Abstract
C28H36O2, monoclinic, P21/c (no. 14), a = 25.4751(13) Å, b = 7.4053(3) Å, c = 6.3520(3) Å, β = 93.923(5)°, V = 1,195.5(1) Å3, Z = 2, R gt(F) = 0.0650, wR ref(F 2) = 0.2007, T = 298 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.26 × 0.24 × 0.21 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.53 mm−1 |
| Diffractometer, scan mode: | Bruker SMART CCD 6000, ω |
| θ max, completeness: | 68.2°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 7,561, 2,166, 0.090 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 802 |
| N(param)refined: | 137 |
| Programs: | Bruker 1 , Olex2 2 , SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.94563 (12) | 0.4544 (5) | −0.3031 (5) | 0.1317 (15) |
| H1A | 0.924252 | 0.483574 | −0.428820 | 0.198* |
| H1B | 0.948281 | 0.325556 | −0.289074 | 0.198* |
| H1C | 0.980119 | 0.504843 | −0.311861 | 0.198* |
| C2 | 0.92108 (10) | 0.5309 (4) | −0.1155 (5) | 0.0972 (11) |
| H2A | 0.919773 | 0.661275 | −0.129094 | 0.117* |
| H2B | 0.943380 | 0.502584 | 0.010045 | 0.117* |
| C3 | 0.86616 (11) | 0.4620 (4) | −0.0861 (5) | 0.0846 (10) |
| H3A | 0.843178 | 0.497108 | −0.207245 | 0.101* |
| H3B | 0.866921 | 0.331107 | −0.080777 | 0.101* |
| C4 | 0.84383 (11) | 0.5322 (4) | 0.1106 (5) | 0.0820 (10) |
| H4A | 0.842455 | 0.662934 | 0.102698 | 0.098* |
| H4B | 0.867660 | 0.500445 | 0.230496 | 0.098* |
| C5 | 0.78992 (11) | 0.4634 (4) | 0.1500 (5) | 0.0746 (9) |
| H5A | 0.764388 | 0.506245 | 0.041122 | 0.089* |
| H5B | 0.789581 | 0.332413 | 0.149166 | 0.089* |
| C6 | 0.72668 (12) | 0.5105 (4) | 0.4092 (5) | 0.0595 (8) |
| C7 | 0.68655 (12) | 0.4232 (3) | 0.2934 (4) | 0.0650 (9) |
| H7 | 0.692653 | 0.368424 | 0.165637 | 0.078* |
| C8 | 0.63680 (12) | 0.4184 (3) | 0.3709 (5) | 0.0640 (8) |
| H8 | 0.609693 | 0.361639 | 0.290616 | 0.077* |
| C9 | 0.62586 (12) | 0.4945 (3) | 0.5626 (5) | 0.0585 (8) |
| C10 | 0.66747 (11) | 0.5828 (3) | 0.6748 (4) | 0.0640 (9) |
| H10 | 0.661606 | 0.637398 | 0.802971 | 0.077* |
| C11 | 0.71675 (11) | 0.5908 (3) | 0.6005 (4) | 0.0645 (9) |
| H11 | 0.743693 | 0.650383 | 0.678514 | 0.077* |
| C12 | 0.57408 (11) | 0.4723 (3) | 0.6417 (5) | 0.0622 (8) |
| H12 | 0.548934 | 0.416946 | 0.550303 | 0.075* |
| C13 | 0.55795 (11) | 0.5216 (3) | 0.8299 (5) | 0.0639 (9) |
| H13 | 0.581141 | 0.588655 | 0.918246 | 0.077* |
| C14 | 0.50789 (11) | 0.4789 (4) | 0.9048 (4) | 0.0659 (9) |
| H14 | 0.484242 | 0.416504 | 0.813511 | 0.079* |
| O1 | 0.77736 (7) | 0.5296 (2) | 0.3513 (3) | 0.0741 (7) |
1 Source of materials
4-Pentyloxybenzaldehyde were purchased from Energy Chemical. Sodium hydride, 60 % dispersion in mineral oil was purchased from J & K Scientific. The (E)-tetraethylbut-2-ene-1,4-diyldiphosphonate was synthesized following the previous report. 5 The (E)-tetraethylbut-2-ene-1,4-diyldiphosphonate (1.15 g, 3.5 mmol) was dissolved in 10 mL of dry tetrahydrofuran (THF). A 60 % dispersion of sodium hydride in mineral oil (0.42 g, 10.5 mmol) was added with 10 mL of dry THF, and the mixture was stirred at room temperature for 30 min. To this mixture a solution of 4-pentyloxybenzaldehyde (1.34 g, 7 mmol) in 20 mL of dry THF was added dropwise over 5 min. The mixture was stirred for 24 h at room temperature. Water was added to the mixture and vigorously stirred for 1 h. The crude product compound was purified by column chromatography on silica gel (dichlormethane/n-hexane = 1/4). Single crystals were grown from toluene by slow evaporation at room temperature in the dark.
