Abstract
C24H27FN6O4, monoclinic, C2 (no. 5), a = 19.7270(4) Å, b = 6.4057(2) Å, c = 36.3058(8) Å, β = 94.602(2)°, V = 4573.0(2) Å3, Z = 8, Rgt (F) = 0.0625, wRref (F 2) = 0.1565, T = 100 K.
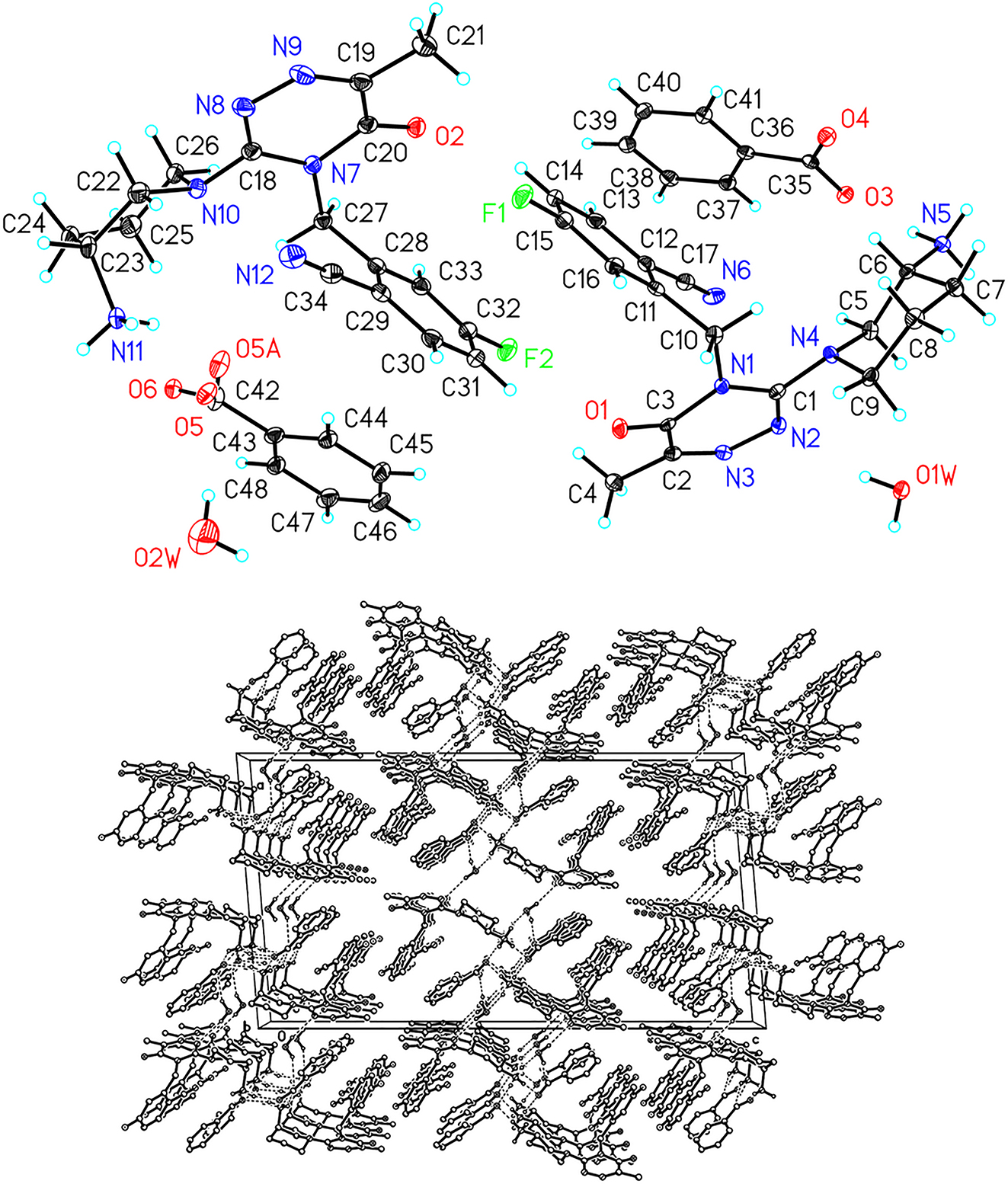
A part of the molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.15 × 0.05 × 0.04 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.86 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 65.0°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 20198, 7780, 0.097 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 6,155 |
| N(param)refined: | 644 |
| Programs: | SHELX 1 , 2 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| F1 | 0.79470 (17) | 0.2212 (7) | 0.74527 (10) | 0.0353 (9) |
| O1 | 0.56100 (19) | 0.6276 (7) | 0.71070 (10) | 0.0235 (9) |
| N1 | 0.5764 (2) | 0.6425 (8) | 0.64925 (12) | 0.0174 (9) |
| N2 | 0.5285 (2) | 0.9239 (8) | 0.61357 (12) | 0.0204 (10) |
| N3 | 0.5184 (2) | 1.0313 (8) | 0.64570 (13) | 0.0209 (10) |
| N4 | 0.5785 (2) | 0.6434 (8) | 0.58474 (12) | 0.0182 (9) |
| N5 | 0.6666 (2) | 0.7837 (8) | 0.50136 (12) | 0.0187 (9) |
| H5A | 0.6923 | 0.8896 | 0.5117 | 0.022* |
| H5B | 0.6303 | 0.8375 | 0.4875 | 0.022* |
| H5C | 0.6922 | 0.7061 | 0.4867 | 0.022* |
| N6 | 0.6855 (2) | 0.9966 (9) | 0.63322 (15) | 0.0278 (11) |
| C1 | 0.5587 (2) | 0.7429 (8) | 0.61588 (15) | 0.0168 (11) |
| C2 | 0.5296 (3) | 0.9392 (9) | 0.67750 (15) | 0.0202 (11) |
| C3 | 0.5547 (2) | 0.7259 (10) | 0.68174 (14) | 0.0187 (11) |
| C4 | 0.5191 (3) | 1.0581 (10) | 0.71241 (16) | 0.0250 (12) |
| H4A | 0.5121 | 1.2063 | 0.7065 | 0.037* |
| H4B | 0.5593 | 1.0423 | 0.7299 | 0.037* |
| H4C | 0.4791 | 1.0031 | 0.7235 | 0.037* |
| C5 | 0.5969 (3) | 0.7770 (8) | 0.55431 (14) | 0.0186 (11) |
| H5D | 0.6215 | 0.9026 | 0.5641 | 0.022* |
| H5E | 0.5556 | 0.8225 | 0.5392 | 0.022* |
| C6 | 0.6422 (3) | 0.6487 (9) | 0.53112 (14) | 0.0194 (11) |
| H6 | 0.6827 | 0.6018 | 0.5474 | 0.023* |
| C7 | 0.6058 (3) | 0.4578 (9) | 0.51555 (15) | 0.0221 (12) |
| H7A | 0.6369 | 0.3737 | 0.5014 | 0.026* |
| H7B | 0.5665 | 0.5002 | 0.4985 | 0.026* |
