Abstract
C25H21CuN3O4, triclinic,
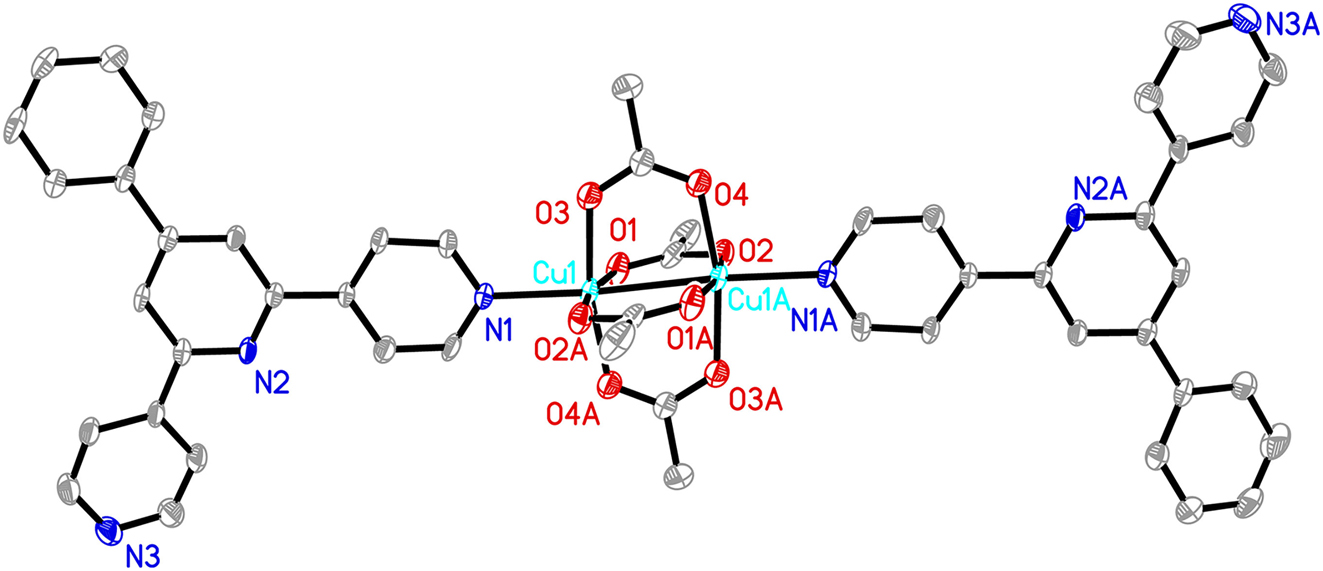
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green block |
| Size: | 0.23 × 0.21 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.00 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 25.0°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 5,790, 3,968, 0.130 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2,513 |
| N(param)refined: | 300 |
| Programs: | Bruker 1 , SHELX 2 , 3 , Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Cu1 | 0.45002 (8) | 0.09534 (6) | 0.93013 (4) | 0.0384 (3) |
| O1 | 0.2215 (5) | 0.0786 (4) | 1.0157 (3) | 0.0540 (10) |
| O2 | 0.3039 (5) | −0.0860 (4) | 1.1312 (3) | 0.0493 (10) |
| O3 | 0.4236 (5) | −0.0512 (4) | 0.8677 (3) | 0.0499 (10) |
| O4 | 0.5022 (5) | −0.2107 (3) | 0.9866 (3) | 0.0535 (11) |
| N1 | 0.3694 (6) | 0.2412 (4) | 0.8072 (3) | 0.0381 (10) |
| N2 | 0.2679 (6) | 0.6262 (4) | 0.5403 (3) | 0.0389 (11) |
| N3 | 0.2472 (8) | 1.1038 (5) | 0.4737 (4) | 0.0680 (16) |
| C1 | 0.3481 (8) | 0.2027 (5) | 0.7250 (4) | 0.0497 (15) |
| H1 | 0.357549 | 0.116000 | 0.726520 | 0.060* |
| C2 | 0.3127 (8) | 0.2835 (5) | 0.6372 (4) | 0.0461 (14) |
| H2 | 0.296185 | 0.250617 | 0.582968 | 0.055* |
| C3 | 0.3024 (6) | 0.4123 (5) | 0.6312 (3) | 0.0329 (12) |
| C4 | 0.3265 (8) | 0.4530 (5) | 0.7172 (4) | 0.0521 (16) |
| H4 | 0.321522 | 0.539086 | 0.717601 | 0.063* |
| C5 | 0.3578 (8) | 0.3645 (6) | 0.8015 (4) | 0.0505 (15) |
| H5 | 0.371470 | 0.394152 | 0.857843 | 0.061* |
| C6 | 0.2686 (6) | 0.5034 (5) | 0.5394 (4) | 0.0339 (12) |
| C7 | 0.2382 (7) | 0.4607 (5) | 0.4544 (4) | 0.0380 (12) |
| H7 | 0.240547 | 0.374407 | 0.455938 | 0.046* |
| C8 | 0.2051 (7) | 0.5468 (5) | 0.3694 (4) | 0.0377 (12) |
| C9 | 0.2038 (7) | 0.6746 (5) | 0.3720 (4) | 0.0404 (13) |
| H9 | 0.181602 | 0.735553 | 0.316335 | 0.048* |
| C10 | 0.2357 (7) | 0.7122 (5) | 0.4574 (4) | 0.0391 (13) |
| C11 | 0.2407 (7) | 0.8464 (5) | 0.4618 (4) | 0.0408 (13) |
| C12 | 0.2672 (8) | 0.9415 (5) | 0.3764 (4) | 0.0502 (15) |
| H12 | 0.283200 | 0.920885 | 0.312887 | 0.060* |
