Abstract
C68H56As3NO5Rh2, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
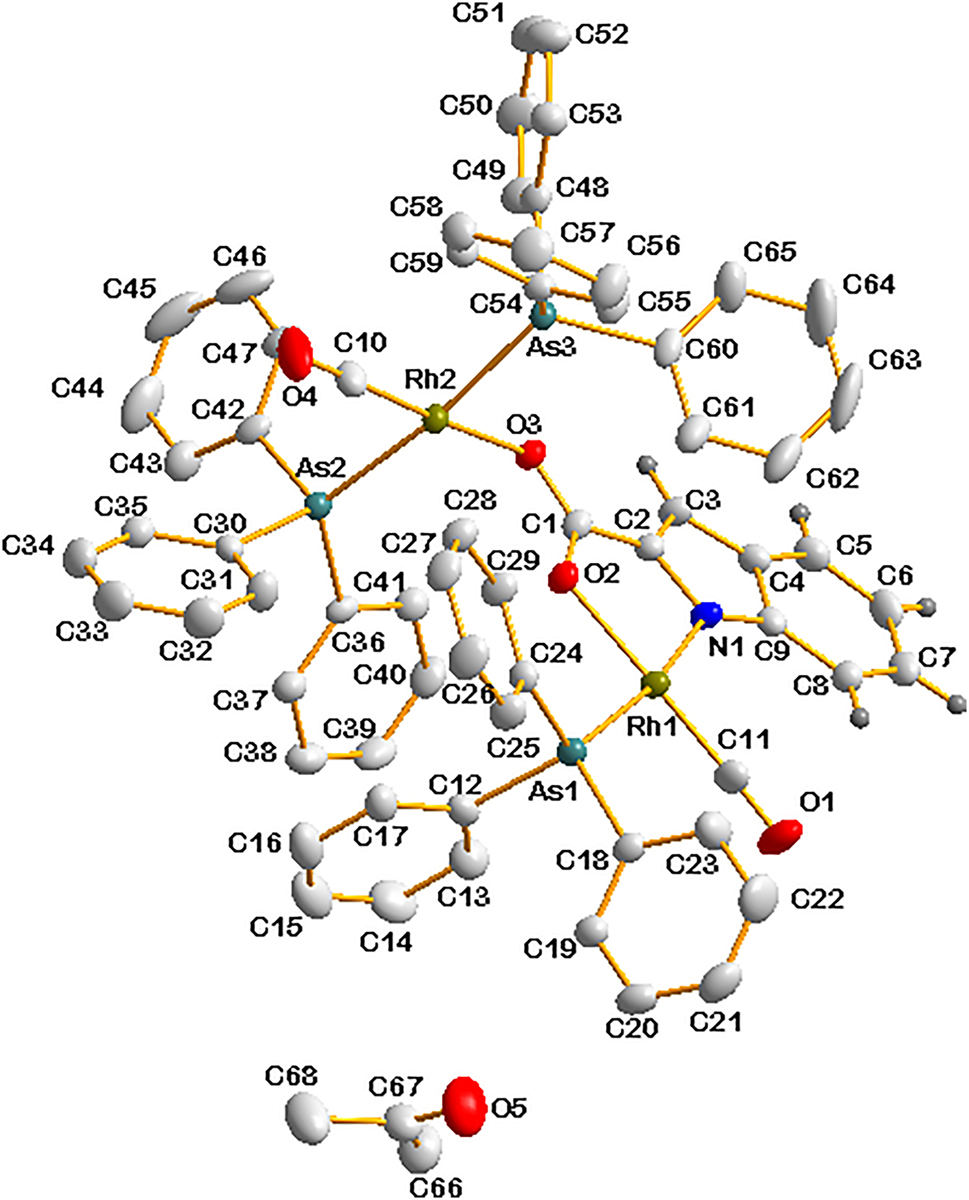
Data collection and handling.
