Abstract
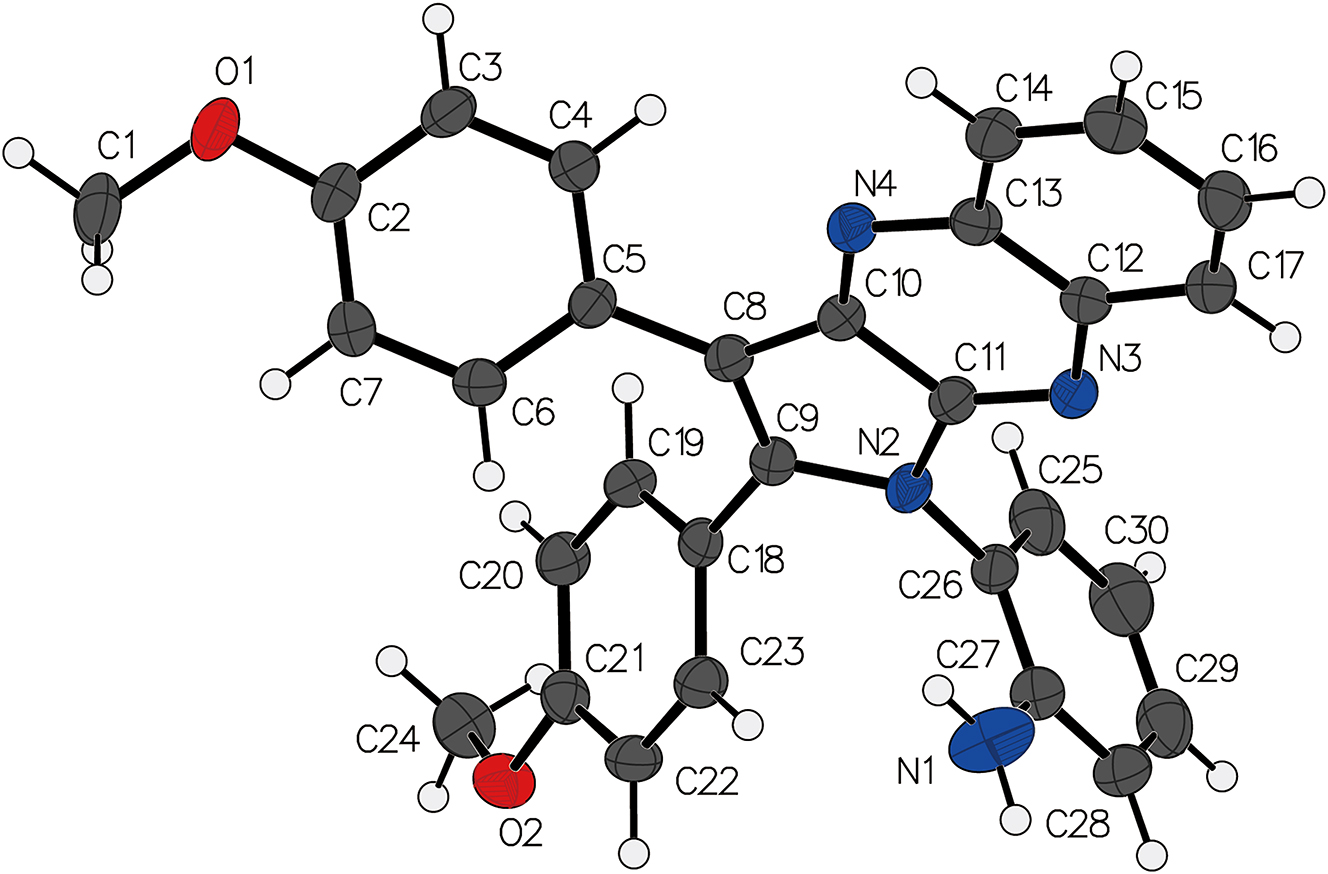
C30H24N4O2, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.06 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 26.4°, 99 % |
| N(hkl)measured, N(hkl)unique, R int: | 13,892, 4,848, 0.043 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3,536 |
| N(param)refined: | 315 |
| Programs: | Bruker, 1 SHELX, 2 , 3 Olex2 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.1689 (3) | 0.4226 (2) | −0.0378 (2) | 0.0423 (7) |
| H1C | 0.119980 | 0.447877 | −0.102694 | 0.063* |
| H1D | 0.097207 | 0.429042 | 0.009757 | 0.063* |
| H1E | 0.185255 | 0.339260 | −0.071944 | 0.063* |
| C2 | 0.4062 (3) | 0.4748 (2) | 0.1329 (2) | 0.0288 (5) |
| C3 | 0.5553 (3) | 0.5477 (2) | 0.2025 (2) | 0.0341 (6) |
| H3 | 0.591757 | 0.608937 | 0.182217 | 0.041* |
| C4 | 0.6502 (3) | 0.5315 (2) | 0.3006 (2) | 0.0313 (5) |
| H4 | 0.751833 | 0.581853 | 0.347244 | 0.038* |
| C5 | 0.5997 (3) | 0.44235 (19) | 0.33293 (19) | 0.0247 (5) |
| C6 | 0.4500 (3) | 0.3709 (2) | 0.2630 (2) | 0.0266 (5) |
| H6 | 0.412624 | 0.310291 | 0.283672 | 0.032* |
| C7 | 0.3537 (3) | 0.3861 (2) | 0.1632 (2) | 0.0278 (5) |
| H7 | 0.252183 | 0.335680 | 0.116005 | 0.033* |
| C8 | 0.7040 (3) | 0.42839 (19) | 0.43949 (19) | 0.0248 (5) |
| C9 | 0.7231 (3) | 0.32409 (19) | 0.45627 (19) | 0.0250 (5) |
| C10 | 0.7993 (3) | 0.52440 (19) | 0.5501 (2) | 0.0244 (5) |
| C11 | 0.8762 (3) | 0.47160 (19) | 0.6313 (2) | 0.0250 (5) |
| C12 | 0.9936 (3) | 0.6516 (2) | 0.7769 (2) | 0.0266 (5) |
| C13 | 0.9132 (3) | 0.70698 (19) | 0.7009 (2) | 0.0257 (5) |
| C14 | 0.9308 (3) | 0.8330 (2) | 0.7472 (2) | 0.0308 (5) |
| H14 | 0.876276 | 0.870829 | 0.697735 | 0.037* |
| C15 | 1.0254 (3) | 0.9013 (2) | 0.8626 (2) | 0.0339 (6) |
| H15 | 1.035731 | 0.986222 | 0.892866 | 0.041* |
| C16 | 1.1077 (3) | 0.8467 (2) | 0.9371 (2) | 0.0337 (6) |
| H16 | 1.174275 | 0.895119 | 1.016887 | 0.040* |