| C13 | 0.2701 (9) | 1.0646 (6) | 0.3848 (5) | 0.0593 (17) |
| H13 | 0.288911 | 1.124716 | 0.325915 | 0.071* |
| C14 | 0.2201 (11) | 1.0120 (7) | 0.5545 (5) | 0.080 (2) |
| H14 | 0.201302 | 1.035577 | 0.617153 | 0.096* |
| C15 | 0.2173 (9) | 0.8867 (6) | 0.5536 (4) | 0.066 (2) |
| H15 | 0.199966 | 0.828642 | 0.613784 | 0.079* |
| C16 | 0.1719 (7) | 0.5038 (5) | 0.2784 (4) | 0.0408 (13) |
| C17 | 0.1118 (8) | 0.3870 (6) | 0.2870 (4) | 0.0501 (15) |
| H17 | 0.088916 | 0.335683 | 0.350117 | 0.060* |
| C18 | 0.0855 (9) | 0.3456 (6) | 0.2030 (5) | 0.0619 (17) |
| H18 | 0.044887 | 0.267074 | 0.210389 | 0.074* |
| C19 | 0.1187 (11) | 0.4194 (8) | 0.1093 (5) | 0.077 (2) |
| H19 | 0.102081 | 0.391109 | 0.052949 | 0.093* |
| C20 | 0.1767 (11) | 0.5353 (8) | 0.0994 (5) | 0.090 (3) |
| H20 | 0.198061 | 0.585944 | 0.035821 | 0.108* |
| C21 | 0.2044 (10) | 0.5792 (6) | 0.1833 (5) | 0.0680 (19) |
| H21 | 0.244292 | 0.658048 | 0.175426 | 0.082* |
| C22 | 0.1902 (7) | −0.0036 (6) | 1.0939 (4) | 0.0465 (15) |
| C23 | 0.0074 (7) | −0.0074 (7) | 1.1497 (5) | 0.071 (2) |
| H23A | −0.030376 | −0.088841 | 1.153035 | 0.107* |
| H23B | −0.066469 | 0.057283 | 1.115021 | 0.107* |
| H23C | 0.002678 | 0.006821 | 1.216679 | 0.107* |
| C24 | 0.4499 (7) | −0.1682 (5) | 0.9078 (4) | 0.0404 (13) |
| C25 | 0.4164 (9) | −0.2601 (6) | 0.8440 (5) | 0.0607 (17) |
| H25A | 0.512991 | −0.267281 | 0.790805 | 0.091* |
| H25B | 0.314072 | −0.228540 | 0.815508 | 0.091* |
| H25C | 0.401309 | −0.341880 | 0.885714 | 0.091* |
1 Source of materials
The reagents were purchased from standard commercial sources and used without further purification. A mixture of CuCl2⋅2H2O (0.017 g, 0.10 mmol), 4264-phtpy (0.031 g, 0.10 mmol) was dispersed in mixed CH3COOH (2 mL) and C2H5OH (8 mL) solutions and ammonia (25 %) were added until a pale blue solution were obtained. The resultant solution was allowed slowly to evaporate under room temperature for two weeks to give green crystals which were isolated by filtration and washed by deionized water and dried in air.