| Crystal: | Yellow cuboid |
| Size: | 0.37 × 0.27 × 0.07 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.28 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 28.3°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 51,426, 14,547, 0.069 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 11,878 |
| N(param)refined: | 714 |
| Programs: | SHELX 1 , Bruker 2 , Olex2 3 , Diamond 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Rh1 | 0.79134 (2) | 0.29834 (2) | 0.22951 (2) | 0.01971 (6) |
| Rh2 | 0.42314 (2) | 0.12525 (2) | 0.27869 (2) | 0.01904 (6) |
| As1 | 0.71298 (2) | 0.39805 (2) | 0.15301 (2) | 0.02027 (7) |
| As2 | 0.49169 (2) | 0.28291 (2) | 0.37905 (2) | 0.01931 (7) |
| As3 | 0.33916 (2) | −0.04416 (2) | 0.18659 (2) | 0.01924 (7) |
| O1 | 0.9990 (2) | 0.3980 (2) | 0.19712 (18) | 0.0471 (7) |
| O2 | 0.65112 (17) | 0.23173 (16) | 0.26069 (12) | 0.0232 (4) |
| O3 | 0.58455 (16) | 0.12646 (16) | 0.33612 (12) | 0.0223 (4) |
| O4 | 0.1902 (2) | 0.1131 (2) | 0.21100 (17) | 0.0518 (8) |
| N1 | 0.84275 (19) | 0.21163 (19) | 0.30648 (14) | 0.0205 (5) |
| C1 | 0.6632 (2) | 0.1763 (2) | 0.31286 (17) | 0.0212 (6) |
| C2 | 0.7725 (2) | 0.1708 (2) | 0.34603 (18) | 0.0218 (6) |
| C3 | 0.8194 (3) | 0.1290 (2) | 0.40890 (19) | 0.0253 (6) |
| H3 | 0.787137 | 0.097484 | 0.444932 | 0.030* |
| C4 | 0.9248 (2) | 0.1427 (2) | 0.40863 (18) | 0.0240 (6) |
| C5 | 1.0115 (3) | 0.1171 (2) | 0.45641 (19) | 0.0287 (7) |
| H5 | 1.006320 | 0.085424 | 0.500967 | 0.034* |
| C6 | 1.1040 (3) | 0.1388 (3) | 0.4375 (2) | 0.0306 (7) |
| H6 | 1.163540 | 0.122383 | 0.469543 | 0.037* |
| C7 | 1.1110 (3) | 0.1851 (2) | 0.3713 (2) | 0.0303 (7) |
| H7 | 1.174292 | 0.197075 | 0.358341 | 0.036* |
| C8 | 1.0293 (2) | 0.2137 (2) | 0.32442 (19) | 0.0253 (6) |
| H8 | 1.036318 | 0.246394 | 0.280616 | 0.030* |
| C9 | 0.9357 (2) | 0.1930 (2) | 0.34368 (17) | 0.0213 (6) |
| C10 | 0.2808 (3) | 0.1173 (2) | 0.2359 (2) | 0.0308 (7) |
| C11 | 0.9166 (3) | 0.3578 (3) | 0.2086 (2) | 0.0290 (7) |
| C12 | 0.7400 (3) | 0.5181 (2) | 0.22645 (18) | 0.0241 (6) |
| C13 | 0.8495 (3) | 0.5752 (3) | 0.2960 (2) | 0.0365 (8) |
| H13 | 0.908498 | 0.555022 | 0.306694 | 0.044* |
| C14 | 0.8726 (4) | 0.6618 (3) | 0.3498 (2) | 0.0449 (9) |
| H14 | 0.947925 | 0.701613 | 0.396792 | 0.054* |
| C15 | 0.7865 (4) | 0.6901 (3) | 0.3353 (2) | 0.0476 (10) |
| H15 | 0.802236 | 0.748914 | 0.372369 | 0.057* |
