Abstract
C44H34Cl2N6O2Zn, triclinic, P
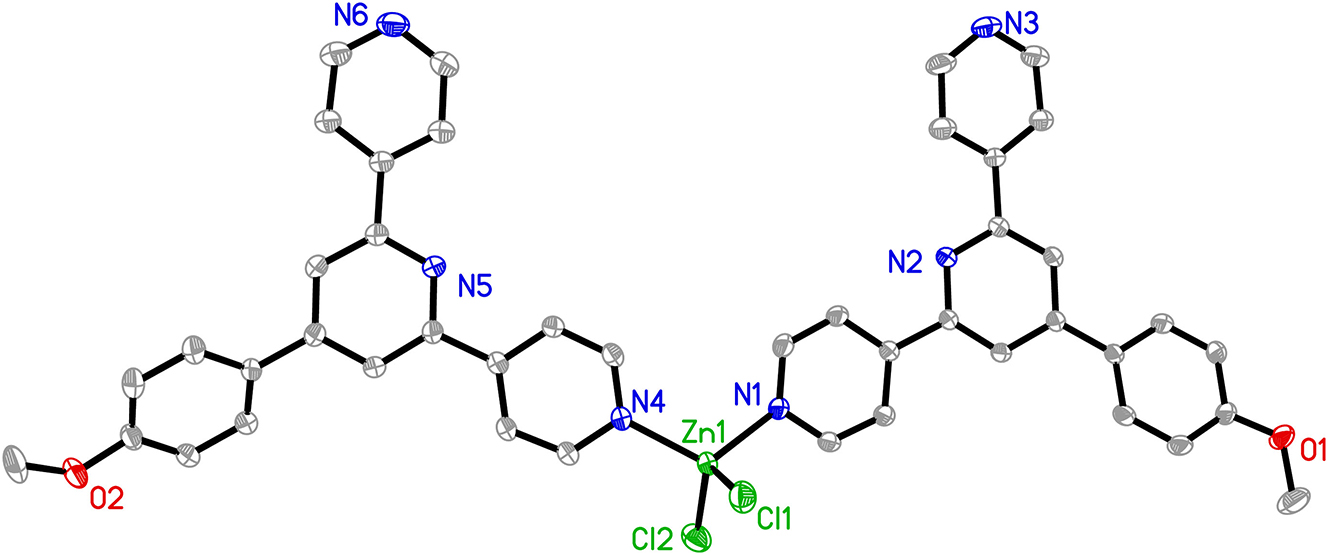
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.27 × 0.23 × 0.19 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.83 mm−1 |
| Diffractometer, scan mode: | Bruker SMART APEX2, φ and ω |
| θ max, completeness: | 25.0°, 98 % |
| N(hkl)measured, N(hkl)unique, R int: | 10,500, 6,597, 0.034 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 4,139 |
| N(param)refined: | 498 |
| Programs: | Bruker 1 , SHELX 2 , 3 , Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Zn1 | 0.79541(4) | 0.32271(4) | 0.29359(3) | 0.04299(16) |
| Cl1 | 0.89579(10) | 0.52398(10) | 0.35097(7) | 0.0619(3) |
| Cl2 | 0.91750(11) | 0.20267(11) | 0.24525(7) | 0.0665(3) |
| O1 | 0.6831(3) | 0.5574(3) | −0.42977(18) | 0.0739(10) |
| O2 | 0.8372(3) | −0.0820(3) | 0.95210(17) | 0.0628(8) |
| N1 | 0.6693(3) | 0.3011(3) | 0.18164(18) | 0.0426(8) |
| N2 | 0.3398(3) | 0.3278(3) | −0.04697(18) | 0.0389(8) |
| N3 | −0.1240(3) | 0.3359(4) | −0.0958(3) | 0.0749(12) |
| N4 | 0.7115(3) | 0.2641(3) | 0.38399(19) | 0.0442(8) |
| N5 | 0.4303(3) | 0.1412(3) | 0.58074(19) | 0.0397(7) |
| N6 | −0.0257(3) | 0.1083(3) | 0.6217(3) | 0.0642(10) |
| C1 | 0.5410(3) | 0.2845(4) | 0.1672(2) | 0.0475(10) |
| H1 | 0.496336 | 0.267950 | 0.209144 | 0.057* |
| C2 | 0.4723(3) | 0.2910(3) | 0.0929(2) | 0.0429(10) |
| H2 | 0.382907 | 0.277492 | 0.085104 | 0.052* |
| C3 | 0.5361(3) | 0.3174(3) | 0.0300(2) | 0.0344(8) |
| C4 | 0.6678(3) | 0.3297(4) | 0.0432(2) | 0.0462(10) |
| H4 | 0.714029 | 0.343124 | 0.001304 | 0.055* |
| C5 | 0.7295(3) | 0.3220(4) | 0.1190(2) | 0.0488(11) |
| H5 | 0.818321 | 0.331845 | 0.127225 | 0.059* |
| C6 | 0.4656(3) | 0.3363(3) | −0.0471(2) | 0.0344(8) |
| C7 | 0.5266(3) | 0.3655(3) | −0.1103(2) | 0.0364(9) |
| H7 | 0.612935 | 0.367429 | −0.108476 | 0.044* |
| C8 | 0.4579(3) | 0.3922(3) | −0.1770(2) | 0.0343(8) |
| C9 | 0.3274(3) | 0.3818(3) | −0.1773(2) | 0.0368(9) |
| H9 | 0.277140 | 0.396649 | −0.221169 | 0.044* |