| C8 | 0.5810 (3) | 0.3271 (9) | 0.54709 (16) | 0.0229 (12) |
| H8A | 0.5525 | 0.2111 | 0.5366 | 0.028* |
| H8B | 0.6208 | 0.2654 | 0.5615 | 0.028* |
| C9 | 0.5401 (3) | 0.4553 (9) | 0.57274 (15) | 0.0208 (12) |
| H9A | 0.4961 | 0.4959 | 0.5597 | 0.025* |
| H9B | 0.5307 | 0.3706 | 0.5946 | 0.025* |
| C10 | 0.6225 (3) | 0.4613 (9) | 0.65253 (15) | 0.0191 (11) |
| H10A | 0.6359 | 0.4232 | 0.6277 | 0.023* |
| H10B | 0.5981 | 0.3409 | 0.6623 | 0.023* |
| C11 | 0.6861 (3) | 0.5060 (10) | 0.67793 (15) | 0.0199 (11) |
| C12 | 0.7231 (3) | 0.6900 (9) | 0.67631 (15) | 0.0211 (12) |
| C13 | 0.7829 (3) | 0.7186 (10) | 0.69921 (16) | 0.0255 (13) |
| H13 | 0.8066 | 0.8474 | 0.6987 | 0.031* |
| C14 | 0.8077 (3) | 0.5613 (10) | 0.72258 (16) | 0.0265 (14) |
| H14 | 0.8487 | 0.5785 | 0.7379 | 0.032* |
| C15 | 0.7711 (3) | 0.3799 (11) | 0.72299 (16) | 0.0255 (13) |
| C16 | 0.7111 (3) | 0.3477 (9) | 0.70156 (15) | 0.0205 (12) |
| H16 | 0.6872 | 0.2196 | 0.7029 | 0.025* |
| C17 | 0.7023 (3) | 0.8581 (9) | 0.65194 (16) | 0.0225 (12) |
| F2 | 0.68405 (18) | 0.9308 (7) | 0.76107 (10) | 0.0382 (9) |
| O2 | 0.9152 (2) | 0.5351 (7) | 0.79227 (11) | 0.0283 (10) |
| N7 | 0.8999 (2) | 0.4828 (8) | 0.85317 (13) | 0.0233 (10) |
| N8 | 0.9496 (2) | 0.1857 (9) | 0.88328 (15) | 0.0314 (12) |
| N9 | 0.9585 (3) | 0.0978 (9) | 0.84931 (16) | 0.0332 (13) |
| N10 | 0.9030 (2) | 0.4457 (9) | 0.91788 (14) | 0.0269 (11) |
| N11 | 0.7908 (2) | 0.3944 (9) | 0.97124 (13) | 0.0270 (11) |
| H11A | 0.7708 | 0.4255 | 0.9923 | 0.032* |
| H11B | 0.7736 | 0.2721 | 0.9618 | 0.032* |
| H11C | 0.7822 | 0.4984 | 0.9544 | 0.032* |
| N12 | 0.7893 (3) | 0.1421 (9) | 0.87114 (17) | 0.0350 (13) |
| C18 | 0.9197 (3) | 0.3667 (10) | 0.88432 (17) | 0.0259 (13) |
| C19 | 0.9466 (3) | 0.2060 (11) | 0.81929 (17) | 0.0288 (13) |
| C20 | 0.9210 (3) | 0.4204 (10) | 0.81928 (16) | 0.0237 (12) |
| C21 | 0.9579 (3) | 0.1079 (11) | 0.78277 (19) | 0.0322 (14) |
| H21A | 0.9973 | 0.1729 | 0.7726 | 0.048* |
| H21B | 0.9175 | 0.1287 | 0.7656 | 0.048* |
| H21C | 0.9662 | −0.0419 | 0.7862 | 0.048* |