| C16 | 0.6776 (4) | 0.6328 (3) | 0.2667 (2) | 0.0419 (9) |
| H16 | 0.618339 | 0.652333 | 0.256550 | 0.050* |
| C17 | 0.6542 (3) | 0.5469 (3) | 0.2126 (2) | 0.0308 (7) |
| H17 | 0.578721 | 0.507497 | 0.165719 | 0.037* |
| C18 | 0.7784 (2) | 0.4639 (2) | 0.07975 (18) | 0.0231 (6) |
| C19 | 0.8257 (3) | 0.5703 (2) | 0.0857 (2) | 0.0274 (7) |
| H19 | 0.825069 | 0.614220 | 0.125797 | 0.033* |
| C20 | 0.8747 (3) | 0.6138 (3) | 0.0329 (2) | 0.0340 (8) |
| H20 | 0.907139 | 0.687378 | 0.037254 | 0.041* |
| C21 | 0.8765 (3) | 0.5513 (3) | −0.0252 (2) | 0.0390 (8) |
| H21 | 0.909904 | 0.581354 | −0.061088 | 0.047* |
| C22 | 0.8295 (4) | 0.4446 (3) | −0.0312 (2) | 0.0484 (10) |
| H22 | 0.831107 | 0.401158 | −0.071177 | 0.058* |
| C23 | 0.7799 (3) | 0.4000 (3) | 0.0207 (2) | 0.0398 (8) |
| H23 | 0.747019 | 0.326360 | 0.016063 | 0.048* |
| C24 | 0.5437 (2) | 0.3325 (2) | 0.08146 (17) | 0.0218 (6) |
| C25 | 0.4995 (3) | 0.3683 (3) | 0.01218 (19) | 0.0304 (7) |
| H25 | 0.551845 | 0.422708 | −0.002825 | 0.036* |
| C26 | 0.3771 (3) | 0.3232 (3) | −0.0348 (2) | 0.0362 (8) |
| H26 | 0.346314 | 0.346630 | −0.082478 | 0.043* |
| C27 | 0.3007 (3) | 0.2455 (3) | −0.01308 (19) | 0.0323 (7) |
| H27 | 0.217579 | 0.216243 | −0.045165 | 0.039* |
| C28 | 0.3443 (3) | 0.2098 (3) | 0.05510 (19) | 0.0294 (7) |
| H28 | 0.291298 | 0.155352 | 0.069613 | 0.035* |
| C29 | 0.4663 (3) | 0.2535 (3) | 0.10298 (18) | 0.0269 (7) |
| H29 | 0.496262 | 0.229094 | 0.150302 | 0.032* |
| C30 | 0.4131 (2) | 0.3624 (2) | 0.33181 (18) | 0.0215 (6) |
| C31 | 0.4105 (3) | 0.3836 (3) | 0.2565 (2) | 0.0318 (7) |
| H31 | 0.451373 | 0.364171 | 0.233229 | 0.038* |
| C32 | 0.3484 (3) | 0.4333 (3) | 0.2150 (2) | 0.0389 (8) |
| H32 | 0.347747 | 0.448319 | 0.163832 | 0.047* |
| C33 | 0.2882 (3) | 0.4606 (3) | 0.2477 (2) | 0.0379 (8) |
| H33 | 0.245143 | 0.493853 | 0.218876 | 0.045* |
| C34 | 0.2899 (3) | 0.4400 (3) | 0.3223 (2) | 0.0345 (8) |
| H34 | 0.248039 | 0.458948 | 0.344819 | 0.041* |
| C35 | 0.3533 (3) | 0.3910 (2) | 0.36498 (19) | 0.0269 (6) |
| H35 | 0.355167 | 0.377429 | 0.416752 | 0.032* |
| C36 | 0.6570 (2) | 0.3884 (2) | 0.44000 (17) | 0.0210 (6) |
| C37 | 0.6932 (3) | 0.4952 (2) | 0.45390 (19) | 0.0269 (6) |
| H37 | 0.636812 | 0.518203 | 0.430565 | 0.032* |
| C38 | 0.8130 (3) | 0.5690 (3) | 0.5024 (2) | 0.0336 (7) |
| H38 | 0.837583 | 0.642204 | 0.512250 | 0.040* |
| C39 | 0.8954 (3) | 0.5363 (3) | 0.5358 (2) | 0.0352 (8) |
