Abstract
C21H18Br3N, monoclinic, P21/c (no. 14), a = 17.0082(5) Å, b = 7.70818(16) Å, c = 15.8792(5) Å, β =
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.14 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 7.46 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy R, ω |
| θ max, completeness: | 73.5°, 96 % |
| N(hkl)measured, N(hkl)unique, R int: | 12,515, 3,884, 0.058 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3,358 |
| N(param)refined: | 229 |
| Programs: | Olex2, 1 Shelx 2 , 3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Br1 | 0.69537 (4) | 1.10083 (7) | 0.28340 (5) | 0.0395 (2) |
| Br2 | 0.90315 (5) | 0.86413 (10) | 0.46962 (5) | 0.0450 (2) |
| Br3 | 0.64850 (4) | 0.79336 (8) | 0.47175 (4) | 0.0385 (2) |
| C1 | 0.6688 (3) | 0.7351 (7) | 0.2742 (4) | 0.0249 (11) |
| C2 | 0.6376 (4) | 0.8954 (7) | 0.2401 (4) | 0.0304 (12) |
| C3 | 0.5653 (4) | 0.9062 (8) | 0.1761 (4) | 0.0338 (13) |
| H3 | 0.5458 | 1.0167 | 0.1533 | 0.041* |
| C4 | 0.5206 (4) | 0.7604 (9) | 0.1446 (4) | 0.0372 (14) |
| C5 | 0.5489 (3) | 0.6005 (8) | 0.1823 (4) | 0.0328 (13) |
| H5 | 0.5176 | 0.4987 | 0.1642 | 0.039* |
| C6 | 0.6223 (3) | 0.5891 (7) | 0.2456 (4) | 0.0294 (12) |
| H6 | 0.6409 | 0.4792 | 0.2699 | 0.035* |
| C7 | 0.4441 (4) | 0.7709 (11) | 0.0701 (5) | 0.0506 (18) |
| H7A | 0.3963 | 0.7537 | 0.0927 | 0.076* |
| H7B | 0.4456 | 0.6806 | 0.0271 | 0.076* |
| H7C | 0.4410 | 0.8852 | 0.0424 | 0.076* |
| C8 | 0.7588 (3) | 0.5947 (7) | 0.4004 (4) | 0.0268 (11) |
| C9 | 0.8117 (3) | 0.4553 (8) | 0.4029 (4) | 0.0320 (12) |
| H9 | 0.8394 | 0.4440 | 0.3585 | 0.038* |
| C10 | 0.8245 (4) | 0.3330 (8) | 0.4692 (5) | 0.0369 (14) |
| H10 | 0.8620 | 0.2412 | 0.4702 | 0.044* |
| C11 | 0.7836 (4) | 0.3424 (8) | 0.5339 (4) | 0.0332 (13) |
| C12 | 0.7299 (3) | 0.4796 (7) | 0.5324 (4) | 0.0297 (12) |
| H12 | 0.7010 | 0.4879 | 0.5759 | 0.036* |
| C13 | 0.7187 (3) | 0.6045 (7) | 0.4669 (4) | 0.0258 (11) |
| C14 | 0.7968 (5) | 0.2083 (9) | 0.6058 (5) | 0.0467 (17) |
| H14A | 0.8387 | 0.2496 | 0.6567 | 0.070* |
| H14B | 0.8146 | 0.0990 | 0.5852 | 0.070* |
| H14C | 0.7457 | 0.1895 | 0.6222 | 0.070* |
| C15 | 0.8144 (3) | 0.7616 (7) | 0.2990 (4) | 0.0262 (11) |
| C16 | 0.8882 (4) | 0.8283 (7) | 0.3497 (4) | 0.0282 (11) |
| C17 | 0.9513 (4) | 0.8702 (8) | 0.3144 (4) | 0.0337 (13) |
| H17 | 1.0006 | 0.9141 | 0.3511 | 0.040* |
| C18 | 0.9444 (4) | 0.8495 (8) | 0.2261 (5) | 0.0348 (13) |
| C19 | 0.8079 (3) | 0.7418 (8) | 0.2106 (4) | 0.0293 (12) |
| H19 | 0.7589 | 0.6968 | 0.1737 | 0.035* |
| C20 | 0.8713 (4) | 0.7861 (8) | 0.1748 (4) | 0.0330 (13) |
| H20 | 0.8645 | 0.7727 | 0.1138 | 0.040* |
| C21 | 1.0145 (4) | 0.8933 (9) | 0.1870 (5) | 0.0434 (16) |
| H21A | 1.0513 | 0.9751 | 0.2252 | 0.065* |
