Abstract
C28H34Cl2N2O2, orthorhombic, Pbcn (no. 60), a = 30.5739(5) Å, b = 8.00310(10) Å, c = 10.5257(2) Å, V = 2575.49(7) Å3, Z = 4, R gt (F) = 0.0374, wR ref (F 2) = 0.1029, T = 100.01(10) K.
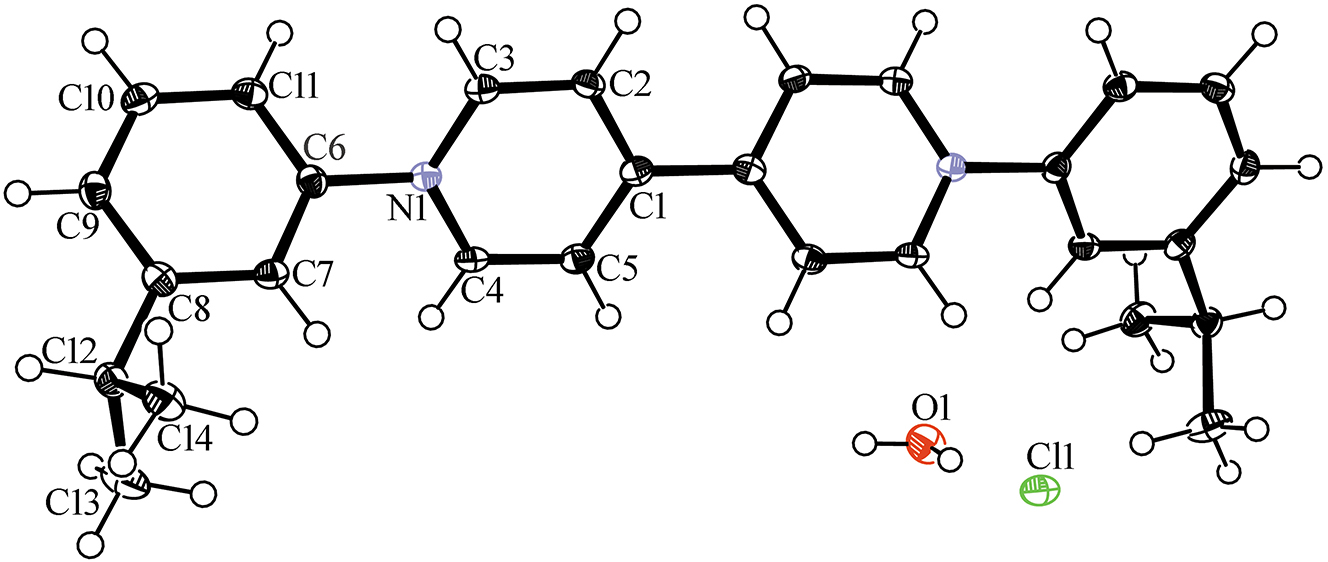
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless plate |
| Size: | 0.20 × 0.20 × 0.10 mm |
| Wavelength: μ: |
Cu Kα radiation (1.54184 Å) 2.48 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
SuperNova, ω
66.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 5450, 2280, 0.025 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 1987 |
| N(param)refined: | 164 |
| Programs: | Olex2, 1 Bruker, 2 SHELX, 3 Diamond 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| N1 | 0.41852 (4) | 0.50900 (18) | 0.48266 (14) | 0.0143 (3) |
| C1 | 0.48299 (5) | 0.5063 (2) | 0.30046 (17) | 0.0149 (4) |
| C2 | 0.45781 (6) | 0.3634 (2) | 0.32321 (17) | 0.0158 (4) |
| H2 | 0.4632 | 0.2635 | 0.2771 | 0.019* |
| C3 | 0.42520 (5) | 0.3689 (2) | 0.41292 (17) | 0.0162 (4) |
| H3 | 0.4072 | 0.2738 | 0.4262 | 0.019* |
| C4 | 0.44379 (5) | 0.6469 (2) | 0.46570 (17) | 0.0152 (4) |
| H4 | 0.4395 | 0.7422 | 0.5181 | 0.018* |
| C5 | 0.47555 (5) | 0.6495 (2) | 0.37326 (17) | 0.0159 (4) |
| H5 | 0.4923 | 0.7477 | 0.3590 | 0.019* |
| C6 | 0.38322 (5) | 0.5128 (2) | 0.57569 (17) | 0.0152 (4) |
| C7 | 0.35568 (5) | 0.6507 (2) | 0.57738 (17) | 0.0157 (4) |
| H7 | 0.3607 | 0.7422 | 0.5217 | 0.019* |
| C8 | 0.32052 (5) | 0.6529 (2) | 0.66214 (17) | 0.0164 (4) |
| C9 | 0.31541 (6) | 0.5194 (2) | 0.74549 (17) | 0.0180 (4) |
| H9 | 0.2922 | 0.5213 | 0.8054 | 0.022* |
| C10 | 0.34372 (6) | 0.3827 (2) | 0.74276 (18) | 0.0184 (4) |
| H10 | 0.3396 | 0.2930 | 0.8006 | 0.022* |
| C11 | 0.37792 (6) | 0.3771 (2) | 0.65610 (17) | 0.0170 (4) |
| H11 | 0.3970 | 0.2836 | 0.6520 | 0.020* |
| C12 | 0.28803 (5) | 0.7966 (2) | 0.65906 (17) | 0.0181 (4) |
| H12 | 0.2652 | 0.7741 | 0.7249 | 0.022* |
| C13 | 0.30987 (6) | 0.9629 (2) | 0.6918 (2) | 0.0275 (5) |
| H13A | 0.3235 | 0.9549 | 0.7758 | 0.041* |
| H13B | 0.2878 | 1.0517 | 0.6925 | 0.041* |
| H13C | 0.3322 | 0.9886 | 0.6281 | 0.041* |
| C14 | 0.26508 (6) | 0.8075 (2) | 0.52974 (18) | 0.0233 (4) |
| H14A | 0.2862 | 0.8415 | 0.4648 | 0.035* |
| H14B | 0.2414 | 0.8899 | 0.5342 | 0.035* |
| H14C | 0.2529 | 0.6980 | 0.5076 | 0.035* |
| Cl1 | 0.44561 (2) | 1.01245 (5) | 0.64618 (4) | 0.02029 (16) |
| O1 | 0.38844 (5) | 0.98547 (18) | 0.40139 (15) | 0.0263 (3) |
| H1A | 0.4046 (8) | 0.983 (3) | 0.338 (3) | 0.029 (7)* |
| H1B | 0.4066 (9) | 0.998 (3) | 0.464 (3) | 0.041 (7)* |
1 Source of materials
Bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride was prepared by Zincke reaction according to the method described in ref. 5. A mixture of N,N-bis(2,4-dinitrophenyl) 4,4′-bipyridinium dichloride (56.0 mg, 0.1 mmol) and 3-isopropylaniline (41.0 mg, 0.3 mmol) was dissolved in ethanol (20 ml) in a round-bottomed flask. The solution was heated for 24 h at 80 °C and cooled to room temperature and the solvent was evaporated under reduced pressure. The crude product was washed by acetone (25 ml × 3). Yellow solids were obtained in vacuum in 73 % yield (34.0 mg).