| H39 | 0.976539 | 0.586442 | 0.569097 | 0.042* |
| C40 | 0.8589 (3) | 0.4299 (3) | 0.52046 (19) | 0.0332 (7) |
| H40 | 0.915910 | 0.407193 | 0.542400 | 0.040* |
| C41 | 0.7400 (3) | 0.3553 (3) | 0.47335 (18) | 0.0262 (6) |
| H41 | 0.715788 | 0.282225 | 0.464066 | 0.031* |
| C42 | 0.4533 (2) | 0.2430 (2) | 0.46968 (18) | 0.0244 (6) |
| C43 | 0.4834 (3) | 0.3162 (3) | 0.5377 (2) | 0.0357 (8) |
| H43 | 0.520937 | 0.389220 | 0.539648 | 0.043* |
| C44 | 0.4592 (3) | 0.2839 (4) | 0.6030 (2) | 0.0527 (11) |
| H44 | 0.477753 | 0.334305 | 0.648871 | 0.063* |
| C45 | 0.4073 (3) | 0.1768 (4) | 0.6009 (2) | 0.0563 (13) |
| H45 | 0.391054 | 0.154000 | 0.645587 | 0.068* |
| C46 | 0.3800 (3) | 0.1049 (4) | 0.5344 (3) | 0.0503 (11) |
| H46 | 0.345797 | 0.032169 | 0.533497 | 0.060* |
| C47 | 0.4017 (3) | 0.1369 (3) | 0.4684 (2) | 0.0348 (8) |
| H47 | 0.381238 | 0.086077 | 0.422034 | 0.042* |
| C48 | 0.2750 (2) | −0.1581 (2) | 0.23482 (18) | 0.0233 (6) |
| C49 | 0.3366 (3) | −0.1442 (3) | 0.3205 (2) | 0.0316 (7) |
| H49 | 0.405663 | −0.078875 | 0.353550 | 0.038* |
| C50 | 0.2974 (3) | −0.2251 (3) | 0.3572 (2) | 0.0406 (9) |
| H50 | 0.339251 | −0.215627 | 0.415511 | 0.049* |
| C51 | 0.1964 (3) | −0.3208 (3) | 0.3085 (2) | 0.0419 (9) |
| H51 | 0.169781 | −0.376892 | 0.333527 | 0.050* |
| C52 | 0.1349 (3) | −0.3343 (3) | 0.2241 (2) | 0.0393 (8) |
| H52 | 0.065383 | −0.399428 | 0.191193 | 0.047* |
| C53 | 0.1741 (3) | −0.2530 (3) | 0.1869 (2) | 0.0289 (7) |
| H53 | 0.131633 | −0.262605 | 0.128648 | 0.035* |
| C54 | 0.2119 (2) | −0.0858 (2) | 0.07508 (17) | 0.0217 (6) |
| C55 | 0.2249 (3) | −0.1097 (3) | 0.00531 (19) | 0.0300 (7) |
| H55 | 0.296509 | −0.107404 | 0.012130 | 0.036* |
| C56 | 0.1344 (3) | −0.1370 (3) | −0.0746 (2) | 0.0383 (8) |
| H56 | 0.144368 | −0.152713 | −0.122074 | 0.046* |
| C57 | 0.0300 (3) | −0.1411 (3) | −0.0847 (2) | 0.0385 (8) |
| H57 | −0.032126 | −0.159820 | −0.139078 | 0.046* |
| C58 | 0.0163 (3) | −0.1181 (3) | −0.0156 (2) | 0.0329 (7) |
| H58 | −0.055649 | −0.120977 | −0.022696 | 0.039* |
| C59 | 0.1063 (3) | −0.0907 (2) | 0.06419 (19) | 0.0271 (7) |
| H59 | 0.095627 | −0.075365 | 0.111416 | 0.033* |
| C60 | 0.4499 (2) | −0.0702 (3) | 0.16362 (18) | 0.0271 (7) |
| C61 | 0.5205 (3) | 0.0054 (3) | 0.1352 (2) | 0.0371 (8) |
| H61 | 0.516667 | 0.068638 | 0.131129 | 0.044* |
| C62 | 0.5962 (3) | −0.0118 (4) | 0.1130 (2) | 0.0477 (10) |
