Abstract
C20H26O5, orthorhombic, P212121 (no. 19), a = 5.2825(10) Å, b = 13.0652(2) Å, c = 26.4176(4) Å, V = 1823.26(5) Å3, Z = 4, R gt (F) = 0.0338, wR ref (F 2) = 0.0821, T = 150 K. CCDC No.: 2377464.
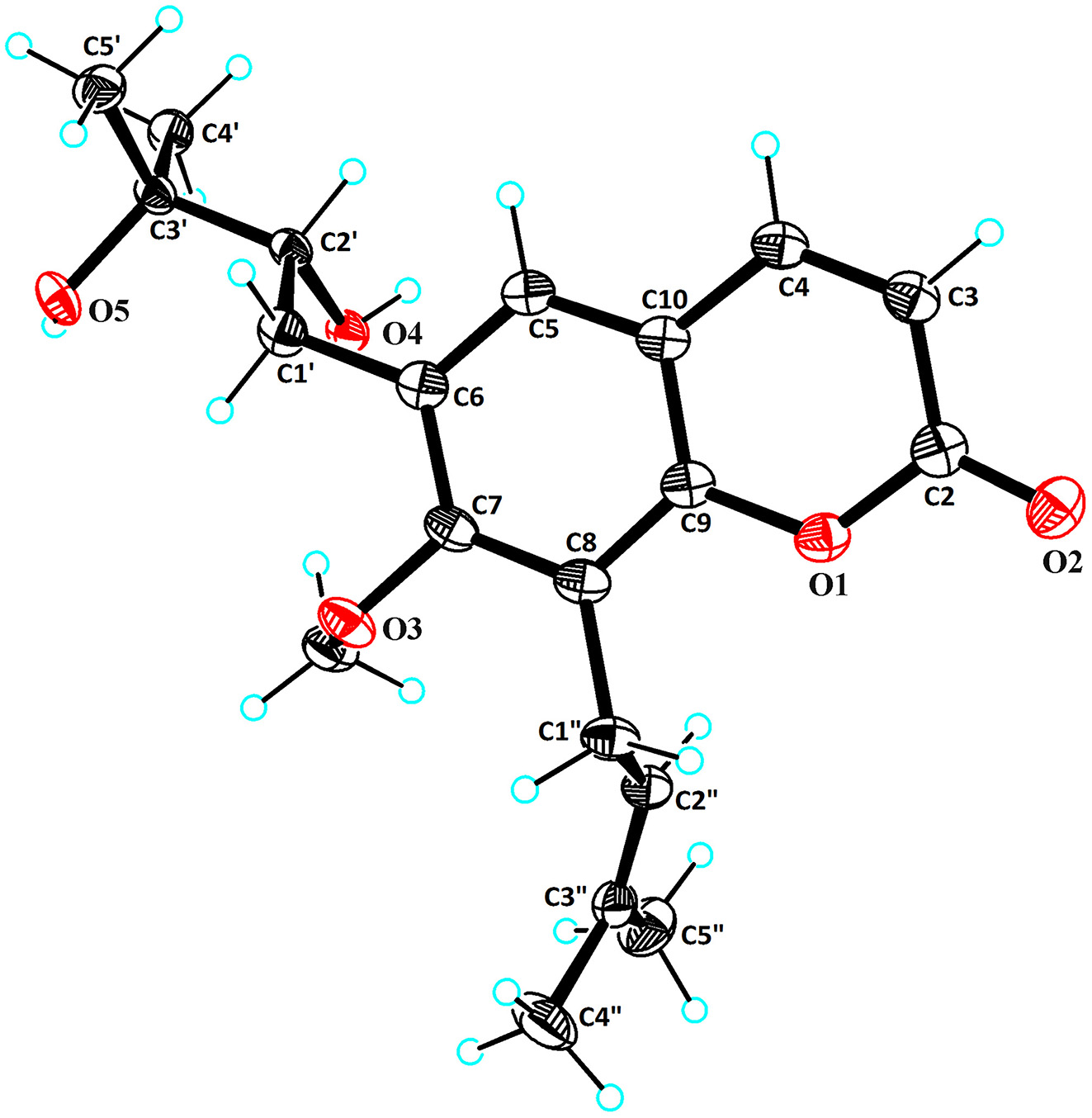
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size | 0.10 × 0.09 × 0.07 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 0.73 mm−1 |
| Diffractometer, scan mode: | XtaLAB AFC12 (RINC), ω |
| θ max, completeness: | 73.9°, >99 % |
| N(hkl)measured , N(hkl)unique, R int: | 17,041, 3,653, 0.046 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3,364 |
| N(param)refined: | 233 |
| Programs: | Olex2, 1 SHELX 2 , 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1′ | 0.2310 (4) | 0.27497 (15) | 0.36932 (7) | 0.0361 (4) |
| H1′A | 0.129627 | 0.234968 | 0.344785 | 0.043* |
| H1′B | 0.113050 | 0.318512 | 0.388927 | 0.043* |
| C1″ | 0.5611 (4) | 0.62884 (15) | 0.32281 (7) | 0.0390 (5) |
| H1″A | 0.625850 | 0.660446 | 0.291301 | 0.047* |
| H1″B | 0.382839 | 0.650401 | 0.327229 | 0.047* |
| C2′ | 0.3644 (3) | 0.20121 (14) | 0.40547 (7) | 0.0280 (4) |
| H2′A | 0.482511 | 0.157884 | 0.385065 | 0.034* |
| C2 | 1.1047 (4) | 0.49086 (16) | 0.23038 (7) | 0.0382 (5) |
| C2″ | 0.7152 (4) | 0.66586 (14) | 0.36701 (8) | 0.0370 (4) |
| H2″ | 0.866809 | 0.629289 | 0.373890 | 0.044* |
| C3′ | 0.1810 (3) | 0.12958 (14) | 0.43342 (7) | 0.0301 (4) |
| C3″ | 0.6636 (4) | 0.74378 (16) | 0.39750 (7) | 0.0384 (4) |
| C3 | 1.1042 (4) | 0.38152 (16) | 0.22168 (7) | 0.0394 (5) |
| H3 | 1.221991 | 0.353033 | 0.198446 | 0.047* |
| C4 | 0.9412 (4) | 0.31937 (15) | 0.24569 (7) | 0.0361 (5) |
| H4 | 0.947316 | 0.247732 | 0.239649 | 0.043* |
| C4′ | 0.3214 (4) | 0.06704 (15) | 0.47310 (8) | 0.0363 (4) |
| H4′A | 0.205009 | 0.017505 | 0.488420 | 0.054* |
| H4′B | 0.387279 | 0.112804 | 0.499375 | 0.054* |
| H4′C | 0.462208 | 0.030542 | 0.457065 | 0.054* |
| C4″ | 0.4483 (6) | 0.8158 (2) | 0.38997 (12) | 0.0685 (8) |
| H4″A | 0.332936 | 0.788024 | 0.364376 | 0.103* |
