Enyi, G. Nasr, A. A. Yahaya, A. A. Ahmadu, and B. Saidu. Water-based drilling fluids for high-temperature applications and water-sensitive and dispersible shale formations. Journal of Petroleum Science and Engineering, Vol. 175, 2019, pp. 1028–1038.10.1016/j.petrol.2019.01.002Search in Google Scholar
[3] Liu, X., R. Jiang, and W. Huang. Experimental study on optimal injection pressure of heavy oil reservoir developed by supercritical CO2 flooding. Fresenius Environmental Bulletin, Vol. 29, No. 4, 2020, pp. 2555–2561.Search in Google Scholar
[4] Luo, X., W. Han, H. Ren, Q. Zhuang. Research on high performance temporary plugging drilling fluid for reservoir protection and field trial. Fresenius Environmental Bulletin, Vol. 28, No. 8, 2019, pp. 6290–6298.Search in Google Scholar
[5] Soltani, A., Head. Reliable level of corrosion inhibitor′s residual concentration in wet gas-condensate pipelines. Eurocorr 2020 Virtual Conference, 2020.Search in Google Scholar
[6] Shamlooh, M., S. Abdelrady, M. Buasali, and K Farouque. Well integrity protection using corrosion inhibitor treatment system for sour gas producers in Bahrain field. SPE Middle East Oil and Gas Show and Conference, 2019.10.2118/194893-MSSearch in Google Scholar
[7] Wylde, J. J., Turnern, and M. Austill. Development, testing and field application of a novel combination foamer-iron sulfide scale inhibitor-corrosion inhibitor in East Texas. SPE International Conference on Oilfield Chemistry, 2017.10.2118/184584-MSSearch in Google Scholar
[8] Danaee, I. and M. N. Khomami. Effect of ethylenediamine on corrosion of AISI 4130 steel alloy in 30% ethylene glycol solution under static and hydrodynamic condition. Materialwissenschaft Und Werkstofftechnik, Vol. 43, No. 11, 2012, pp. 942–949.10.1002/mawe.201200020Search in Google Scholar
[9] Vishwanatham, S. and A. Kumar. Corrosion inhibition of mild steel in binary acid mixture. Corrosion Reviews, Vol. 23, No. 2–3, 2005, pp. 181–194.10.1515/CORRREV.2005.23.2-3.181Search in Google Scholar
[10] Ghasemi, O., I. Danaee, G. R. Rashed, M. RashvandAvei, and M. H. Maddahy. Inhibition effect of a synthesized N, N'-bis(2-hydroxybenzaldehyde)-1, 3-propandiimine on corrosion of mild steel in HCl. Journal of Central South University, Vol. 20, No. 2, 2013, pp. 301–311.10.1007/s11771-013-1488-9Search in Google Scholar
[11] Bentiss, F., M. Lebrini, H. Vezin, and M. Lagrenée. Experimental and theoretical study of 3-pyridyl-substituted 1,2,4-thiadiazole and 1,3,4-thiadiazole as corrosion inhibitors of mild steel in acidic media. Materials Chemistry and Physics, Vol. 87, 2004, pp. 18–23.10.1016/j.matchemphys.2004.05.040Search in Google Scholar
[12] Scully, J. R., S. B. Inman, A. Y. Gerard, C. D. Taylor, W. Windl, D. K. Schreiber, et al. Controlling the corrosion resistance of multi-principal element alloys. Scripta Materialia, Vol. 188, 2020, pp. 96–101.10.1016/j.scriptamat.2020.06.065Search in Google Scholar
[13] Duarte, M. J., J. Klemm, S. O. Klemm, K. J. J. Mayrhofer, M. Stratmann, S. Borodin, et al. Element-resolved corrosion analysis of stainless-type glass-forming steels. Science, Vol. 341, No. 6144, 2013 Jul 26, pp. 372–376.10.1126/science.1230081Search in Google Scholar PubMed
[14] Qian, Y., Y. Li, and S. Jungwirth. The application of anti-corrosion coating for preserving the value of equipment asset in chloride-laden environments: a review. International Journal of Electrochemical Science, Vol. 10, 2015, pp. 10756–10780.10.1016/S1452-3981(23)11298-3Search in Google Scholar
[15] Cui, G., Z. Bi, R. Zhang, J. Liu, X. Yu, and Z. Li. A comprehensive review on graphene-based anti-corrosive coatings. Chemical Engineering Journal, Vol. 373, 2019, pp. 104–121.10.1016/j.cej.2019.05.034Search in Google Scholar
[16] Goyal, A., E. Ganjian, H. S. Pouya, and M. Tyrer. Inhibitor efficiency of migratory corrosion inhibitors to reduce corrosion in reinforced concrete exposed to high chloride environment. Construction and Building Materials, Vol. 303, 2021, id. 124461.10.1016/j.conbuildmat.2021.124461Search in Google Scholar