| H62 | 0.645535 | 0.039820 | 0.094383 | 0.057* |
| C63 | 0.5995 (4) | −0.1046 (4) | 0.1182 (2) | 0.0615 (14) |
| H63 | 0.650718 | −0.116966 | 0.102064 | 0.074* |
| C64 | 0.5303 (4) | −0.1793 (4) | 0.1461 (3) | 0.0625 (13) |
| H64 | 0.533749 | −0.242781 | 0.149316 | 0.075* |
| C65 | 0.4547 (3) | −0.1620 (3) | 0.1699 (2) | 0.0404 (9) |
| H65 | 0.407298 | −0.212844 | 0.190210 | 0.048* |
| O00O | 0.9584 (3) | 0.8245 (2) | 0.18113 (17) | 0.0591 (8) |
| C66 | 1.0860 (3) | 0.9061 (3) | 0.3235 (2) | 0.0496 (10) |
| H66A | 1.126773 | 0.869169 | 0.315783 | 0.074* |
| H66B | 1.062660 | 0.885824 | 0.367744 | 0.074* |
| H66C | 1.139373 | 0.982386 | 0.339211 | 0.074* |
| C67 | 0.9784 (3) | 0.8770 (3) | 0.2440 (2) | 0.0374 (8) |
| C68 | 0.8977 (3) | 0.9164 (3) | 0.2465 (3) | 0.0570 (12) |
| H68A | 0.933528 | 0.991875 | 0.248864 | 0.085* |
| H68B | 0.884681 | 0.905010 | 0.295976 | 0.085* |
| H68C | 0.821671 | 0.878145 | 0.196151 | 0.085* |
1 Source of materials
The complex was synthesized starting with the reduction of hydrated RhCl3 in DMF which was refluxed for approximately 20 min to give a yellow solution of di-μ-chloro-tetra-carbonyldirhodium(I), [RhCl(CO)2]2. 5 , 6 , 7 The addition of an equivalent amount of indol-2-carboxylic acid (indol(H2)) to the aforementioned yellow solution followed by addition of ice water yielded the dicarbonylrhodium(I) complex, [RhI(indol)(CO)2] by precipitation. The anion formed by double deprotonation of the indole-2-carboxylic acid (indol(H2)) ligand displays an unprecedented and unexpected mode of coordination with Rh(I) by forming bridging ligand to two rhodium centers; the ketonic oxygen atom coordinating (κO) to the Rh2 atom, while the N,O atoms bind to a second rhodium atom Rh1 atom in the more familiar bidentate (κN,O) fashion. The dinuclear title complex simplifies as [Rh(indol)(CO)(AsPh3)Rh(CO)(AsPh3)2]. 8 [Rh(indol)(CO)(AsPh3)Rh(CO)(AsPh3)2] was synthesized by dissolving [Rh(indol)(CO)2] (0.0412 g, 0.1291 mmol) in 5 cm3 of acetone. Triphenylarsine (AsPh3) (0.0396 g, 0.1291 mmol) was added to the solution with stirring, resulting in the immediate evolution of CO gas. After a few minutes the reaction was completed as determined by IR spectroscopy. Ice water was added drop-wise to the solution. A yellow precipitate was filtered off and dried. Yellow cuboid crystals were obtained from recrystallization in acetone and a few drops of water.