| C17 | 1.0925 (3) | 0.7246 (2) | 0.8954 (2) | 0.0297 (5) |
| H17 | 1.148791 | 0.688707 | 0.946224 | 0.036* |
| C18 | 0.6509 (3) | 0.1996 (2) | 0.3751 (2) | 0.0249 (5) |
| C19 | 0.6641 (3) | 0.1507 (2) | 0.2613 (2) | 0.0275 (5) |
| H19 | 0.719063 | 0.199304 | 0.235867 | 0.033* |
| C20 | 0.5989 (3) | 0.0325 (2) | 0.1841 (2) | 0.0293 (5) |
| H20 | 0.609535 | 0.000436 | 0.106660 | 0.035* |
| C21 | 0.5180 (3) | −0.03888 (19) | 0.2205 (2) | 0.0276 (5) |
| C22 | 0.5011 (3) | 0.0091 (2) | 0.3331 (2) | 0.0324 (6) |
| H22 | 0.443845 | −0.039139 | 0.357538 | 0.039* |
| C23 | 0.5674 (3) | 0.1267 (2) | 0.4097 (2) | 0.0312 (5) |
| H23 | 0.556141 | 0.158604 | 0.486942 | 0.037* |
| C24 | 0.5021 (3) | −0.2179 (2) | 0.0505 (2) | 0.0371 (6) |
| H24A | 0.462467 | −0.184873 | −0.009549 | 0.056* |
| H24B | 0.458282 | −0.303655 | 0.015691 | 0.056* |
| H24C | 0.620275 | −0.206822 | 0.076296 | 0.056* |
| C25 | 1.02230 (19) | 0.21448 (14) | 0.57623 (14) | 0.0383 (6) |
| H25 | 1.056822 | 0.236017 | 0.521514 | 0.046* |
| C26 | 0.89901 (18) | 0.26484 (13) | 0.61519 (14) | 0.0280 (5) |
| C27 | 0.84850 (18) | 0.23332 (14) | 0.69525 (14) | 0.0348 (6) |
| C28 | 0.9213 (2) | 0.15145 (15) | 0.73635 (14) | 0.0424 (6) |
| H28 | 0.886752 | 0.129913 | 0.791061 | 0.051* |
| C29 | 1.0446 (2) | 0.10109 (14) | 0.69738 (17) | 0.0478 (7) |
| H29 | 1.094303 | 0.045135 | 0.725470 | 0.057* |
| C30 | 1.09507 (18) | 0.13261 (15) | 0.61733 (17) | 0.0474 (7) |
| H30 | 1.179337 | 0.098187 | 0.590697 | 0.057* |
| N1 | 0.7232 (3) | 0.2803 (2) | 0.7291 (2) | 0.0561 (7) |
| H1A | 0.687663 | 0.258825 | 0.777503 | 0.067* |
| H1B | 0.678062 | 0.332083 | 0.702537 | 0.067* |
| N2 | 0.8273 (2) | 0.34932 (16) | 0.57284 (16) | 0.0265 (4) |
| N3 | 0.9740 (2) | 0.52937 (16) | 0.74079 (17) | 0.0274 (4) |
| N4 | 0.8169 (2) | 0.64207 (16) | 0.58424 (17) | 0.0264 (4) |
| O1 | 0.3208 (2) | 0.49790 (16) | 0.03709 (15) | 0.0394 (4) |