| C10 | 0.2719(3) | 0.3496(3) | −0.1127(2) | 0.0365(9) |
| C11 | 0.1336(3) | 0.3425(3) | −0.1085(2) | 0.0405(9) |
| C12 | 0.0867(4) | 0.3277(5) | −0.0376(3) | 0.0739(15) |
| H12 | 0.139852 | 0.319265 | 0.008073 | 0.089* |
| C13 | −0.0398(4) | 0.3254(6) | −0.0345(3) | 0.095(2) |
| H13 | −0.068499 | 0.315802 | 0.014628 | 0.114* |
| C14 | −0.0783(4) | 0.3466(4) | −0.1644(3) | 0.0650(13) |
| H14 | −0.134522 | 0.352352 | −0.209781 | 0.078* |
| C15 | 0.0470(4) | 0.3499(4) | −0.1743(3) | 0.0536(11) |
| H15 | 0.072316 | 0.357105 | −0.224902 | 0.064* |
| C16 | 0.5206(3) | 0.4328(3) | −0.2433(2) | 0.0356(9) |
| C17 | 0.4471(3) | 0.4314(3) | −0.3195(2) | 0.0407(9) |
| H17 | 0.356870 | 0.401959 | −0.329698 | 0.049* |
| C18 | 0.5038(3) | 0.4721(4) | −0.3798(2) | 0.0445(10) |
| H18 | 0.452002 | 0.468449 | −0.430711 | 0.053* |
| C19 | 0.6377(4) | 0.5188(4) | −0.3656(2) | 0.0487(11) |
| C20 | 0.7130(4) | 0.5229(4) | −0.2903(2) | 0.0612(13) |
| H20 | 0.803090 | 0.554334 | −0.279718 | 0.073* |
| C21 | 0.6540(3) | 0.4800(4) | −0.2303(2) | 0.0534(11) |
| H21 | 0.705846 | 0.483036 | −0.179717 | 0.064* |
| C22 | 0.8188(4) | 0.5960(6) | −0.4242(3) | 0.104(2) |
| H22A | 0.835559 | 0.612227 | −0.476390 | 0.156* |
| H22B | 0.854603 | 0.531886 | −0.416643 | 0.156* |
| H22C | 0.858409 | 0.670234 | −0.375822 | 0.156* |
| C23 | 0.7809(4) | 0.2293(5) | 0.4396(3) | 0.0725(15) |
| H23 | 0.865123 | 0.230599 | 0.433975 | 0.087* |
| C24 | 0.7364(4) | 0.1914(4) | 0.5050(3) | 0.0646(14) |
| H24 | 0.790440 | 0.168712 | 0.542217 | 0.077* |
| C25 | 0.6117(3) | 0.1870(3) | 0.5157(2) | 0.0377(9) |
| C26 | 0.5402(4) | 0.2242(4) | 0.4587(2) | 0.0475(10) |
| H26 | 0.455754 | 0.223988 | 0.463075 | 0.057* |
| C27 | 0.5918(4) | 0.2618(4) | 0.3955(2) | 0.0501(11) |
| H27 | 0.540543 | 0.287110 | 0.358438 | 0.060* |
| C28 | 0.5550(3) | 0.1440(3) | 0.5832(2) | 0.0367(9) |
| C29 | 0.6243(3) | 0.1082(3) | 0.6439(2) | 0.0402(9) |
| H29 | 0.710192 | 0.110242 | 0.643140 | 0.048* |