2 Experimental details
Using Olex2, 1 the structure was solved using Charge Flipping and refined with the ShelXL 3 refinement. All hydrogen atoms were positioned geometrically, with the d(C–H) = 0.97–0.99 Å, U iso (H) = 1.2 times U eq (C) and U iso (H) = 1.5 times U eq(O).
3 Comment
Disubstituated bipyridinium ions, which also named viologen derivatives (RV2+), have been widely studied in electrochemistry,
6
conductivity and optoelectronic materials
7
,
8
,
9
attributed to their electron acceptor property. For example, the color change of viologens is mainly ascribed to the electron transfer from the electron-donating moieties to the viologen dication to form the corresponding radical cation (
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: a Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Bruker. Apex2, Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
5. Chen, L.; Willcock, H.; Wedge, C. J.; Hartl, F.; Colquhoun, H. M.; Greenland, B. W. Efficient Access to Conjugated 4,4′-bipyridinium Oligomers Using the Zincke Reaction: Synthesis, Spectroscopic and Electrochemical Properties. Org. Biomol. Chem. 2016, 14, 980–988; https://doi.org/10.1039/c5ob02211h.Search in Google Scholar PubMed
6. Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-Inspired Functional Materials: Synthetic Strategies and Applications. J. Mater. Chem., A2019 (7), 23337–23360; https://doi.org/10.1039/c9ta01724k.Search in Google Scholar
7. Li, J.; Pudar, S.; Yu, H.; Li, S.; Moore, J. S.; Rodríguez-López, J.; Jackson, N. E.; Schroeder, C. M. Reversible Switching of Molecular Conductance in Viologens Is Controlled by the Electrochemical Environment. J. Phys. Chem. C 2021, 125, 21862–21872; https://doi.org/10.1021/acs.jpcc.1c06942.Search in Google Scholar
8. Ciobanu, M.; Asaftei, S. Nucleobase Appended Viologens: Building Blocks for New Optoelectronic Materials. Opt. Mater. 2015, 42, 262–269; https://doi.org/10.1016/j.optmat.2014.12.044.Search in Google Scholar
9. Papadakis, R. Mono- and Di-quaternized 4,4′-Bipyridine Derivatives as Key Building Blocks for Medium- and Environment-Responsive Compounds and Materials. Molecules 2020, 25, 1; https://doi.org/10.3390/molecules25010001.Search in Google Scholar PubMed PubMed Central
10. Nie, H.; Rao, Y.; Song, J.; Ni, X.-L. Through-Space Conjugated Supramolecular Polymer Radicals from Spatial Organization of Cucurbit[8]uril: An Efficient Approach for Electron Transfer and Smart Photochromism Materials. Chem. Mater. 2022, 34, 8925–8934; https://doi.org/10.1021/acs.chemmater.2c02251.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

