Abstract
C20H22N2O6, triclinic,
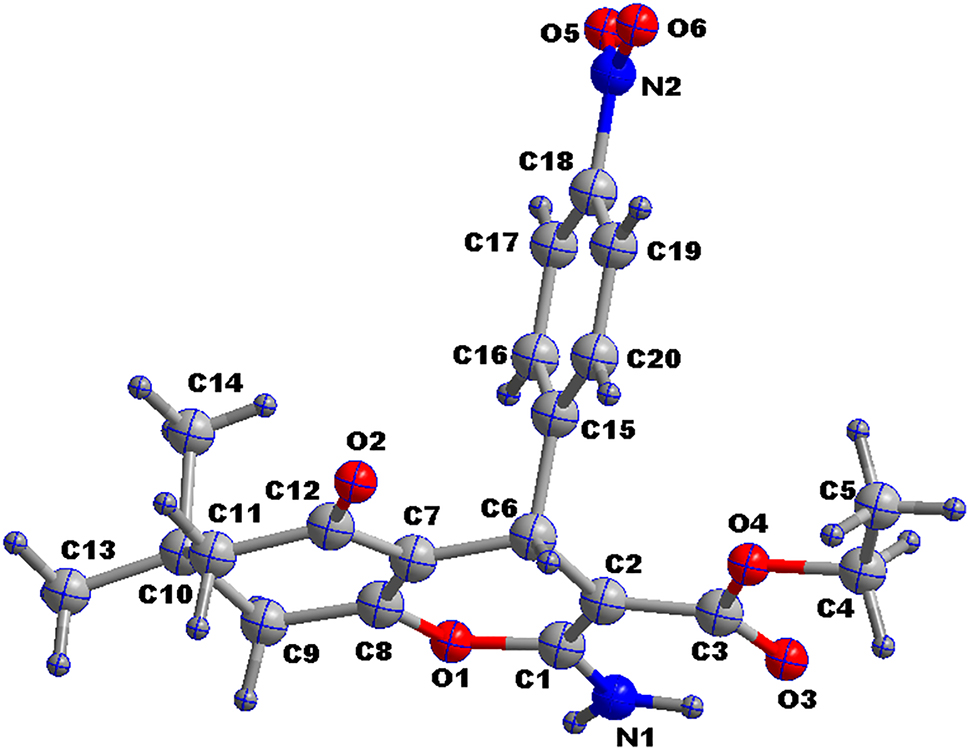
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.26 × 0.21 × 0.17 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.2°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 5,146, 3,514, 0.027 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2,131 |
| N(param)refined: | 265 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.8844 (4) | 0.1081 (2) | 0.81131 (13) | 0.0483 (6) |
| C2 | 0.6909 (4) | 0.1710 (2) | 0.84062 (13) | 0.0479 (6) |
| C3 | 0.6465 (5) | 0.1600 (2) | 0.92973 (15) | 0.0608 (7) |
| C4 | 0.3848 (6) | 0.2245 (3) | 1.03631 (16) | 0.0936 (11) |
| H4A | 0.507098 | 0.251691 | 1.059762 | 0.112* |
| H4B | 0.357182 | 0.135883 | 1.068011 | 0.112* |
| C5 | 0.1784 (7) | 0.3125 (4) | 1.0422 (2) | 0.1410 (19) |
| H5A | 0.207477 | 0.399930 | 1.010920 | 0.212* |
| H5B | 0.132641 | 0.311395 | 1.100545 | 0.212* |
| H5C | 0.058114 | 0.284516 | 1.019225 | 0.212* |
| C6 | 0.5271 (4) | 0.2564 (2) | 0.78153 (13) | 0.0436 (6) |
| H6 | 0.370899 | 0.235097 | 0.804111 | 0.052* |
| C7 | 0.5778 (3) | 0.22779 (19) | 0.69623 (13) | 0.0415 (5) |
| C8 | 0.7729 (4) | 0.16253 (19) | 0.67389 (13) | 0.0419 (5) |
| C9 | 0.8430 (4) | 0.1372 (2) | 0.58979 (13) | 0.0517 (6) |
| H9A | 0.819030 | 0.047381 | 0.592566 | 0.062* |
| H9B | 1.005674 | 0.146094 | 0.575573 | 0.062* |
| C10 | 0.7141 (4) | 0.2281 (2) | 0.52066 (14) | 0.0533 (6) |
| C11 | 0.4647 (4) | 0.2388 (3) | 0.55227 (16) | 0.0673 (8) |
| H11A | 0.383566 | 0.303986 | 0.509944 | 0.081* |
| H11B | 0.406018 | 0.155312 | 0.558059 | 0.081* |
| C12 | 0.4113 (4) | 0.2752 (2) | 0.63501 (14) | 0.0492 (6) |
| C13 | 0.7546 (5) | 0.1786 (3) | 0.44148 (17) | 0.0877 (10) |
| H13A | 0.916354 | 0.167901 | 0.424846 | 0.132* |
| H13B | 0.680403 | 0.240722 | 0.396801 | 0.132* |
| H13C | 0.692883 | 0.095685 | 0.452486 | 0.132* |
| C14 | 0.8010 (5) | 0.3647 (3) | 0.49890 (18) | 0.0796 (9) |
| H14A | 0.778497 | 0.397962 | 0.548488 | 0.119* |
| H14B | 0.717321 | 0.423114 | 0.455333 | 0.119* |
| H14C | 0.961276 | 0.358332 | 0.479302 | 0.119* |