2 Experimental details
The structure was solved by Direct Methods with the SHELXT-2018 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2U eq(C)) using a riding model with C–H = 0.93 and 0.97 Å.
3 Comment
In the past decades, there are a growing interest in the design of organic ligands and syntheses of metal-organic frameworks for their diverse structures and wide applications. 5 , 6 , 7 , 8 Terpyridine possesses 48 isomers, among which the bis-chelating 2,2′:6′,2″-terpyridine is the best known and the adoption of 3,2′:6′,3″-terpyridine and 4,2′:6′,4″-terpyridine in construction of coordination complexes have become more widespread over the last twenty years. 9 , 10 , 11 , 12 , 13 Just as the design and syntheses of organic ligands in some cases are time-consuming and laborious, usually with strict synthesis conditions, low yields, and high costs, an alternative strategy is to introduce metalloligands 14 which are preassembled by common organic ligands and metal ions. The metalloligand still possess unsaturated coordination groups, which are important to direct the assembly of large molecular arrays and one-, two- and three-dimensional coordination polymers and networks. 15 , 16 A Cu(II) metalloligand was obtained with 4′-phenyl-4,2′:6′,4″-terpyridine(phtpy) and its structure has been determined.
The asymmetric unit contains one Cu(II) ion, one phtpy ligand, and two acetate anions. As shown in the figure, each Cu(II) ion is five coordinated by one N atom from phtpy and four O atoms from four acetates, comprising a Cu2(CH3CO2)4 paddle-wheel core axially bound by two terminal phtpy ligands. In this complex, the Cu–O distances ranged from 1.971(4) to 2.010(4) Å, the Cu–N distance is 2.193(4) Å and the distance between two nearby Cu(II) ions is 2.6640(12) Å showing weak interaction. All these bond lengths are similar with other paddle-wheel Cu(II) complexes. 17 , 18
In the crystal, three C atoms, C1, C2 and C12 acted as hydrogen donors, contributing hydrogen atoms H1, H2 and H12 to O3, N3 and O2 to form non-classic hydrogen bonds, C1–H1⋯O3, with d(C1⋯O3) = 3.092(7) Å, ∠(C1–H1⋯O3) = 126°, C2–H2⋯N3_1545, with d(C2⋯N3_1545) = 3.422(8) Å, ∠(C2–H2⋯N3_1545) = 163°, C12–H12⋯O2_1564, with d(C12⋯O2_1564) = 3.408(7) Å, ∠(C12–H12⋯O2_1564) = 168°. In addition, there are three kinds of offset face to face π–π stacking interactions with center to center distances of 3.789(5) Å, 3.870(4) Å, 3.871(4) Å, between pyridine rings. The discrete complexes were further extended into 3D network mainly by the hydrogen bonding interaction and the π–π stacking interactions.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the 2023 Innovation and Entrepreneurship Training Program for Nanyue College of Hengyang Normal University students (No. NYD202319), and the Scientific Research Project of Hengyang Normal University (2023HSKFJJ013).
