Abstract
C14H14AsNO5, monoclinic, P21 (no. 4), a = 8.5830(5) Å, b = 7.0799(5) Å, c = 11.6011(7) Å, β = 100.059(2)∘, V = 694.13(8) Å3, Z = 2, R gt(F) = 0.0288, wR ref(F 2) = 0.0603, T = 150.0 K.
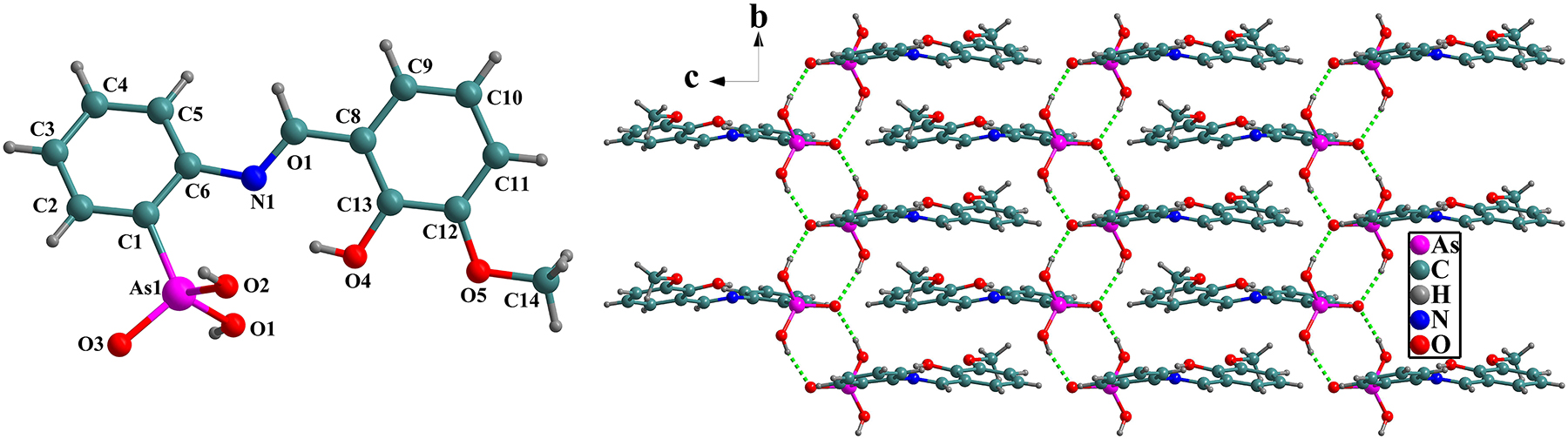
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.25 × 0.21 × 0.12 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 2.47 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
Bruker APEX-II, φ and ω
25.2°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 4678, 2475, 0.031 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2219 |
| N(param)refined: | 192 |
| Programs: | Bruker, 1 Olex2, 2 SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| As1 | 0.49360 (5) | 0.50505 (12) | 0.09895 (4) | 0.02447 (14) |
| C1 | 0.2953 (6) | 0.5274 (14) | 0.1486 (4) | 0.0283 (14) |
| C2 | 0.1597 (6) | 0.5263 (16) | 0.0643 (5) | 0.0389 (16) |
| H2 | 0.166279 | 0.505822 | −0.015668 | 0.047* |
| C3 | 0.0148 (7) | 0.5552 (9) | 0.0975 (5) | 0.047 (2) |
| H3 | −0.079027 | 0.553853 | 0.040310 | 0.056* |
| C4 | 0.0058 (7) | 0.5859 (8) | 0.2133 (6) | 0.0393 (15) |
| H4 | −0.094202 | 0.608804 | 0.235104 | 0.047* |
| C5 | 0.1401 (7) | 0.5837 (8) | 0.2980 (5) | 0.0346 (14) |
| H5 | 0.131606 | 0.604770 | 0.377518 | 0.041* |
| C6 | 0.2895 (6) | 0.5508 (6) | 0.2679 (5) | 0.0275 (15) |
| C7 | 0.4383 (6) | 0.5221 (14) | 0.4558 (4) | 0.0275 (12) |
| H7 | 0.343100 | 0.485514 | 0.480863 | 0.033* |
| C8 | 0.5805 (6) | 0.5402 (8) | 0.5427 (5) | 0.0273 (16) |
| C9 | 0.5751 (6) | 0.5097 (16) | 0.6610 (4) | 0.0318 (10) |
| H9 | 0.477585 | 0.474423 | 0.683031 | 0.038* |
| C10 | 0.7056 (7) | 0.5292 (14) | 0.7454 (5) | 0.0349 (17) |
| H10 | 0.698095 | 0.509402 | 0.825243 | 0.042* |
| C11 | 0.8510 (7) | 0.5785 (8) | 0.7148 (5) | 0.0368 (15) |
| H11 | 0.942633 | 0.588322 | 0.773701 | 0.044* |
| C12 | 0.8617 (7) | 0.6129 (8) | 0.5986 (5) | 0.0324 (13) |
| C13 | 0.7255 (7) | 0.5968 (7) | 0.5120 (5) | 0.0281 (12) |
| C14 | 1.1372 (7) | 0.6738 (10) | 0.6432 (5) | 0.0448 (16) |
| H14A | 1.223002 | 0.724614 | 0.606567 | 0.067* |
| H14B | 1.164919 | 0.546187 | 0.672423 | 0.067* |
| H14C | 1.121593 | 0.755399 | 0.708436 | 0.067* |
| N1 | 0.4347 (5) | 0.5534 (5) | 0.3459 (4) | 0.0268 (13) |
| O1 | 0.6180 (6) | 0.6844 (7) | 0.1519 (5) | 0.0294 (13) |
| H1 | 0.579005 | 0.787774 | 0.125553 | 0.044* |
| O2 | 0.6023 (7) | 0.3181 (8) | 0.1582 (5) | 0.0303 (13) |
| H2A | 0.554996 | 0.217393 | 0.135300 | 0.045* |
| O3 | 0.4667 (4) | 0.4986 (10) | −0.0465 (2) | 0.0295 (7) |
| O4 | 0.7418 (4) | 0.6384 (6) | 0.4009 (3) | 0.0349 (10) |
| H4A | 0.653628 | 0.628949 | 0.356558 | 0.052* |
| O5 | 0.9951 (4) | 0.6671 (6) | 0.5589 (3) | 0.0386 (10) |
1 Source of materials