| C15 | 0.5390 (4) | 0.4008 (2) | 0.77267 (12) | 0.0410 (5) |
| C16 | 0.7375 (4) | 0.4579 (2) | 0.73913 (14) | 0.0506 (6) |
| H16 | 0.865120 | 0.406393 | 0.722265 | 0.061* |
| C17 | 0.7525 (4) | 0.5889 (2) | 0.72983 (15) | 0.0587 (7) |
| H17 | 0.888037 | 0.626453 | 0.707177 | 0.070* |
| C18 | 0.5623 (5) | 0.6629 (2) | 0.75484 (14) | 0.0533 (6) |
| C19 | 0.3620 (4) | 0.6103 (2) | 0.78824 (15) | 0.0594 (7) |
| H19 | 0.234708 | 0.662382 | 0.804735 | 0.071* |
| C20 | 0.3516 (4) | 0.4798 (2) | 0.79707 (14) | 0.0532 (6) |
| H20 | 0.215570 | 0.442974 | 0.819990 | 0.064* |
| N1 | 1.0508 (4) | 0.0369 (2) | 0.85494 (16) | 0.0681 (7) |
| H1A | 1.023 (5) | 0.026 (3) | 0.9156 (18) | 0.093 (10)* |
| H1B | 1.143 (5) | 0.003 (3) | 0.8274 (17) | 0.075 (10)* |
| N2 | 0.5749 (5) | 0.8027 (2) | 0.74494 (14) | 0.0748 (7) |
| O1 | 0.9358 (2) | 0.10961 (14) | 0.72781 (9) | 0.0499 (4) |
| O2 | 0.2328 (3) | 0.33832 (17) | 0.65114 (10) | 0.0616 (5) |
| O3 | 0.7662 (3) | 0.0980 (2) | 0.98416 (10) | 0.0848 (7) |
| O4 | 0.4489 (3) | 0.22822 (17) | 0.94891 (9) | 0.0694 (6) |
| O5 | 0.7604 (5) | 0.8446 (2) | 0.72364 (19) | 0.1305 (11) |
| O6 | 0.4020 (4) | 0.8695 (2) | 0.75925 (15) | 0.1112 (9) |
1 Source of materials
The synthesis of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate adopts the following method by a three-component reaction: 4-nitrobenzaldehyde (10 mmol), ethyl 2-cyanoacetate (10 mmol), and cyclohexane-1,3-dione (10 mmol) were mixed in a 100 mL ethanol solution, then DMAP (1 mmol) used as the catalyst was added and stirred. The mixture was heated at 353 K for 6 h and cooled naturally. The precipitate was filtered and recrystallized from an ethanol solution to give yellow crystals of the goal product, yield 62.5 % (based on 4-nitrobenzaldehyde).
2 Experimental details
The structure was solved by Direct Methods with the SHELXL-2014 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2U eq(C) or U eq(N)) using a riding model with C–H = 0.930, 0.960, 0.970 and 0.980 Å, respectively. The H-atoms from N atoms were positioned with Q peaks and refined freely with N–H = 0.955 and 0.785 Å.