2 Experimental details
The SHELXT program was used to determine the initial structure. SHELXL program was carried out to refine the structure. The H atoms were positioned ideally with isotropic thermal parameters.
3 Comment
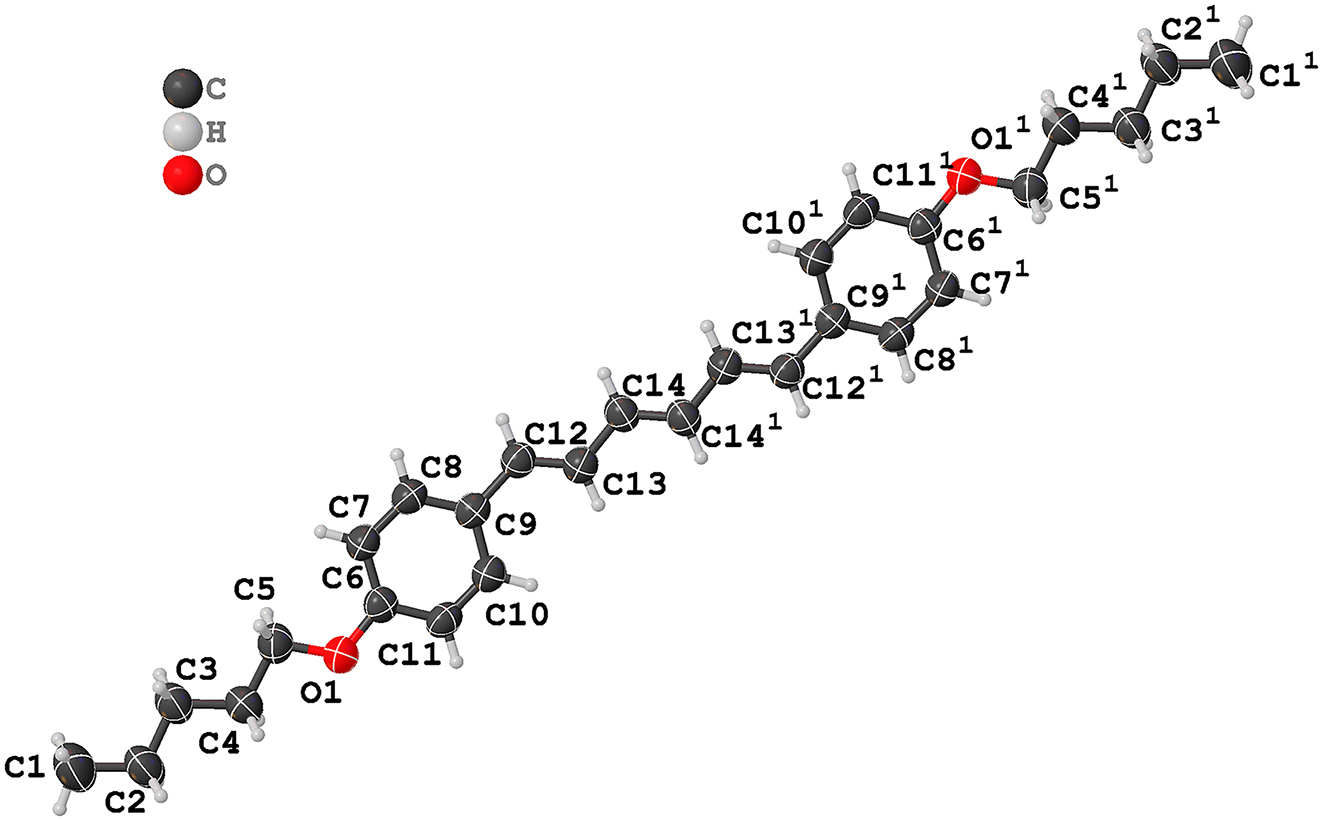
(E,E,E)-1,6-diphenyl-1,3,5-hexatriene (DPH) shows good singlet fission property. 6 The function of DPH molecules is of interest, particularly when substituents are attached or benzene rings are replaced with other aromatic rings. These modifications can alter the relative orientation and distance between adjacent molecules in the solid state, potentially affecting the efficiency and yield of singlet fission. 7 , 8 So far, only limited DPH-based crystal structures were reported. To extend the family of singlet fission chromophores, the title compound was synthesized. The asymmetric unit contains half a DPH-OC5H11 molecule. In the triene segment of central DPH, carbon-carbon (C–C) single bonds and C–C double bonds alternate. These conjugated bonds have bond lengths within the range of 1.337(5) – 1.453(4) Å and bond angles within the range of 124.9(3) – 128.2(3)°. In addition, the torsion angles of C14–C13–C12–C9, C12–C13–C14–C141−x,1−y,2−z and C13–C14–C141−x,1−y,2−z –C131−x,1−y,2−z are of −173.3(2)°, 177.2(3)° and 180°. The carbon atoms in the part of the pentyloxy connected to the central benzene ring only have single bonds, with C–O bond lengths, C–C bond lengths and bond angles in the range of 1.374(3) – 1.426(3) Å, 1.494(3) – 1.513(4) Å and 107.3(3) – 115.1(3)°, respectively. All the C–C and C–O bond lengths are in the normal range and comparable to the reported DPH-based crystal structures. 9 , 10 , 11 , 12 Furthermore, from the perspective of the crystal structure, the molecule demonstrates good planarity. Further analysis indicates that the formation of the 3D crystal structure is dominated by pi-pi interactions between the benzene rings (Cg: C6–C7–C8–C9–C10–C11), centroid-centroid distance: 4.8404(15) – 4.9161(15) Å and C–H⋯pi interactions (C7–H7⋯Cg x,1/2−y,−1/2+z , H7⋯Cg x,1/2−y,−1/2+z distance: 3.00 Å, C7⋯Cg x,1/2−y,−1/2+z distance: 3.716(3) Å; C10–H10⋯Cg x,3/2−y,1/2+z , H10⋯Cg x,3/2−y,1/2+z distance: 2.92 Å, C10⋯Cg x,3/2−y,1/2+z distance: 3.643(3) Å).
-
Research funding: Scientific Research Fund for Doctor of Weifang Univerisity (2020BS17).