[17] Kobzar, Y. L. and K. Fatyeyeva. Ionic liquids as green and sustainable steel corrosion inhibitors: recent developments. Chemical Engineering Journal, Vol. 425, 2021, id. 131480.10.1016/j.cej.2021.131480Search in Google Scholar
[18] Baskar, R., H. Lgaz, and R. Salghi. Heterocyclic compounds as corrosion inhibitors for mild steel: a review. Chemical Science & Engineering Research, Vol. 1, No. 1, 2019, pp. 32–54.10.36686/Ariviyal.CSER.2019.01.01.005Search in Google Scholar
[19] Saraswat, V. Improved corrosion resistant performance of mild steel under acid environment by novel carbon dots as green corrosion inhibitor. Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 627, No. 7, 2021, id. 127172.10.1016/j.colsurfa.2021.127172Search in Google Scholar
[20] Shamsa, A., E. Barmatov, and T. L. Hughes. Hydrolysis of imidazoline based corrosion inhibitor and effects on inhibition performance of X65 steel in CO2 saturated brine. Journal of Petroleum Science and Engineering, Vol. 208(B), 2022, id. 109235.10.1016/j.petrol.2021.109235Search in Google Scholar
[21] Shamsa, A., R. Barker, Y. Hua, E. Barmatov, T. L. Hughes, and A. Neville. Performance evaluation of an imidazoline corrosion inhibitor in a CO2-saturated environment with emphasis on localised corrosion. Corrosion Science, Vol. 176, 2020, id. 108916.10.1016/j.corsci.2020.108916Search in Google Scholar
[22] Hu, J., Z. H. Zhang, Z. Zhu, J. Chen, X. Hu, and H. Chen. Molecular dynamic simulation and experimental investigation on the synergistic mechanism and synergistic effect of oleic acid imidazoline and l-cysteine corrosion inhibitors. Corrosion Science, Vol. 185, 2021, id. 109414.10.1016/j.corsci.2021.109414Search in Google Scholar
[23] Jiang, D. S., X. P. Li, K. W. Jiang, B. Chen, and C. Sun. Study on diverting acid conductivity of heterogeneous reservoir in Gaomo area. Chemical Engineering of Oil & Gas, Vol. 50, No. 4, 2021, pp. 92–95 + 113.Search in Google Scholar
[24] Xiong, Y., Y. Q. Liu, and Z. H. Mei. Slick water technology of high salinity resistance for shale gas development in Sichuan. Chemical Engineering of Oil & Gas, Vol. 48, No. 3, 2019, pp. 62–65 + 71.Search in Google Scholar
[25] Chooto, P. and S. Manaboot. Electrochemical, spectrochemical and quantum chemical studies on dimedone as corrosion inhibitor for copper in acetonitrile. Journal of Scientific Research & reports, Vol. 15, No. 2, 2017, pp. 1–13.10.9734/JSRR/2017/34929Search in Google Scholar
[26] Mutombo, P. and N. Hackerman. The effect of some organophosphorous compounds on the corrosion behaviour of iron in 6 M HCl. Anti-corrosion Methods and Materials, Vol. 45, No. 6, 1998, pp. 413–418.10.1108/00035599810236559Search in Google Scholar
[27] Song-Qing, H., H. Jian-Chun, F. Cheng-Cheng, M. Si-Qi, Z. Jun, and G. Wen-Yue. Corrosion inhibition of Q235 steel by a novel imidazoline compound under H2S and CO2 coexistence. Acta Physico-chemica Sinica, Vol. 26, No. 8, 2010, pp. 2163–2170.10.3866/PKU.WHXB20100809Search in Google Scholar
[28] Zhong, H. Y., X. Z. Kong, S. Chen, B. Grady, and Z. S. Qiu. Preparation, characterization and filtration control properties of crosslinked starch nanospheres in water-based drilling fluids. Journal of Molecular Liquids, Vol. 325, 2021, id. 115221.10.1016/j.molliq.2020.115221Search in Google Scholar
[29] Cui, G. D., L. Zhu, Q. Zhou, S. R. Ren, and J. Y. Wang. Geochemical reactions and their effect on CO2 storage efficiency during the whole process of CO2 EOR and subsequent storage. International Journal of Greenhouse Gas Control, Vol. 108, 2021, id. 103335.10.1016/j.ijggc.2021.103335Search in Google Scholar
[30] Schmid, G. M. and H. J. Huang. Spectro-electrochemical studies of the inhibition effect of 4, 7-diphenyl-1, 10-phenanthroline on the corrosion of 304 stainless steel. Corrosion Science, Vol. 20, No. 8–9, 1980, pp. 1041–1057.10.1016/0010-938X(80)90083-9Search in Google Scholar