| C30 | 0.5655(3) | 0.0696(3) | 0.7054(2) | 0.0388(9) |
| C31 | 0.4365(3) | 0.0696(3) | 0.7034(2) | 0.0402(9) |
| H31 | 0.393718 | 0.045933 | 0.744158 | 0.048* |
| C32 | 0.3723(3) | 0.1052(3) | 0.6402(2) | 0.0384(9) |
| C33 | 0.2347(3) | 0.1056(3) | 0.6348(2) | 0.0424(10) |
| C34 | 0.1590(4) | 0.0659(4) | 0.6878(3) | 0.0676(14) |
| H34 | 0.193089 | 0.036077 | 0.729632 | 0.081* |
| C35 | 0.0324(4) | 0.0700(5) | 0.6796(3) | 0.0769(15) |
| H35 | −0.015125 | 0.043852 | 0.717611 | 0.092* |
| C36 | 0.0483(4) | 0.1473(4) | 0.5711(3) | 0.0665(13) |
| H36 | 0.011444 | 0.176680 | 0.529991 | 0.080* |
| C37 | 0.1757(4) | 0.1478(4) | 0.5748(3) | 0.0571(12) |
| H37 | 0.221677 | 0.176454 | 0.536982 | 0.069* |
| C38 | 0.6346(3) | 0.0281(3) | 0.7705(2) | 0.0404(9) |
| C39 | 0.7213(3) | −0.0379(4) | 0.7511(3) | 0.0493(10) |
| H39 | 0.736318 | −0.058278 | 0.695373 | 0.059* |
| C40 | 0.7865(4) | −0.0745(4) | 0.8124(2) | 0.0526(11) |
| H40 | 0.842837 | −0.120791 | 0.797483 | 0.063* |
| C41 | 0.7678(4) | −0.0421(4) | 0.8956(3) | 0.0472(10) |
| C42 | 0.6818(4) | 0.0241(4) | 0.9173(3) | 0.0537(11) |
| H42 | 0.668181 | 0.045208 | 0.973355 | 0.064* |
| C43 | 0.6159(4) | 0.0587(4) | 0.8548(2) | 0.0511(11) |
| H43 | 0.558054 | 0.103331 | 0.869526 | 0.061* |
| C44 | 0.8401(5) | −0.0316(5) | 1.0421(3) | 0.0764(15) |
| H44A | 0.755147 | −0.061289 | 1.052811 | 0.115* |
| H44B | 0.865848 | 0.057328 | 1.058664 | 0.115* |
| H44C | 0.901249 | −0.056885 | 1.075231 | 0.115* |
1 Source of materials
The reagents were purchased from standard commercial sources and used without further purification. A mixture of ZnCl2 (0.014 g, 0.10 mmol), meophtpy (0.034 g, 0.10 mmol) was dispersed in C2H5OH (7 mL) solution and ammonia (25 %) was added dropwise until a clear colorless solution was obtained. The resultant solution was allowed slowly to evaporate under room temperature for two weeks to give light yellow crystals which were isolated by filtration and washed by deionized water and dried in air.