| O2 | 0.4515 (2) | −0.15715 (14) | 0.15169 (15) | 0.0345 (4) |
1 Source of materials
To a solution of 3,4-bis(4-methoxyphenyl)cyclobut-3-ene-1,2-dione (2.94 g, 10 mmol) and benzene-1,2-diamine (2.38 g, 22 mmol) in dimethyl sulfoxide (20 mL) was added potassium hydroxide (1.68 g, 30 mmol) and copper powder (32 mg, 0.5 mmol). The mixture was stirred at 120 °C for 12 h, until the TLC indicated the reaction was completed. The mixture was diluted with brine (30 mL), and then extracted with ethyl acetate (3 × 30 mL). The organic phase was washed with brine (30 mL), dried with anhydrous sodium sulphate, and then concentrated under pressure. The title compound was separated by silica-gel column chromatography with methanol – dichloromethane (5 %) gradient solvent system. The target product was obtained as a light red solid. Yield: 68 %. For crystal growth, the product was dissolved in a minimal amount of hot ethanol and slowly cooled to room temperature.
2 Experimental details
Single crystals were obtained by slow evaporation from a solvent mixture. Data collection was performed on a Bruker D8 Venture diffractometer with MoKα radiation (λ = 0.71073 Å). 1 The structure was solved by Direct Methods and refined using SHELX-2014. 2 , 3 , 4 All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated positions and refined using a riding model.
3 Comment
The previous research has demonstrated that pyrrolo[3,2-b]quinoxaline derivatives exhibit low nanomolar affinity towards Eph kinases in vitro and display excellent selectivity within a panel of 453 human kinases (395 non-mutant). 5 To date, only a limited number of crystal structures of pyrrolo[3,2-b]quinoxaline derivatives have been reported. 6 , 7 , 8 , 9 Exploring the crystal structures of pyrrolo[3,2-b]quinoxaline derivatives is crucial for the development of novel therapeutics.