[31] Annand, R. R. Theory of organic corrosion inhibitors adsorption and linear free energy relationships. Journal of the Electrochemical Society, Vol. 113, No. 6, 1966, id. 633.10.1149/1.2424059Search in Google Scholar
[32] Nakonieczny, D. S., M. Antonowicz, and Z. Paszenda. Surface modification methods of ceramic filler in ceramic-carbon fibre composites for bioengineering applications – a systematic review. Reviews on Advanced Materials Science, Vol. 59, No. 1, 2020, pp. 586–605.10.1515/rams-2020-0024Search in Google Scholar
[33] Khamis, E., F. Bellucci, R. M. Latanision, and E. S. H. El-Ashry. Acid corrosion inhibition of nickel by 2-(triphenosphoranylidene) succinic anhydride. Corrosion, Vol. 47, No. 9, 1991, pp. 677–686.10.5006/1.3585307Search in Google Scholar
[34] Bentiss, F., M. Traisnel, N. Chaibi, B. Mernari, H. Vezin, and M. Lagrenée. 2,5-Bis(n-methoxyphenyl)-1,3,4-oxadiazoles used as corrosion inhibitors in acidic media: correlation between inhibition efficiency and chemical structure. Corrosion Science, Vol. 44, No. 10, 2002, pp. 2271–2289.10.1016/S0010-938X(02)00037-9Search in Google Scholar
© 2022 Yunfeng Liu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Review Articles
- State of the art, challenges, and emerging trends: Geopolymer composite reinforced by dispersed steel fibers
- A review on the properties of concrete reinforced with recycled steel fiber from waste tires
- Copper ternary oxides as photocathodes for solar-driven CO2 reduction
- Properties of fresh and hardened self-compacting concrete incorporating rice husk ash: A review
- Basic mechanical and fatigue properties of rubber materials and components for railway vehicles: A literature survey
- Research progress on durability of marine concrete under the combined action of Cl− erosion, carbonation, and dry–wet cycles
- Delivery systems in nanocosmeceuticals
- Study on the preparation process and sintering performance of doped nano-silver paste
- Analysis of the interactions between nonoxide reinforcements and Al–Si–Cu–Mg matrices
- Research Articles
- Study on the influence of structural form and parameters on vibration characteristics of typical ship structures
- Deterioration characteristics of recycled aggregate concrete subjected to coupling effect with salt and frost
- Novel approach to improve shale stability using super-amphiphobic nanoscale materials in water-based drilling fluids and its field application
- Research on the low-frequency multiline spectrum vibration control of offshore platforms
- Multiple wide band gaps in a convex-like holey phononic crystal strip
- Response analysis and optimization of the air spring with epistemic uncertainties
- Molecular dynamics of C–S–H production in graphene oxide environment
- Residual stress relief mechanisms of 2219 Al–Cu alloy by thermal stress relief method
- Characteristics and microstructures of the GFRP waste powder/GGBS-based geopolymer paste and concrete
- Development and performance evaluation of a novel environmentally friendly adsorbent for waste water-based drilling fluids
- Determination of shear stresses in the measurement area of a modified wood sample
- Influence of ettringite on the crack self-repairing of cement-based materials in a hydraulic environment
- Multiple load recognition and fatigue assessment on longitudinal stop of railway freight car
- Synthesis and characterization of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt used as acidizing corrosion inhibitor
- Perforated steel for realizing extraordinary ductility under compression: Testing and finite element modeling
- The influence of oiled fiber, freeze-thawing cycle, and sulfate attack on strain hardening cement-based composites
- Perforated steel block of realizing large ductility under compression: Parametric study and stress–strain modeling
- Study on dynamic viscoelastic constitutive model of nonwater reacted polyurethane grouting materials based on DMA