2 Experimental details
The structure was solved by Direct Methods with the SHELXT-2018 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2U eq(C)) using a riding model with C–H = 0.93 and 0.97 Å.
3 Comment
In the past few years, metal-organic frameworks (MOFs) have been extensively studied for their diversity of structures, topologies, properties and potential application in different fields like gas sorption, fluorescence sensing, magnetism and photodegradation, etc. 5 , 6 , 7 , 8 The successful synthesis of MOFs mainly relies on the careful selection of metal ions and organic links. In this text, Zn(II) ion is accepted for its success in the assembly of fluorescence complexes 9 , 10 and 4,2′:6′,4″-terpyridine ligand is accepted as it contains two terminal pyridine N atoms which would behave as bridges among metal ions. 11 , 12 A Zn(II) complex was obtained with 4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine (meophtpy) as ligand and its structure has been determined. Just as it still possesses unsaturated coordination groups, the title complex may also behave as metalloligand, which are better alternative to direct the assembly of large molecular arrays and 1D, 2D, 3D coordination polymers and networks. 13 , 14
As shown in the figure, the asymmetric unit contains one Zn(II) ion, two meophtpy ligands, and two Cl− anions. Each Zn(II) ion is coordinated by two pyridyl N atoms from two meophtpy ligands, Zn1–N1 = 2.036(3) Å, Zn1–N4 = 2.062(3) Å and two chlorides, Zn1–Cl1 = 2.2340(18) Å, Zn1–Cl2 = 2.211(2) Å to furnish a distorted tetrahedral geometry. In this complex, the Cl–Zn–Cl bond angle is 116.55(7)°, the N–Zn–N bond angle is 115.56(14)° and the N–Zn–Cl bond angles range from 102.32(12)° to 108.65(11)°. All these bond lengths and angles are similar with other complexes with ZnN2Cl2 coordination environment. 12 , 13 , 14 , 15 , 16 , 17
In the crystal structure, five C atoms, C4, C15, C18, C22 and C24 act as hydrogen donors, contributing hydrogen atoms H4, H15, H18, H22C and H12 to N3, Cl1, O1, Cl2, and N6 to form non-classic hydrogen bonds between molecules. In addition, there are two kinds of offset face to face π–π stacking interactions with center to center distances of 3.641(4) Å and 3.896(4) Å, between pyridine rings. The discrete complexes were further extended into 3D network mainly by the hydrogen bonding interaction and the π–π stacking interactions.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the 2023 Innovation and Entrepreneurship Training Program for Nanyue College of Hengyang Normal University Students (No. NYD202319), Hunan Provincial Training Program of Innovation and Entrepreneurship for Undergraduates (No. S202412659002) and the Scientific Research Project of Hengyang Normal University (2023HSKFJJ013).