| H4″B | 0.513166 | 0.882187 | 0.378596 | 0.103* |
| H4″C | 0.357217 | 0.824518 | 0.421999 | 0.103* |
| C5 | 0.5869 (4) | 0.29991 (15) | 0.30770 (7) | 0.0326 (4) |
| H5 | 0.590302 | 0.227703 | 0.303537 | 0.039* |
| C5′ | 0.0477 (4) | 0.05945 (16) | 0.39589 (8) | 0.0419 (5) |
| H5′A | −0.064601 | 0.100107 | 0.374272 | 0.063* |
| H5′B | −0.052000 | 0.008435 | 0.414371 | 0.063* |
| H5′C | 0.174085 | 0.024683 | 0.374856 | 0.063* |
| C5″ | 0.8246 (6) | 0.7657 (2) | 0.44297 (9) | 0.0576 (7) |
| H5″A | 0.973951 | 0.721209 | 0.442509 | 0.086* |
| H5″B | 0.726621 | 0.752800 | 0.473809 | 0.086* |
| H5″C | 0.878578 | 0.837468 | 0.442296 | 0.086* |
| C6 | 0.4125 (4) | 0.34266 (15) | 0.34071 (7) | 0.0336 (4) |
| C7 | 0.4091 (4) | 0.44986 (15) | 0.34505 (7) | 0.0346 (4) |
| C8 | 0.5728 (4) | 0.51340 (14) | 0.31806 (7) | 0.0344 (4) |
| C9 | 0.7493 (4) | 0.46614 (14) | 0.28684 (7) | 0.0331 (4) |
| C10 | 0.7577 (4) | 0.35997 (14) | 0.28039 (6) | 0.0320 (4) |
| C11 | 0.2921 (5) | 0.50260 (17) | 0.42739 (8) | 0.0453 (5) |
| H11A | 0.149495 | 0.531230 | 0.446417 | 0.068* |
| H11B | 0.440317 | 0.547013 | 0.431351 | 0.068* |
| H11C | 0.331613 | 0.434126 | 0.440312 | 0.068* |
| O1′ | 0.5132 (2) | 0.25973 (10) | 0.44047 (5) | 0.0309 (3) |
| H1′ | 0.661104 | 0.236204 | 0.441405 | 0.046* |
| O1 | 0.9186 (3) | 0.52903 (10) | 0.26183 (5) | 0.0378 (3) |
| O2′ | −0.0158 (3) | 0.18863 (12) | 0.45742 (5) | 0.0375 (3) |
| H2′ | 0.020309 | 0.197435 | 0.488078 | 0.056* |
| O2 | 1.2502 (3) | 0.55211 (12) | 0.21228 (5) | 0.0474 (4) |
| O3 | 0.2264 (3) | 0.49593 (11) | 0.37495 (5) | 0.0439 (4) |
1 Source of material
The fruits of Rosa roxburghii Tratt were extracted with 95 % ethanol under reflux. The crude extract (914.5 g) was loaded onto a silica gel column and eluted with a solvent system of dichloromethane/methanol (1:0 to 1:1, v/v) to afford eight fractions (Fr. A–H). The colourless block crystals were isolated from fraction B and recrystallized with ethyl acetate/methanol (1:5, v/v). The title compound (23 mg) was obtained after 3 days.