- Mechanical behavior and mechanism investigation on the optimized and novel bio-inspired nonpneumatic composite tires
- Effect of cooling rate on the microstructure and thermal expansion properties of Al–Mn–Fe alloy
- Research on process optimization and rapid prediction method of thermal vibration stress relief for 2219 aluminum alloy rings
- Failure prevention of seafloor composite pipelines using enhanced strain-based design
- Deterioration of concrete under the coupling action of freeze–thaw cycles and salt solution erosion
- Creep rupture behavior of 2.25Cr1Mo0.25V steel and weld for hydrogenation reactors under different stress levels
- Statistical damage constitutive model for the two-component foaming polymer grouting material
- Nano-structural and nano-constraint behavior of mortar containing silica aggregates
- Influence of recycled clay brick aggregate on the mechanical properties of concrete
- Effect of LDH on the dissolution and adsorption behaviors of sulfate in Portland cement early hydration process
- Comparison of properties of colorless and transparent polyimide films using various diamine monomers
- Study in the parameter influence on underwater acoustic radiation characteristics of cylindrical shells
- Experimental study on basic mechanical properties of recycled steel fiber reinforced concrete
- Dynamic characteristic analysis of acoustic black hole in typical raft structure
- A semi-analytical method for dynamic analysis of a rectangular plate with general boundary conditions based on FSDT
- Research on modification of mechanical properties of recycled aggregate concrete by replacing sand with graphite tailings
- Dynamic response of Voronoi structures with gradient perpendicular to the impact direction
- Deposition mechanisms and characteristics of nano-modified multimodal Cr3C2–NiCr coatings sprayed by HVOF
- Effect of excitation type on vibration characteristics of typical ship grillage structure
- Study on the nanoscale mechanical properties of graphene oxide–enhanced shear resisting cement
- Experimental investigation on static compressive toughness of steel fiber rubber concrete
- Study on the stress field concentration at the tip of elliptical cracks
- Corrosion resistance of 6061-T6 aluminium alloy and its feasibility of near-surface reinforcements in concrete structure
- Effect of the synthesis method on the MnCo2O4 towards the photocatalytic production of H2
- Experimental study of the shear strength criterion of rock structural plane based on three-dimensional surface description
- Evaluation of wear and corrosion properties of FSWed aluminum alloy plates of AA2020-T4 with heat treatment under different aging periods
- Thermal–mechanical coupling deformation difference analysis for the flexspline of a harmonic drive
- Frost resistance of fiber-reinforced self-compacting recycled concrete
- High-temperature treated TiO2 modified with 3-aminopropyltriethoxysilane as photoactive nanomaterials
- Effect of nano Al2O3 particles on the mechanical and wear properties of Al/Al2O3 composites manufactured via ARB
- Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors
- Effect of freeze–thaw cycles on deformation properties of deep foundation pit supported by pile-anchor in Harbin
- Temperature-porosity-dependent elastic modulus model for metallic materials
- Effect of diffusion on interfacial properties of polyurethane-modified asphalt–aggregate using molecular dynamic simulation
- Experimental study on comprehensive improvement of shear strength and erosion resistance of yellow mud in Qiang Village
- A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism
- In situ bow reduction during sublimation growth of cubic silicon carbide
- Adhesion behaviour of 3D printed polyamide–carbon fibre composite filament
- An experimental investigation and machine learning-based prediction for seismic performance of steel tubular column filled with recycled aggregate concrete
- Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel
- Application research of acoustic black hole in floating raft vibration isolation system
- Multi-objective parametric optimization on the EDM machining of hybrid SiCp/Grp/aluminum nanocomposites using Non-dominating Sorting Genetic Algorithm (NSGA-II): Fabrication and microstructural characterizations
- Estimating of cutting force and surface roughness in turning of GFRP composites with different orientation angles using artificial neural network
- Displacement recovery and energy dissipation of crimped NiTi SMA fibers during cyclic pullout tests
Articles in the same Issue
- Review Articles
- State of the art, challenges, and emerging trends: Geopolymer composite reinforced by dispersed steel fibers
- A review on the properties of concrete reinforced with recycled steel fiber from waste tires
- Copper ternary oxides as photocathodes for solar-driven CO2 reduction
- Properties of fresh and hardened self-compacting concrete incorporating rice husk ash: A review
- Basic mechanical and fatigue properties of rubber materials and components for railway vehicles: A literature survey
- Research progress on durability of marine concrete under the combined action of Cl− erosion, carbonation, and dry–wet cycles
- Delivery systems in nanocosmeceuticals
- Study on the preparation process and sintering performance of doped nano-silver paste
- Analysis of the interactions between nonoxide reinforcements and Al–Si–Cu–Mg matrices
- Research Articles
- Study on the influence of structural form and parameters on vibration characteristics of typical ship structures
- Deterioration characteristics of recycled aggregate concrete subjected to coupling effect with salt and frost
- Novel approach to improve shale stability using super-amphiphobic nanoscale materials in water-based drilling fluids and its field application
- Research on the low-frequency multiline spectrum vibration control of offshore platforms
- Multiple wide band gaps in a convex-like holey phononic crystal strip
- Response analysis and optimization of the air spring with epistemic uncertainties
- Molecular dynamics of C–S–H production in graphene oxide environment
- Residual stress relief mechanisms of 2219 Al–Cu alloy by thermal stress relief method
- Characteristics and microstructures of the GFRP waste powder/GGBS-based geopolymer paste and concrete
- Development and performance evaluation of a novel environmentally friendly adsorbent for waste water-based drilling fluids
- Determination of shear stresses in the measurement area of a modified wood sample
- Influence of ettringite on the crack self-repairing of cement-based materials in a hydraulic environment
- Multiple load recognition and fatigue assessment on longitudinal stop of railway freight car
- Synthesis and characterization of nano-SiO2@octadecylbisimidazoline quaternary ammonium salt used as acidizing corrosion inhibitor
- Perforated steel for realizing extraordinary ductility under compression: Testing and finite element modeling
- The influence of oiled fiber, freeze-thawing cycle, and sulfate attack on strain hardening cement-based composites
- Perforated steel block of realizing large ductility under compression: Parametric study and stress–strain modeling
- Study on dynamic viscoelastic constitutive model of nonwater reacted polyurethane grouting materials based on DMA
- Mechanical behavior and mechanism investigation on the optimized and novel bio-inspired nonpneumatic composite tires
- Effect of cooling rate on the microstructure and thermal expansion properties of Al–Mn–Fe alloy
- Research on process optimization and rapid prediction method of thermal vibration stress relief for 2219 aluminum alloy rings
- Failure prevention of seafloor composite pipelines using enhanced strain-based design
- Deterioration of concrete under the coupling action of freeze–thaw cycles and salt solution erosion
- Creep rupture behavior of 2.25Cr1Mo0.25V steel and weld for hydrogenation reactors under different stress levels