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Kauffman, J. M.; Moyna, G. Diarylamino Groups as Photostable Auxofluors in 2-Benzoxazolylfluorene, 2,5-Diphenyloxazoles, 1,3,5-Hexatrienes, 1,4-Distyrylbenzenes, and 2,7-Distyrylfluorenes. J. Org. Chem. 2003, 68, 839–853. https://doi.org/10.1002/chin.200320028.Search in Google Scholar
6. Dillon, R. J.; Piland, G. B.; Bardeen, C. J. Different Rates of Singlet Fission in Monoclinic Versus Orthorhombic Crystal Forms of Diphenylhexatriene. J. Am. Chem. Soc. 2013, 135, 17278–17281. https://doi.org/10.1021/ja409266s.Search in Google Scholar PubMed
7. Sonoda, Y.; Katoh, R.; Tohnai, N.; Yago, T.; Wakasa, M. Singlet Fission in Solid 1,6-Diphenyl-1,3,5-Hexatriene Dicarboxylic Acids and Esters: Effects of Meta and Para Substitution. J. Phys. Chem. C 2022, 126, 8742–8751. https://doi.org/10.1021/acs.jpcc.2c01474.Search in Google Scholar
8. Fallon, K. J.; Sawhney, N.; Toolan, D. T. W.; Sharma, A.; Zeng, W.; Montanaro, S.; Leventis, A.; Dowland, S.; Millington, O.; Congrave, D.; Bond, A.; Friend, R.; Rao, A.; Bronstein, H. Quantitative Singlet Fission in Solution-Processable Dithienohexatrienes. J. Am. Chem. Soc. 2022, 144, 23516–23521. https://doi.org/10.1021/jacs.2c10254.Search in Google Scholar PubMed PubMed Central
9. Sonoda, Y.; Goto, M.; Norikane, Y.; Azum, R. Crystal Structures and Fluorescence Spectroscopic Properties of Cyano-Substituted Diphenylhexatrienes. Cryst. Growth Des. 2014, 14, 4781–4789. https://doi.org/10.1021/cg5009363.Search in Google Scholar
10. Sonoda, Y.; Goto, M.; Matsumoto, Y.; Shimoi, Y.; Sasaki, F.; Furube, A. Halogenated (F, Cl, Br, or I) Diphenylhexatrienes: Crystal Structures, Fluorescence Spectroscopic Properties, and Quantum Chemical Calculations. Cryst. Growth Des. 2016, 16, 4060–4071. https://doi.org/10.1021/acs.cgd.6b00590.Search in Google Scholar
11. Katoh, R.; Hashimoto, M.; Takahashi, A.; Sonoda, Y.; Yago, T.; Wakasa, M. Singlet Fission in Fluorinated Diphenylhexatrienes. J. Phys. Chem. C 2017, 121, 25666–25671. https://doi.org/10.1021/acs.jpcc.7b06905.Search in Google Scholar
12. Sonoda, Y.; Goto, M.; Tsuzuki, S.; Tamaoki, N. Fluorescence Spectroscopic Properties and Crystal Structure of a Series of Donor-Acceptor Diphenylpolyenes. J. Phys. Chem. A 2006, 110, 13379–13387. https://doi.org/10.1021/jp064937j.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