A 50 mL ethanol solution of 2.17 g 2-aminophenylarsonic acid (1.0 mmol) and 1.52 g o-vanillin (1.0 mmol) was stirred at 333.15 K for 2 h using 3 drops of concentrated hydrochloric acid as a catalyst, then yellow precipitates were filtered out and dried under the vacuum. The title compound was purified by the recrystallization from the ethanol solution. Yellow crystals suitable for single X-ray diffraction measurement were obtained from the filtrate under slow evaporation in air. Yield: 78.6 % (based on 2-aminophenylarsonic acid).
2 Experimental details
The structure was solved by Direct Methods with the SHELXS-2018 program. All H-atoms from C were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2 U eq(C)) using a riding model with C–H = 0.950 (for benzene ring) and 0.980 Å (for methyl). The H-atoms from O atoms were positioned with idealized geometry (if they were positioned with Q peaks, unreasonable O–H bond lengths were reported) and refined isotropically with U iso(H) = 1.5 U eq(O) and O–H = 0.840 Å.
3 Comment
To date, some single crystal structures of 2-aminophenylarsonic acid, 5 its salts, 6 and its 2-amino-modified derivatives have been reported. 7 , 8 , 9 , 10 , 11 Furthermore, some of them were used as organic components to build zinc, cobalt, cadium, and copper, etc. complexes 12 , 13 , 14 , 15 , 16 , 17 and polyoxometalates, including polyoxovandates, 18 polyoxomolybdates, 19 , 20 and polyoxotungstates. 21 , 22 A convenient way to modify 2-amino group is through Schiff base reaction because there are a large number of aromatic aldehydes to choose. In 2015, Percino and co-workers reported the synthesis and crystal structures reacting 2-amonophenylarsonic acid with equimolar salicylaldehyde in ethanol solution in the presence of HCl as a catalyst. 23 In 2019, they reported three (E)-(2-(substituted benzylidene)aminophenyl)arsonic acids. 24
As shown in the figure, the asymmetrical unit consists of one whole (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid. The four bond lengths of As–C and As–O are 1.895, 1.663, 1.699 and 1.700 Å, respectively, which are similar to the reported results. 23 , 24 The o-vanillin residue is almost coplanar that is favor to the strong intramolecular O–H···N interaction (D···Adistance 2.679 Å) 25 between the N1 atom and the substituted –OH group in ortho position of benzene ring, which is distinguished from the rotation or planarity of the molecules due to the intermolecular hydrogen bonds (O1–H1···O3 and O2–H2A···O3) of the 2-aminophenylarsonic acid residue. The two kinds of hydrogen bonds mentioned above (right part of the figure, green dashed lines) are linked to generate a 1D chain-like structure.