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Jiao, L.; Seow, J. Y. R.; Skinner, W. S.; Wang, Z. U.; Jiang, H. L. Metal-Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. https://doi.org/10.1016/j.mattod.2018.10.038.Search in Google Scholar
6. Wu, T.; Gao, X. J.; Ge, F.; Zheng, H. G. Metal-Organic Frameworks (MOFs) as Fluorescence Sensors: Principles, Development and Prospects. CrystEngComm 2022, 24, 7881–7901. https://doi.org/10.1039/d2ce01159j.Search in Google Scholar
7. Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. https://doi.org/10.1002/gch2.202300244.Search in Google Scholar PubMed PubMed Central
8. Chia, Y. Y.; Tay, M. G. An Insight into Fluorescent Transition Metal Complexes. Dalton Trans. 2014, 43, 13159–13168. https://doi.org/10.1039/c4dt01098a.Search in Google Scholar PubMed
9. Sun, Y. X.; Han, W. Y.; Deng, Z. P.; Sun, Y. G.; Jia, Y. H.; Sun, Y.; Zhang, S. Z. Zn-Based Metal-Organic-Framework as a Multifunctional Fluorescent Sensor for HSO4−, Acidic and Basic Amino Acids. Inorg. Chim. Acta 2023, 556, 121643. https://doi.org/10.1016/j.ica.2023.121643.Search in Google Scholar
10. Qi, S.; Li, Z.; Jia, Y.; Li, D.; Hu, M. A Zn-Coordination Polymer as a Multifunctional Fluorescent Probe for the Detection of V2O74−, Fe3+, and p-Nitrotoluene. Phys. Chem. Chem. Phys. 2023, 25, 10090–10096. https://doi.org/10.1039/d2cp05921e.Search in Google Scholar PubMed
11. Feng, J.; Li, H. M.; Yang, Q. L.; Wei, S. C.; Zhang, J. Y.; Su, C. Y. A Two-Dimensional Flexible Porous Coordination Polymer Based on Co(II) and Terpyridyl Phosphine Oxide. Inorg. Chem. Front. 2015, 2, 388–394. https://doi.org/10.1039/c4qi00138a.Search in Google Scholar
12. Wen, L. L.; Ke, X. H.; Qiu, L.; Zou, Y.; Zhou, L.; Zhao, J. B.; Li, D. F. Assembly of Two Porous Cadmium(II) Frameworks: Selective Adsorption and Luminescent Property. Cryst. Growth Des. 2012, 12, 4083–4089. https://doi.org/10.1021/cg300566x.Search in Google Scholar
13. Lan, B. L.; Luo, A. Y.; Shao, B.; Gao, L. N.; Wei, Q.; Liang, Y. N.; Huang, J.; Zhang, Z. Structural Evolution from Preorganized Mononuclear Triazamacrocyclic Metalloligands to Polynuclear Metallocages and Heterometallic 2D Layers: Modular Architectures, Assembly Tracking and Magnetic Properties. Dalton Trans. 2022, 51, 16158–16169. https://doi.org/10.1039/d2dt02186b.Search in Google Scholar PubMed
14. Planes, O. M.; Jansze, S. M.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. Two-Step Synthesis of Linear and Bent Dicarboxylic Acid Metalloligands with Lengths of up to 3 nm. Inorg. Chem. 2020, 59, 14544–14548. https://doi.org/10.1021/acs.inorgchem.0c02358.Search in Google Scholar PubMed
15. Li, M. J.; Nie, J. J.; Xu, D. J. Dichloridobis(isoquinoline- κN)zinc(II). Acta Crystallogr. 2010, e66, m876. https://doi.org/10.1107/s1600536810024803.Search in Google Scholar PubMed PubMed Central
16. Haleel, A.; Arthi, P.; Dastagiri Reddy, N.; Veena, V.; Sakthivel, N.; Arun, Y.; Perumal, P. T.; Kalilur, R. A. DNA Binding, Molecular Docking and Apoptotic Inducing Activity of Nickel(II), Copper(II) and Zinc(II) Complexes of Pyridine-Based Tetrazolo[1,5-a]pyrimidine Ligands. RSC Adv. 2014, 4, 60816–60830. https://doi.org/10.1039/c4ra11197d.Search in Google Scholar
17. Hou, L.; Li, D. A New Ligand 4′-Phenyl-4,2′:6′,4″-Terpyridine and its 1D Helical Zinc(II) Coordination Polymer: Syntheses, Structures and Photoluminescent Properties. Inorg. Chem. Commun. 2005, 8, 190–193. https://doi.org/10.1016/j.inoche.2004.11.030.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