| C22 | 0.9005 (3) | 0.2874 (12) | 0.94707 (18) | 0.0331 (14) |
| H22A | 0.9473 | 0.2422 | 0.9552 | 0.040* |
| H22B | 0.8750 | 0.1639 | 0.9371 | 0.040* |
| C23 | 0.8663 (3) | 0.3731 (12) | 0.97984 (16) | 0.0319 (15) |
| H23 | 0.8739 | 0.2703 | 1.0005 | 0.038* |
| C24 | 0.8973 (3) | 0.5792 (13) | 0.99318 (18) | 0.0381 (17) |
| H24A | 0.9427 | 0.5523 | 1.0060 | 0.046* |
| H24B | 0.8684 | 0.6415 | 1.0114 | 0.046* |
| C25 | 0.9048 (3) | 0.7360 (12) | 0.96192 (19) | 0.0366 (16) |
| H25A | 0.8593 | 0.7862 | 0.9524 | 0.044* |
| H25B | 0.9315 | 0.8578 | 0.9716 | 0.044* |
| C26 | 0.9401 (3) | 0.6368 (12) | 0.93080 (17) | 0.0311 (14) |
| H26A | 0.9415 | 0.7368 | 0.9101 | 0.037* |
| H26B | 0.9875 | 0.6006 | 0.9396 | 0.037* |
| C27 | 0.8534 (3) | 0.6642 (10) | 0.85332 (16) | 0.0231 (12) |
| H27A | 0.8774 | 0.7904 | 0.8455 | 0.028* |
| H27B | 0.8404 | 0.6878 | 0.8788 | 0.028* |
| C28 | 0.7902 (3) | 0.6312 (10) | 0.82787 (16) | 0.0234 (12) |
| C29 | 0.7512 (3) | 0.4517 (10) | 0.82854 (16) | 0.0243 (12) |
| C30 | 0.6911 (3) | 0.4291 (11) | 0.80562 (17) | 0.0282 (13) |
| H30 | 0.6660 | 0.3025 | 0.8057 | 0.034* |
| C31 | 0.6684 (3) | 0.5917 (11) | 0.78286 (17) | 0.0306 (15) |
| H31 | 0.6272 | 0.5803 | 0.7675 | 0.037* |
| C32 | 0.7067 (3) | 0.7688 (11) | 0.78297 (17) | 0.0284 (14) |
| C33 | 0.7672 (3) | 0.7938 (10) | 0.80455 (16) | 0.0250 (12) |
| H33 | 0.7927 | 0.9192 | 0.8035 | 0.030* |
| C34 | 0.7724 (3) | 0.2812 (10) | 0.85291 (17) | 0.0267 (13) |
| O5Aa | 0.7356 (8) | 0.697 (2) | 0.9246 (4) | 0.037 (3) |
| O5a | 0.7092 (6) | 0.689 (3) | 0.9343 (4) | 0.031 (3) |
| O6 | 0.7394 (2) | 0.9971 (7) | 0.95724 (11) | 0.0270 (9) |
| C42 | 0.7115 (3) | 0.8809 (11) | 0.93235 (17) | 0.0324 (15) |
| C43 | 0.6614 (3) | 0.9792 (11) | 0.90366 (17) | 0.0276 (13) |
| C44 | 0.6378 (3) | 0.8635 (12) | 0.87290 (17) | 0.0307 (15) |
| H44 | 0.6532 | 0.7243 | 0.8701 | 0.037* |
| C45 | 0.5918 (3) | 0.9495 (13) | 0.84621 (18) | 0.0385 (18) |
| H45 | 0.5771 | 0.8709 | 0.8249 | 0.046* |
| C46 | 0.5676 (3) | 1.1466 (13) | 0.8506 (2) | 0.0409 (18) |
| H46 | 0.5352 | 1.2039 | 0.8326 | 0.049* |