2 Experimental details
The carbon-bound hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms with d (C–H) = 0.95–0.99 Å, U iso (H) = 1.5 times U eq (C) and 1.2 times U eq (O).
3 Comment
Recent studies have shown that a series of coumarins with various structures have been found in R. roxburghii fruit, and exhibit multifarious biological activities, including antioxidant, 4 antibacterial, 5 anti-inflammatory, 6 anticancer, 7 neuroprotective 8 effects. The title compound contains two hydroxyl groups, one double bond, a methoxy and four methyl groups. The hydroxyl was confirmed by the distances d(C2′–O4) = 1.434(2) Å and d(C3′–O5) = 1.442(2) Å, the olefinic bond was identified by the distance d(C2″–C3″) = 1.326(3) Å, the methoxy was confirmed by the distances d(C7–O3) = 1.385(2) Å, respectively. And the structural characteristics of the title compound are similar to those of 7-methoxy-8-(3-methyl-2-butenyl)coumarin 9 and Buntansin C. 10 and related compounds. 11
Acknowledgements
The authors gratefully acknowledge support from: National Natural Science Foundation of China [No. 32160104], The construction of innovation capacity of scientific research institutions in Guizhou province [No. QJHFQ[2024]005], Guizhou Provincial Basic Research Program (No. 2023-239), Science and Technology Program of Guizhou Province (No. [2023]077).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: National Natural Science Foundation of China [No. 32160104], The construction of innovation capacity of scientific research institutions in Guizhou province [No. QJHFQ[2024]005], Guizhou Provincial Basic Research Program (No. 2023-239), Science and Technology Program of Guizhou Province (No. [2023]077).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. SHELXTL – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Wu, C. R.; Huang, M. Y.; Lin, Y. T.; Ju, H. J.; Ching, H. Antioxidant Properties of Cortex Fraxini and its Simple Coumarins. Food Chem. 2007, 104, 1464–1471; https://doi.org/10.1016/j.foodchem.2007.02.023.Search in Google Scholar
5. Yang, L.; Ding, W.; Xu, Y. Q.; Wu, D. S.; Li, S. L.; Chen, J. N.; Guo, B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia Solanacearum. Molecules 2016, 21, 468; https://doi.org/10.3390/molecules21040468.Search in Google Scholar PubMed PubMed Central
6. Cho, J. Y.; Hwang, T. L.; Chang, T. H.; Lim, Y. P.; Sung, P. J.; Lee, T. H.; Chen, J. J. New Coumarins and Anti-inflammatory Constituents from Zanthoxylum Avicennae. Food Chem. 2012, 135, 17–23; https://doi.org/10.1016/j.foodchem.2012.04.025.Search in Google Scholar
7. Jantamat, P.; Weerapreeyakul, N.; Puthongking, P. Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena Harmandiana. Molecules 2019, 24, 3385; https://doi.org/10.3390/molecules24183385.Search in Google Scholar PubMed PubMed Central
8. Tran, N. K. S.; Trinh, T. A.; Pyo, J.; Kim, C. G.; Park, J. G.; Kang, K. S. Neuroprotective Potential of Pyranocoumarins from Angelica gigas Nakai on Glutamate-Induced Hippocampal Cell Death. Antioxidants 2023, 12, 1651; https://doi.org/10.3390/antiox12081651.Search in Google Scholar PubMed PubMed Central
9. Borowiak, T.; Wolska, I. Structure of 7-Methoxy-8-(3-Methyl-2- Butenyl)Coumarin. Acta Crystallogr. 1989, 45, 620–622; https://doi.org/10.1107/s010827018800945x.Search in Google Scholar
10. Wu, T. S.; Huang, S. C.; Lai, J. S. Stem Bark Coumarins of Citrus Grandis. Phytochemistry 1994, 36, 217–219; https://doi.org/10.1016/s0031-9422(00)97040-7.Search in Google Scholar
11. Gridunova, G. V.; Yufit, D. S.; Struchkov, Yu. T.; Reznichenko, A. I.; Khrolova, O. R.; Komlev, I. V. Kristallografiya 1992, 37, 359.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