| H21B | 0.9931 | 0.9460 | 0.1294 | 0.065* |
| H21C | 1.0442 | 0.7870 | 0.1811 | 0.065* |
| N1 | 0.7477 (3) | 0.7236 (6) | 0.3347 (3) | 0.0254 (9) |
1 Source of materials
All chemicals were purchased from commercial sources and used as received. Procedures for the synthesis of tris(2-bromo-4-methylphenyl)amine was adapted from the reported paper. 4 In a 250 mL round-bottom flask, 2.72 g (7.15 mmol) of tris(p-tolyl)amine, 3.82 g (21.5 mmol) of N-bromosuccinimide (NBS), 0.519 g (2.14 mmol) of benzoyl peroxide, and 100 mL of carbon tetrachloride were combined. The mixture was refluxed at 70 °C for 6 h. Once the reaction was finished by TLC analysis, the solid residue was removed through vacuum filtration, and the filtrate was evaporated to yield crude products. The resulting white products were then purified using column chromatography on silica gel, with petroleum ether (PE) as the eluent, resulting in a white product weighing 2 g, which corresponds to a yield of 78 %. 1H NMR (500 MHz, Chloroform-d) d 7.40 (d, J = 2.0 Hz, 3H), 6.99 (dd, J = 8.3, 1.9 Hz, 3H), 6.69 (d, J = 8.1 Hz, 3H), 2.30 (s, 9H). Single crystals of the desired product were obtained using the solvent thermal method over a period of two days at 85 °C, with a solvent mixture of ethanol and water in a 4:1 ratio.
2 Experimental details
Single-crystal X-ray diffraction data for the title structure was collected on XtaLAB Synergy R, DW system, HyPix diffractometer using Olex2, 1 the structure was solved with ShelXS 2 structure solution program using Direct Methods and refined with the ShelXL 3 refinement package. The positions of the hydrogen atoms were generated geometrically. The cif-file of the title compound can be obtained free of charge from the Cambridge Crystallographic Data Center via http://www.ccdc.cam.ac.uk/data_request/cif (2379568).
3 Comment
The photophysical characteristics of highly reactive free radicals across different states have garnered significant attention in the research community. 5 , 6 , 7 Triaromatic amine derivatives are acknowledged for their exceptional electroactive and photoactive properties, enabling them to effectively generate free radicals via electrochemical processes or ultraviolet irradiation. Consequently, these materials are extensively employed in optoelectronic applications. 8 , 9 This study reports the synthesis of brominated trianiline crystals, which hold considerable promise for the advancement of optical and sensor devices.
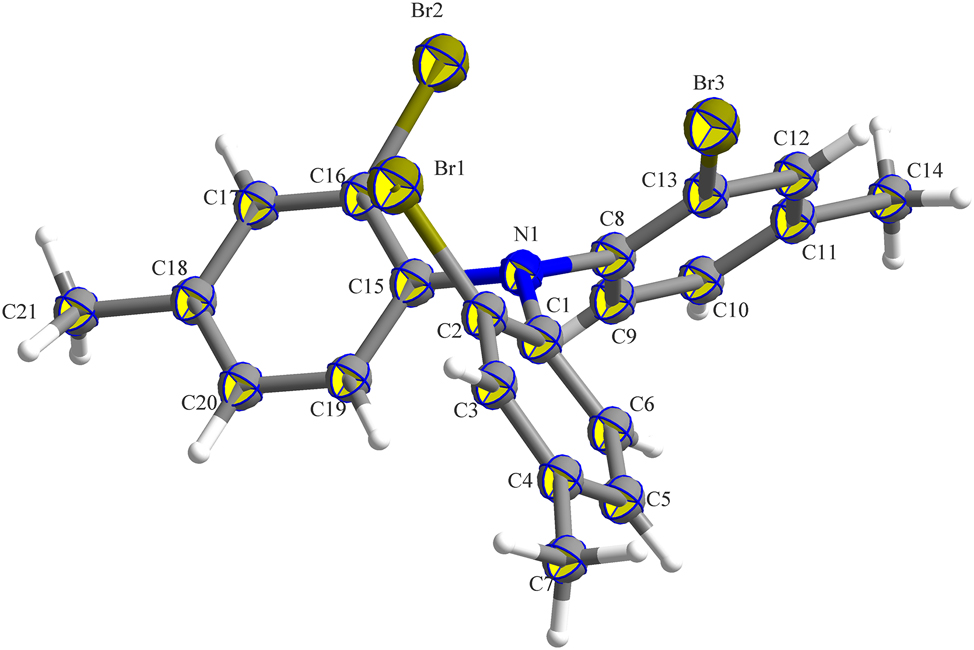