| C47 | 0.5902 (3) | 1.2619 (13) | 0.8809 (2) | 0.0383 (17) |
| H47 | 0.5733 | 1.3993 | 0.8839 | 0.046* |
| C48 | 0.6374 (3) | 1.1799 (12) | 0.90740 (18) | 0.0321 (15) |
| H48 | 0.6533 | 1.2619 | 0.9281 | 0.039* |
| O3 | 0.75915 (18) | 1.0244 (6) | 0.54135 (10) | 0.0210 (8) |
| O4 | 0.80815 (19) | 0.7205 (7) | 0.55702 (11) | 0.0255 (9) |
| C35 | 0.7981 (3) | 0.9073 (9) | 0.56232 (14) | 0.0177 (11) |
| C36 | 0.8332 (3) | 1.0138 (9) | 0.59578 (15) | 0.0181 (11) |
| C37 | 0.8180 (3) | 1.2164 (10) | 0.60424 (16) | 0.0243 (12) |
| H37 | 0.7881 | 1.2950 | 0.5878 | 0.029* |
| C38 | 0.8462 (3) | 1.3079 (10) | 0.63690 (17) | 0.0260 (13) |
| H38 | 0.8360 | 1.4490 | 0.6424 | 0.031* |
| C39 | 0.8888 (3) | 1.1930 (11) | 0.66130 (16) | 0.0269 (13) |
| H39 | 0.9058 | 1.2521 | 0.6842 | 0.032* |
| C40 | 0.9066 (3) | 0.9917 (11) | 0.65223 (16) | 0.0269 (13) |
| H40 | 0.9381 | 0.9153 | 0.6682 | 0.032* |
| C41 | 0.8782 (3) | 0.9008 (9) | 0.61963 (15) | 0.0203 (11) |
| H41 | 0.8895 | 0.7614 | 0.6137 | 0.024* |
| O1W | 0.42813 (19) | 1.0158 (7) | 0.54708 (11) | 0.0254 (9) |
| H1C | 0.4521 | 0.9960 | 0.5673 | 0.038* |
| H1D | 0.3931 | 1.0801 | 0.5533 | 0.038* |
| O2W | 0.5643 (4) | 0.5946 (14) | 0.9474 (2) | 0.084 (2) |
| H2C | 0.6042 | 0.6243 | 0.9418 | 0.125* |
| H2D | 0.5363 | 0.6221 | 0.9290 | 0.125* |
-
aOccupancy: 0.5.
1 Source of materials
The title compound was added to isopropyl acetate, stirred for half an hour to form a saturated solution, then filtered and allowed to evaporate at room temperature to yield colorless prism crystals. The NMR spectra were acquired on a Bruker Avance III HD 400 MHz spectrometer. X-ray powder diffraction (XRD) intensities were measured at 293 K on a empyrean/Bragg-BrentanoHD diffractometer (CuKα, λ = 1.54056 Å). 1 H NMR (400 MHz, DMSO, ppm): δ 7.96 (m, 1H), 7.36 (br, 1H), 7.29 (d, 1H), 5.23 (s, 2H), 3.15 (m, 3H), 2.72 (m, 2H), 2.23 (s, 3H), 1.78 (d, 1H), 1.64 (d, 1H), 1.47 (m, 1H), 1.12 (m, 1H). PXRD: θ = 8.87, 9.74, 12.58, 14.54, 18.87, 19.51, 20.13, 20.77, 22.13, 23.35, 24.08, 25.36, 26.74, 27.83, 29.53, 30.38, 31.75, 33.25°.