- Statistical damage constitutive model for the two-component foaming polymer grouting material
- Nano-structural and nano-constraint behavior of mortar containing silica aggregates
- Influence of recycled clay brick aggregate on the mechanical properties of concrete
- Effect of LDH on the dissolution and adsorption behaviors of sulfate in Portland cement early hydration process
- Comparison of properties of colorless and transparent polyimide films using various diamine monomers
- Study in the parameter influence on underwater acoustic radiation characteristics of cylindrical shells
- Experimental study on basic mechanical properties of recycled steel fiber reinforced concrete
- Dynamic characteristic analysis of acoustic black hole in typical raft structure
- A semi-analytical method for dynamic analysis of a rectangular plate with general boundary conditions based on FSDT
- Research on modification of mechanical properties of recycled aggregate concrete by replacing sand with graphite tailings
- Dynamic response of Voronoi structures with gradient perpendicular to the impact direction
- Deposition mechanisms and characteristics of nano-modified multimodal Cr3C2–NiCr coatings sprayed by HVOF
- Effect of excitation type on vibration characteristics of typical ship grillage structure
- Study on the nanoscale mechanical properties of graphene oxide–enhanced shear resisting cement
- Experimental investigation on static compressive toughness of steel fiber rubber concrete
- Study on the stress field concentration at the tip of elliptical cracks
- Corrosion resistance of 6061-T6 aluminium alloy and its feasibility of near-surface reinforcements in concrete structure
- Effect of the synthesis method on the MnCo2O4 towards the photocatalytic production of H2
- Experimental study of the shear strength criterion of rock structural plane based on three-dimensional surface description
- Evaluation of wear and corrosion properties of FSWed aluminum alloy plates of AA2020-T4 with heat treatment under different aging periods
- Thermal–mechanical coupling deformation difference analysis for the flexspline of a harmonic drive
- Frost resistance of fiber-reinforced self-compacting recycled concrete
- High-temperature treated TiO2 modified with 3-aminopropyltriethoxysilane as photoactive nanomaterials
- Effect of nano Al2O3 particles on the mechanical and wear properties of Al/Al2O3 composites manufactured via ARB
- Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors
- Effect of freeze–thaw cycles on deformation properties of deep foundation pit supported by pile-anchor in Harbin
- Temperature-porosity-dependent elastic modulus model for metallic materials
- Effect of diffusion on interfacial properties of polyurethane-modified asphalt–aggregate using molecular dynamic simulation
- Experimental study on comprehensive improvement of shear strength and erosion resistance of yellow mud in Qiang Village
- A novel method for low-cost and rapid preparation of nanoporous phenolic aerogels and its performance regulation mechanism
- In situ bow reduction during sublimation growth of cubic silicon carbide
- Adhesion behaviour of 3D printed polyamide–carbon fibre composite filament
- An experimental investigation and machine learning-based prediction for seismic performance of steel tubular column filled with recycled aggregate concrete
- Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel
- Application research of acoustic black hole in floating raft vibration isolation system
- Multi-objective parametric optimization on the EDM machining of hybrid SiCp/Grp/aluminum nanocomposites using Non-dominating Sorting Genetic Algorithm (NSGA-II): Fabrication and microstructural characterizations
- Estimating of cutting force and surface roughness in turning of GFRP composites with different orientation angles using artificial neural network
- Displacement recovery and energy dissipation of crimped NiTi SMA fibers during cyclic pullout tests