2 Experimental details
All H-atoms were positioned on geometrically idealized positions and refined using the riding model with fixed C–H distances for aromatic C–H of 0.93 Å (C–H) [U iso(H) = 1.2U eq], for methine C–H of 0.98 Å (C–H) [U iso(H) = 1.5U eq]. The graphics were obtained using the DIAMOND 4 program with 50 % probability ellipsoids. The highest peak is located 0.87 Å from As3 and the deepest hole is situated 0.70 Å from Rh2 respectively.
3 Comment
The rhodium(I) complexes and their oxidative additive (RhIII-alkyl) and migratory insertion (RhIII-acyl) products have been extensively studied in the past decade. The precursor [Rh I (I)2(CO)2]− in the Monsanto process is relatively unstable and by manipulating the precursor by introducing different bidentate ligands forming [Rh(BID)(CO)2] complexes (where BID denote different monocharged bidentate ligands such as 2-oxopyridine N-oxide, 8-hydroxyquinoline, indoline-2-carboxylic acid, etc.) One of the carbonyl ligands in this precursor may be replaced by monophosphine or arsine or stibine ligands to form [Rh(L,L’-BID)(CO)(AX3)] complexes (where A = P, As, Sb and X3 = Ph3, Ph2Cy, PhCy2, Cy3, etc.) Generally, this substitution with asymmetrical bidentate ligands might form two isomers, with complexes where AX3 is trans with respect to the strongest donor atom of the bidentate ligand as the preferred isomer. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 The title complex is isomorphous and isostructural with the corresponding triphenylphospnine complex. 8 The individual indol2− ligand is coordinated to both rhodium atoms in a distorted square planar geometry, as seen in (a) the small 79.61(16)° bite angle of the five membered ring as well the angles C11–Rh1–As: 90.27(18)°, O2–Rh1–As1: 91.36(15)°, C11–Rh1–N1: 98.58(17)°, around Rh1, as well as (b) the angles C10–Rh2–As3: 90.16(17)°, As3–Rh2–O3: 89.40(13)°, O3–Rh2–As2: 92.94(16)°, and As2–Rh2–C19: 86.86(14)° around Rh2, respectively.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: The authors acknowledge funding under the Swiss–South Africa joint research program (SSAJRP) from the SANRF (A. Roodt: UID: 107802) as well as from the Competitive Program for Rated Researchers of the SANRF (A. Roodt: UID: 111698), from the South African Department of Science Innovation (DSI) and the Department of Science and Technology (DST) respectively, “Department of Science and Innovation, Republic of South Africa” and “Department of Science and Technology, Republic of South Africa”.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
2. Bruker. SAINT–Plus (Version 7.12) and SADABS (Version 2004/1); Bruker AXS Inc.: Madison, WI, USA, 2004.Search in Google Scholar
3. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. 3.0c; Crystal Impact: Bonn, Germany, 2005.Search in Google Scholar
5. Roodt, A.; Visser, H. G.; Brink, A. Structure/Reactivity Relationships and Mechanism from X-Ray Data and Spectroscopic Kinetic Analysis. Crystallogr. Rev. 2011, 17, 241–280. https://doi.org/10.1080/0889311x.2011.593032.Search in Google Scholar