2 Experimental details
The diffraction data obtained were processed using the SHELXT software 1 to solve the crystal structure. This structure was further refined using full-matrix least-squares procedures in SHELXL. 2 Anisotropic refinement was applied to non-hydrogen atoms, while hydrogen atoms were refined isotropically.
3 Comment
Diabetes is a chronic disease where prolonged hyperglycemia can cause damage to various organs of the body, leading to various complications. Therefore, controlling blood glucose levels is the primary goal of diabetes treatment. 3 The title compound belongs to the class of DPP-IV inhibitors, used for the treatment of type II diabetes. Inhibition of DPP-IV in the body can increase the levels of endogenous GLP 1(7–36) and reduce the production of its antagonist GLP 1(9–36). Thus, DPP-IV inhibitors may be effective against diseases associated with DPP-IV activity, such as type II diabetes, diabetic dyslipidemia, impaired glucose tolerance (IGT), impaired fasting plasma glucose (IFG), metabolic acidosis, ketosis, appetite regulation, and obesity. 4 , 5 , 6 DPP-IV inhibitors are currently considered a new approach for the treatment of type 2 diabetes. 7 , 8
The X-ray structural analysis of the compound reveals that the asymmetric unit contains two ((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl)methyl)-4-fluorobenzonitrile cations and two benzoic acid anions and two water molecules. This cation can be seen as an aminopiperidinyl group and a 4-fluoro-2-methylbenzonitrile group (with a dihedral angle of approximately 86.6°) connected to the adjacent positions of an oxo-1,2,4-triazinyl group. The pKa value of benzoic acid is 3.9, and another component’s pKa value is 8.6, indicating that both are prone to forming salt compound. In the infrared spectrum, the stretching vibrations at 3,056 cm−1 and 2,624 cm−1, as well as the deformation vibration at 1,592 cm−1, can be attributed to the –NH3 + groups. As shown in figure (below), along the crystallographic b direction, the two main components of the compound form one-dimensional ribbon-like supramolecule chains relying on the abundant hydrogen bonds between their amino groups and carboxyl groups (N⋯O = 2.719–2.789 Å). Hydrogen bonds between the carboxyl/triazinyl groups and the surrounding guest water molecules (O⋯O = 2.755–2.996 Å and O⋯N = 2.876–3.169 Å) play a role in stabilizing the parallel packing of these one-dimensional chains.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. SHELXTL – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
2. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
3. Bastaki, A. Diabetes Mellitus and its Treatment. Int. J. Diabetes Metabol. 2005, 13, 111–134. https://doi.org/10.1159/000497580.Search in Google Scholar
4. Bharatam, P.; Patel, D.; Adane, L.; Mittal, A.; Sundriyal, S. Modeling and Informatics in Designing Anti-Diabetic Agents. Curr. Pharm. Des. 2007, 13, 3518–3530. https://doi.org/10.2174/138161207782794239.Search in Google Scholar PubMed
5. Demuth, H.-U.; McIntosh, C. H.; Pederson, R. A. Type 2 Diabetes − Therapy with Dipeptidyl Peptidase IV Inhibitors. Biochim. Biophys. Acta 2005, 1751, 33–34. https://doi.org/10.1016/j.bbapap.2005.05.010.Search in Google Scholar PubMed
6. Polgar, L.; Szeltner, Z. Structure, Function and Biological Relevance of Prolyl Oligopeptidase. Curr. Protein Pept. Sci. 2008, 9, 96–107. https://doi.org/10.2174/138920308783565723.Search in Google Scholar PubMed
7. Kumar, S.; Mittal, A.; Mittal, A. A Review Upon Medicinal Perspective and Designing Rationale of DPP-4 Inhibitors. Bioorg. Med. Chem. 2021, 46, 116354. https://doi.org/10.1016/j.bmc.2021.116354.Search in Google Scholar PubMed
8. Fang, L.; Gao, Z.; Wu, S.; Jia, S.; Wang, J.; Rohani, S.; Gong, J. Ultrasound-Assisted Solution Crystallization of Fotagliptin Benzoate: Process Intensification and Crystal Product Optimization. Ultrason. Sonochem. 2021, 76, 105634. https://doi.org/10.1016/j.ultsonch.2021.105634.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