3 Comment
To date, many single crystal structures of ethyl 2-amino-(4-phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyran-3-carboxylate 5 , 6 and their derivatives have been studied anywhere because of their pharmacological activities, such as 3-cyanophenyl, 7 3-fluorophenyl, 8 3,4-dimethylphenyl, 9 3,5-difluorophenyl, 10 4-fluorophenyl, 11 , 12 , 13 , 14 4-[4-(methanesulfonyl)phenyl], 15 and 4-methylphenyl. 16
The title compound crystallizes in triclinic system,
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT v7.60A; Bruker AXS Inc: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. Using Phases to Determine the Space Group. Acta Crystallogr. 2018, A74, A353; https://doi.org/10.1107/s0108767318096472.Suche in Google Scholar
5. Khan, A. T.; Lal, M.; Ali, S.; Khan, Md. M. One-pot Three Component Reaction for the Synthesis of Pyran Annulated Heterocyclic Compounds Using DMAP as a Catalyst. Tetrahedron Lett. 2011, 52, 5327–5332; https://doi.org/10.1016/j.tetlet.2011.08.019.Suche in Google Scholar
6. Ramireddy, N.; Abbaraju, S.; Ding, D.; Arman, H.; Zhao, J. C.-G. Organocatalyzed Enantioselective Synthesis of 2-Amino-4H-Chromene Derivatives. J. Heterocycl. Chem. 2017, 54, 677–691; https://doi.org/10.1002/jhet.2641.Suche in Google Scholar
7. Yang, D.-Q.; Li, Z.-M. Crystal Structure of Ethyl 2-Amino-4-(3-cyanophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C21H22N2O4. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 903–904; https://doi.org/10.1515/ncrs-2018-0089.Suche in Google Scholar
8. Sharanina, L. G.; Nesterov, V. N.; Klokol, G. V.; Rodinovskaya, L. A.; Shklover, V. E.; Sharanin, Yu. A.; Struchkov, Yu T.; Promonenkov, V. K. Synthesis of Condensed 2-Amino-4H-Pyrans and the Molecular Structure of 2-amino-7, 7-dimethyl-4-(3-fluorophenyl)-5-Oxo-3-Ethoxycarbonyl-5, 6, 7, 8-Tetrahydro-4h-Benzo [b] Pyran. J. Org. Chem. 1986, 22, 1185–1191.10.1002/chin.198645207Suche in Google Scholar
9. Chen, H.; Yang, G.; Liao, X.; Bao, Z.; Zhan, X. Crystal Structure and Anti-lung Cancer Activity of Novel Pyran Derivatives from Traditional Chinese Medicine. Lat. Am. J. Pharm. 2018, 37, 359–362.Suche in Google Scholar
10. Shin, S. Y.; Yoo, M.; Koh, D. The Crystal Structure of Ethyl 2-amino-4-(3,5-difluorophenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-Carboxylate, C20H21F2NO4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 307–309; https://doi.org/10.1515/ncrs-2020-0566.Suche in Google Scholar
11. Shi, D.; Zhang, S.; Wang, X.; Zhuang, Q. Ethyl 2-Amino-4-(4-fluorophenyl)-7,7-dimethyl 5-oxo-5,6,7,8-tetrahydro-4H-benzo[b]pyran-3-carboxylate. Acta Crystallogr. 2003, E59, o1501–o1502; https://doi.org/10.1107/s1600536803019834.Suche in Google Scholar
12. Yao, C.-S.; Li, T.-J.; Yu, C.-X.; Tu, S.-J. Crystal Structure of 2-Amino-5,10-dioxo-4-aryl-5,10-dihydro-4H-Naphtho [2,3-b]pyran-3-Carboxylic Acid Ethyl Ester. Chin. J. Struct. Chem. 2009, 28, 951–956.Suche in Google Scholar
13. Zhuang, Q.-Y.; Zhang, S.; Shi, D.-Q.; Tu, S.-J.; Wang, X.-S. Synthesis and Crystal Structure of Ethyl 2-amino-7,7-dimethyl-4-(4′-fluorophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-benzo [b] pyran-2-carboxylate in Water. J. Xuzhou Normal Univ. (Nat. Sci.) 2003, 21, 46–49.Suche in Google Scholar
14. Shi, D. –Q.; Zhang, S.; Zhuang, Q. –Y. Efficient Synthesis of 2-Amino-4-aryl-7,7-dimethyl-5-oxo-4H-5,6,7,8-tetrahydrobenzo[b]pyran-3-Carboxylate in Aqueous Media. Chin. J. Org. Chem. 2005, 25, 1570–1574.10.1002/chin.200525133Suche in Google Scholar
15. Xiao, Y.-S.; Zhang, L.; Hu, W.-L.; Zhao, Y.-B.; Hu, H. In Vitro Activity of Synthesized Pyran Derivatives-Inhibitor against Prostate Cancer. Lat. Am. J. Pharm. 2017, 36, 609–612.Suche in Google Scholar
16. Nongrum, R.; Nongthombam, G. R.; Kharkongor, M.; Rani, J. W. S.; Rahman, N.; Kathing, C.; Myrboh, B.; Nongkhlaw, R. A Nano-Organo Catalyzed Route towards the Efficient Synthesis of Benzo[b]pyran Derivatives under Ultrasonic Irradiation. RSC Adv. 2016, 6, 108384–108392; https://doi.org/10.1039/c6ra24108e.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3