Acknowledgments
We acknowledge the fund support from Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD65).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2023JD65).
References
1. Bruker, SAINT v8.40A; Bruker AXS Inc: Madison, Wisconsin, USA, 2015.Search in Google Scholar
2. Bourhis, L. J.; Dolomanov, O. V.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. The Anatomy of a Comprehensive Constrained, Restrained Refinement Program for the Modern Computing Environment–Olex2 Dissected. Acta Crystallogr. 2015, A71, 59–75; https://doi.org/10.1107/s2053273314022207.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. Using Phases to Determine the Space Group. Acta Crystallogr. 2018, A74, A353; https://doi.org/10.1107/s0108767318096472.Search in Google Scholar
5. Chatterjee, A.; Sengupta, S. P. o-Aminophenylarsonic Acid. Acta Crystallogr. 1977, B33, 164–167; https://doi.org/10.1107/s0567740877002921.Search in Google Scholar
6. Wojtas, L.; Milart, P.; Stadnicka, K. Crystal and Molecular Structure of 3-Methyl-4-(2,4,6-Triphenylpyridinium-1-yl)-Phenolate Salts with o-Arsanilic and Perchloric Acids. J. Mol. Struct. 2006, 782, 157–164; https://doi.org/10.1016/j.molstruc.2005.08.007.Search in Google Scholar
7. Zenki, M.; Shibahara, T.; Yamasaki, M.; Kushi, Y. Crystal Structure of Arsenazo I. Anal. Sci. 1990, 6, 153–154; https://doi.org/10.2116/analsci.6.153.Search in Google Scholar
8. Shkol’nikova, L. M.; Fundamenskii, V. S.; Poznyak, A. L. Structure of the New Complexones Including Arsonic Groups- Crystal and Molecular-Structure of Ortho-Arsonophenylglycine and Ortho-Arsonophenylalanine Nonohydrate. Kristallografiya 1992, 37, 684–691.Search in Google Scholar
9. Percino, M. J.; Chapela, V. M.; Zayas, T.; de Barbarin, C. R. Crystal and Molecular Structure of o-Methacryloylaminophenylarsonic Acid. J. Chem. Cryst. 2002, 32, 307–314; https://doi.org/10.1023/a:1020257524892.10.1023/A:1020257524892Search in Google Scholar
10. Herrera, A. M.; Garcia-Serrano, J.; Alvarado-Rodriguez, J. A.; Rivas-Silva, J. F.; Pa, U. (2-Acryloylaminophenyl)arsonic Acid. Acta Crystallogr. 2005, E61, m2752–m2754; https://doi.org/10.1107/s1600536805038857.Search in Google Scholar
11. Ennaceur, N.; Henchiri, R.; Jalel, B.; Cordier, M.; Ledoux-Rak, I.; Elaloui, E. Synthesis, Crystal Structure, and Spectroscopic Characterization Supported by DFT Calculations of Organoarsenic Compound. J. Mol. Struct. 2017, 1144, 25–32; https://doi.org/10.1016/j.molstruc.2017.05.007.Search in Google Scholar
12. Shkol’nikova, L. M.; Sotman, S. S.; Poznyak, A. L.; Dashevskaya, E. E.; Pavlovskii, V. I. Structure of the New Complex Included Arsonic Groups-Synthesis, Crystalline and Molecular-Structures of [ortho-Aminophenylarsonato](-1) bis(ethylenediamine)] Cobalt (3+) Dinitrate. Kristallografiya 1992, 37, 1200–1209.Search in Google Scholar
13. Xie, Y.-P.; Yang, J.; Ma, J.-F.; Zhang, L.-P.; Song, S.-Y.; Su, Z.-M. Tin-Oxo Clusters Based on Aryl Arsonate Anions. Chem. Eur. J. 2008, 14, 4093–4103; https://doi.org/10.1002/chem.200701498.Search in Google Scholar PubMed
14. Xie, Y.-P.; Ma, J.-F.; Yang, J.; Liu, Y.-Y.; Ma, J.-C.; Su, M.-Z. Penta-hexa-and Heptanuclear Organotin-Oxygen Arsonate Clusters Constructed from an Acetate Drum Cluster Precursor and Different Arsonate Anions. Eur. J. Inorg. Chem. 2009, 2009, 2144–2152; https://doi.org/10.1002/ejic.200900135.Search in Google Scholar