6. Warsink, S.; Kotze, P. D. R.; van Rensburg, J. M. J.; Venter, J. A.; Otto, S.; Botha, E.; Roodt, A. Kinetic-Mechanistic and Solid-State Study of the Oxidative Addition and Migratory Insertion of Iodomethane to [Rhodium(S,O-BdiPT or N,O-ox)(CO)(PR1R2R3)] Complexes. Eur. J. Inorg. Chem. 2018, 2018, 3615–3625. https://doi.org/10.1002/ejic.201800293.Search in Google Scholar
7. Elmakki, M. A.; Koen, R.; Venter, J. A.; Drost, R. Crystal Structure of Dicarbonyl(pyridine-2-olate-1-oxido-κ2O,O′)rhodium(I), C7H4NO4Rh. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 703–705. https://doi.org/10.1515/ncrs-2015-0240.Search in Google Scholar
8. Elmakki, M. A. E.; Alexander, O. T.; Venter, G. J. S.; Venter, J. A.; Roodt, A. Structural Study and Iodomethane Oxidative Addition Mechanistic Investigation on Model Rhodium(I) Carbonylation Catalysts Activated by Indole-2-/Indoline-2-Carboxylate Bidentate Ligands. Inorganics 2022, 10, 1–24.10.3390/inorganics10120251Search in Google Scholar
9. Brink, A.; Roodt, A.; Steyl, G.; Visser, H. G. Steric vs. Electronic Anomaly Observed from Iodomethane Oxidative Addition to Tertiary Phosphine Modified Rhodium(I) Acetylacetonato Complexes Following Progressive Phenyl Replacement by Cyclohexyl [PR3 = PPh3, PPh2Cy, PPhCy2, PCy3]. Dalton Trans. 2010, 39, 5572–5578. https://doi.org/10.1039/b922083f.Search in Google Scholar PubMed
10. Basson, S. S.; Leipoldt, J. G.; Roodt, A.; Venter, J. A. Crystal Structure of Carbonyl(N-hydroxy-N-nitrosobenzenaminato-κ2o,o’τ)-Triphenylphosphinerhodium(I). Inorg. Chim. Acta 1986, 118, L45–L47. https://doi.org/10.1016/s0020-1693(00)81365-1.Search in Google Scholar
11. Basson, S. S.; Leipoldt, J. G.; Roodt, A.; Venter, J. A. Mechanism for the Oxidative Addition of Iodomethane to Carbonyl(N-Hydroxy-N-Nitrosobenzenaminato-O, O′)-triarylphosphinerhodium(I) Complexes and Crystal Structure of [Rh(cupf)(CO)(CH3)(I)(PPh3)]. Inorg. Chim. Acta 1987, 128, 31–37.10.1016/S0020-1693(00)84691-5Search in Google Scholar
12. Basson, S. S.; Leipoldt, J. G.; Venter, J. A. Structure of Pentacoordinated B-Carbonyl-Cd-(n-Hydroxy-N-Nitrosobenzenaminato-O, O′)-ae-bis(triphenylphosphine)rhodium(I). Acta Crystallogr. 1990, C46, 1324–1326. https://doi.org/10.1107/s0108270190002074.Search in Google Scholar
13. Elmakki, M. A.; Alexander, O. T.; Venter, J. A.; Roodt, A. Crystal Structure of Carbonyl(2-oxopyridin-1(2H)-olato-κ2O,O′)(triphenylarsine-κAs)Rhodium(I), C24H19AsNO3Rh. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 223–225. https://doi.org/10.1515/ncrs-2020-0490.Search in Google Scholar
14. Elmakki, M. A.; Alexander, O. T.; Venter, J. A.; Roodt, A. Crystal Structure of Carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 215–217. https://doi.org/10.1515/ncrs-2020-0494.Search in Google Scholar
15. Elmakki, M. A. E.; Alexander, O. T.; Venter, G. J. S.; Venter, J. A.; Roodt, A. Synthesis and Structural Determination of [Rh(opo)(CO)(PR3)] Complexes (Opo- = 2-Oxopyridin-1-olate) and In Situ Isomeric Behavior from Preliminary Kinetic Study of Iodomethane Oxidative Addition. J. Coord. Chem. 2021, 74, 444–466. https://doi.org/10.1080/00958972.2021.1879385.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