References
1. Bruker. SAINT APEX2 and SADABS; Bruker AXS inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Ahlén, M.; Cheung, O.; Xu, C. Low-Concentration CO2 Capture Using Metal-Organic Frameworks-Current Status and Future Perspectives. Dalton Trans. 2023, 52, 1841–1856; https://doi.org/10.1039/d2dt04088c.Search in Google Scholar PubMed
6. Ding, M. L.; Flaig, R. W.; Jiang, H. L.; Yaghi, O. M. Carbon Capture and Conversion Using Metal-Organic Frameworks and MOF-Based Materials. Chem. Soc. Rev. 2019, 48, 2783–2828; https://doi.org/10.1039/c8cs00829a.Search in Google Scholar PubMed
7. Yi, F. Y.; Chen, D.; Wu, M. K.; Han, L.; Jiang, H. L. Chemical Sensors Based on Metal–Organic Frameworks. ChemPlusChem 2016, 81, 675–690; https://doi.org/10.1002/cplu.201600137.Search in Google Scholar PubMed
8. Wang, J. X.; Yin, J.; Shekhah, O.; Bakr, O. M.; Eddaoudi, M.; Mohammed, O. F. Energy Transfer in Metal–Organic Frameworks for Fluorescence Sensing. ACS Appl. Mater. Interfaces 2022, 14, 9970–9986; https://doi.org/10.1021/acsami.1c24759.Search in Google Scholar PubMed PubMed Central
9. Anito, D. A.; Wang, T. X.; Liang, H. P.; Ding, X. S.; Han, B. H. Bis(terpyridine) Ru(III) Complex Functionalized Porous Polycarbazole for Visible-Light Driven Chemical Reactions. Polym. Chem. 2021, 12, 4557–4564; https://doi.org/10.1039/d1py00527h.Search in Google Scholar
10. Chen, X. L.; Shang, L.; Huang, M. P.; Tong, Y. Q.; Zhang, J. N.; Xue, W. N. Two Complexes Based on Terpyridine/Benzotricarboxylic Acid Ligands: Synthesis, Structures and Properties. Chin. J. Inorg. Chem. 2021, 37, 340–350.Search in Google Scholar
11. Manfroni, G.; Prescimone, A.; Constable, E. C.; Housecroft, C. E. Stars and Stripes: Hexatopic Tris(3,2′:6′,3″-terpyridine) Ligands that Unexpectedly Form One-Dimensional Coordination Polymers. CrystEngComm 2022, 24, 491–503, https://doi.org/10.1039/d1ce01531a.Search in Google Scholar PubMed PubMed Central
12. Zhang, J. F.; Xu, B.; Luo, F. M.; Tang, G. D.; Zhang, C., Two 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine-based Luminescent Zn(II) Coordination Polymers for Detection of 2,4,6-trinitrophenol. Polyhedron 2019, 169, 51–57, https://doi.org/10.1016/j.poly.2019.04.049.Search in Google Scholar
13. Zhu, S.; Dai, X. J.; Wang, X. G.; Cao, Y. Y.; Zhao, X. J.; Yang, E. C. Two Bulky Conjugated 4′-(4–Hydroxyphenyl)-4,2′:6′,4″-terpyridine-based Layered Complexes: Synthesis, Structure, and Photocatalytic Hydrogen Evolution Activity. Z. Anorg. Allg. Chem. 2019, 645, 516–522, https://doi.org/10.1002/zaac.201800455.Search in Google Scholar
14. Zhang, S. R.; Du, D. Y.; Qin, J. S.; Li, S. L.; He, W. W.; Lan, Y. Q.; Su, Z. M. 2D Cd(II)-Lanthanide(III) Heterometallic–Organic Frameworks Based on Metalloligands for Tunable Luminescence and Highly Selective, Sensitive, and Recyclable Detection of Nitrobenzene. Inorg. Chem. 2014, 53, 8105–8113; https://doi.org/10.1021/ic5011083.Search in Google Scholar PubMed
15. Sun, D.; Zhang, L.; Yan, Z.; Sun, D. Stepwise Construction of a Ag9I–Cu4II Heterometallic Cluster Incorporating Two Unusual Vertex–Shared Trigonal–Bipyramidal Silver Polyhedra. Chem.–Asian J. 2012, 7, 1558–1561; https://doi.org/10.1002/asia.201200181.Search in Google Scholar PubMed
16. Kong, X.; Hu, K.; Mei, L.; Wu, Q.; Huang, Z.; Liu, K.; Chai, Z.; Nie, C.; Shi, W. Construction of Hybrid Bimetallic Uranyl Compounds Based on a Preassembled Terpyridine Metalloligand. Chem. Eur. J. 2021, 27, 2124–2130; https://doi.org/10.1002/chem.202004344.Search in Google Scholar PubMed
17. Zhang, J.; Li, F.; Zhao, Y. Y.; Zhao, X. H.; Li, T. H.; Li, X. Synthesis, Crystal Structures and Electrocatalytic Properties of Ni(II), Cu(II) Complexes with Pymetrozine Ligands. Chin. J. Inorg. Chem. 2014, 30, 2394–2400.Search in Google Scholar
18. Zhu, H. B. Yang, W. N. Tetra-μ-acetato-κ8O:O′-bis{[2-methylsulfanyl-4-(pyridin-4-yl-κN)pyrimidine]Copper(II)}(Cu–Cu). Acta Crystallogr. 2011, E67, m1168.10.1107/S1600536811029837Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3