15. Zhou, T.-H.; Zhang, J.; Zhang, H.-X.; Feng, R.; Mao, J.-G. A Ligand-Conformation Driving Chiral Generation and Symmetry-Breaking Crystallization of a Zinc(ii) Organoarsonate. Chem. Commun. 2011, 47, 8862–8864; https://doi.org/10.1039/c1cc12914g.Search in Google Scholar PubMed
16. Kopylovich, M. N.; Nunes, A. C. C.; Mahmudov, K. T.; Haukka, M.; MacLeod, T. C. O.; Martins, L. M. D. R. S.; Kuznetsov, M. L.; Pombeiro, A. J. L. Complexes of Copper(ii) with 3-(ortho-Substituted Phenylhydrazo)pentane-2,4-Diones: Syntheses, Properties and Catalytic Activity for Cyclohexane Oxidation. Dalton Trans. 2011, 40, 2822–2836; https://doi.org/10.1039/c0dt01527j.Search in Google Scholar PubMed
17. Lin, H.; Deng, X.; Sun, Y.; Chen, S.; Zhou, T. Effect of N-Donor Ancillary Ligand on Zinc/cadmium-Organic Arsonates: Structural Analysis and Photoluminescence. J. Solid State Chem. 2022, 311, 123148; https://doi.org/10.1016/j.jssc.2022.123148.Search in Google Scholar
18. Chen, B.; Wang, B.; Lin, Z.; Fan, L.; Gao, Y.; Chi, Y.; Hu, C. Controlled Solvothermal Synthesis of Novel Organic Functionalized Polyoxovanadates. Dalton Trans. 2012, 41, 6910–6913; https://doi.org/10.1039/c2dt30660c.Search in Google Scholar PubMed
19. Liu, B.-Y.; Wang, X.; Xie, G.-Y.; Ku, Y.-T. Crystal and Molecular Structure of Guanidinium Bis(o-Aminophenylarsenic) Hexamolybdate (CN3H6)4[(o-NH2C6H4As)2Mo6O24]. Chin. J. Struct. Chem. 1990, 9, 211–216.Search in Google Scholar
20. Chang, Y.-D.; Zubieta, J. Investigations into the Syntheses and Structures of Clusters of the Mo O REO2−3 Systems (E = P and As). Inorg. Chim. Acta 1996, 245, 177–198; https://doi.org/10.1016/0020-1693(95)04810-3.Search in Google Scholar
21. Faassen, F.; Izarova, N. V.; van Leusen, J.; Kogerler, P. Bifunctionalized Polyoxotungstates: Dilacunary Keggin Clusters Incorporating FeIII and Organoarsonate Constituents. Cryst. Growth Des. 2022, 23, 434–441; https://doi.org/10.1021/acs.cgd.2c01100.Search in Google Scholar
22. Iftikhar, T.; Izarova, N. V.; Kogerler, P. Organoarsonates Enable Single-Site Condensation of Hexalacunary {P2W12} Polyoxotungstates. Inorg. Chem. 2024, 63, 99–107; https://doi.org/10.1021/acs.inorgchem.3c01051.Search in Google Scholar PubMed
23. Percino, M. J.; Ceron, M.; Castro, M. E.; Ramirez, R.; Soriano, G.; Chapela, V. M. (E)-2-[(2-hydroxybenzylidene)amino]phenylarsonic Acid Schiff Base: Synthesis, Characterization and Theoretical Studies. J. Mol. Struct. 2015, 1081, 193–200.10.1016/j.molstruc.2014.10.030Search in Google Scholar
24. Venkatesan, P.; Ceron, M.; Ceballos, P.; Perez-Gutierrez, E.; Thamotharan, S.; Percino, M. J. Experimental Study and DFT Calculation for the Strength of Intermolecular Interactions in Schiff Base with the Phenylarsonic Acid Scaffold. J. Mol. Struct. 2019, 1196, 306–322; https://doi.org/10.1016/j.molstruc.2019.06.073.Search in Google Scholar
25. Li, P. P.; Ma, J. X.; Li, Q. L.; Zhao, J. X.; Zhao, L. Crystal Structure of (E)-1-(4-(((E)-2-Hydroxy-3-Methoxybenzylidene)Amino)Phenyl)Ethan-1-One Oxime, C16H16N2O3. Z. Kristallogr. – N. Cryst. Struct. 2018, 233, 777–778; https://doi.org/10.1515/ncrs-2017-0385.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