The asymmetric unit contains one molecule of tris(2-bromo-4-methylphenyl)amine (see the figure). The bond lengths and angles within the molecule fall within the expected ranges. 10 The steric hindrance caused by the bromine atom prevents the nitrogen atom at the center of the molecule from achieving a coplanar arrangement with the adjacent carbon atoms. The bond angles involving nitrogen and the connected carbon atoms C1–N1–C18, C1–N1–C8, and C8–N1–C18 measure 115.06°, 117.26°, and 118.03°, respectively. Consequently, the nitrogen and the benzene ring ultimately form a spiral, fan-like configuration. The distances of Br1–C2, Br3–C13, Br2–C16, N2–C1, N1–C15, and N1–C18 are 1.896(6) Å, 1.897(6) Å, 1.435(7) Å, 1.425(7) Å, and 1.418(7) Å.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Science and Technology Planning Project of Guangxi Zhuang autonomous region (Gui ke AD19245096), Youth Fund of Guangxi University of Chinese Medicine (2023QN005) and College Student Innovation and Entrepreneurship Program Training Program (S202310600066, S202410600143).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2018, A64, 112–122.10.1107/S0108767307043930Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Beneto, A. J.; Sivamani, J.; Ashokkumar, V.; Balasaravanan, R.; Duraimurugan, K.; Siva, A. Highly Enantioselective Michael Addition Reactions with New Trimeric Chiral Phase Transfer Catalysts. New J. Chem. 2015, 39, 3098–3104; https://doi.org/10.1039/c4nj02395a.Search in Google Scholar
5. Zhang, L.; Wu, Z.-Q.; Jiao, L. Photoinduced Radical Borylation of Alkyl Bromides Catalyzed by 4-Phenylpyridine. Angew. Chem., Int. Ed. 2020, 132, 2111–2115; https://doi.org/10.1002/ange.201912564.Search in Google Scholar
6. Tang, Q.; Li, L.; Song, Y.; Liu, Y.; Li, H.; Xu, W.; Liu, Y.; Hu, W.; Zhu, D. Photoswitches and Phototransistors from Organic Single-Crystalline Sub-Micro/Nanometer Ribbons. Adv. Mater. 2007, 519, 2624–2628; https://doi.org/10.1002/adma.200700208.Search in Google Scholar
7. Moulin, E.; Niess, F.; Maaloum, M.; Buhler, E.; Nyrkova, I.; Giuseppone, N. The Hierarchical Self-Assembly of Charge Nanocarriers: A Highly Cooperative Process Promoted by Visible Light. Angew. Chem., Int. Ed. 2010, 49, 6974–6978; https://doi.org/10.1002/anie.201001833.Search in Google Scholar PubMed
8. Armao, J. J.; Maaloum, M.; Ellis, T.; Fuks, G.; Rawiso, M.; Moulin, E.; Giuseppone, N. Healable Supramolecular Polymers as Organic Metals. J. Am. Chem. Soc. 2014, 139, 11382–11388; https://doi.org/10.1021/ja5044006.Search in Google Scholar PubMed
9. Matsunaga, Y.; Goto, K.; Kubono, K.; Sako, K.; Shinmyozu, T. Photoinduced Color Change and Photomechanical Effect of Naphthalene Diimides Bearing Alkylamine Moieties in the Solid State. Chem. Eur. J. 2014, 20, 7309–7316; https://doi.org/10.1002/chem.201304849.Search in Google Scholar PubMed
10. Hu, L.; Tumanov, N.; Wouters, J.; Berionni, G. CSD Communication, 2022.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