As illustrated in the compound under study, two anisole groups substitute the hydrogen atoms on the C atoms of the pyrrolo[3,2-b]quinoxaline heterocyclic ring, with methoxy groups positioned para. An aniline group replaces the hydrogen atom on the N atom of the pyrrolo[3,2-b]quinoxaline heterocyclic ring, with the amino group located ortho on the benzene ring. The framework atoms of the pyrrolo[3,2-b]quinoxaline moiety are nearly coplanar, consistent with previously reported crystal structures of pyrrolo[3,2-b]quinoxaline derivatives. 10 , 11 , 12 The two anisole and one aniline groups show deviations relative to the pyrrolo[3,2-b]quinoxaline plane, with dihedral angles measured at 37.0°, 55.4°, and 80.1°, respectively.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Natural Science Foundation of Shannxi Province (2024JC–YBMS-733), Scientific research plan project of Shaanxi Provincial Department of Education (23JK0321), the Xianyang key laboratory of molecular imaging and drug synthesis (2021QXNL–PT-0008), the 2023 key research and development project of the Xianyang Science and Technology Bureau (L2023–ZDYF–SF-030).
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Unzue, A.; Dong, J.; Lafleur, K.; Zhao, H.; Frugier, E.; Caflisch, A.; Nevado, C. Pyrrolo[3,2-b]quinoxaline Derivatives as Types I1/2 and II Eph Tyrosine Kinase Inhibitors: Structure–Based Design, Synthesis, and In Vivo Validation. J. Med. Chem. 2014, 57, 6834–6844; https://doi.org/10.1021/jm5009242.Search in Google Scholar PubMed
6. Prasad, B.; Shiva Kumar, K.; Vijaya Babu, P.; Anusha, K.; Rambabu, D.; Kandale, A.; Vanaja, G. R.; Kalle, A. M.; Pal, M. AlCl3 Induced C–N Bond Formation Followed by Pd/C–Cu Mediated Coupling-Cyclization Strategy: Synthesis of Pyrrolo[2,3-B]quinoxalines as Anticancer Agents. Tetrahedron Lett. 2012, 53, 6059–6066; https://doi.org/10.1016/j.tetlet.2012.08.119.Search in Google Scholar
7. Gryko, D. T.; Piechowska, J.; Vetokhina, V.; Wójcik, D. Fluorescent Dyes with 2-Amino-4,7-Diazaindole Skeleton: Synthesis and Spectroscopy. Bull. Chem. Soc. Jpn. 2009, 82, 1514–1519; https://doi.org/10.1246/bcsj.82.1514.Search in Google Scholar
8. Gryko, D. T.; Piechowska, J.; Tasior, M.; Waluk, J.; Orzanowska, G. From Bifunctional Nucleophilic Behavior of DBU to a New Heterocyclic Fluorescent Platform. Org. Lett. 2006, 8, 4747–4750; https://doi.org/10.1021/ol061827m.Search in Google Scholar PubMed
9. Mackenroth, A. V.; Antoni, P. W.; Rominger, F.; Rudolph, M.; Hashmi, A. S. K. Gold-Catalyzed [3,3]-Sigmatropic Rearrangement of Ortho-Alkynyl-S,S-Diarylsulfilimines. Org. Lett. 2023, 25, 2907–2912; https://doi.org/10.1021/acs.orglett.3c00953.Search in Google Scholar PubMed
10. Bloch, W. M.; Derwent-Smith, S. M.; Issa, F.; Morris, J. C.; Rendina, L. M.; Sumby, C. J. Fused Pyrazino[2,3-B]indolizine and Indolizino[2,3-B]quinoxaline Derivatives; Synthesis, Structures, and Properties. Tetrahedron 2011, 67, 9368–9375; https://doi.org/10.1016/j.tet.2011.09.133.Search in Google Scholar
11. Keivanloo, A.; Besharati–Seidani, T.; Kaboudin, B.; Yoshida, A.; Yokomatsu, T. One-pot Synthesis of Biologically Active 1,2,3-Trisubstituted Pyrrolo[2,3-B]quinoxalines through a Palladium-Catalyzed Reaction with Internal Alkyne Moieties. Mol. Div. 2018, 22, 879–891; https://doi.org/10.1007/s11030-018-9838-z.Search in Google Scholar PubMed
12. Nakhi, A.; Rahman, M. S.; Kishore, R.; Meda, C. L. T.; Deora, G. S.; Parsa, K. V. L.; Pal, M. Pyrrolo[2,3-b]quinoxalines as Inhibitors of Firefly Luciferase: Their Cu-Mediated Synthesis and Evaluation as False Positives in a Reporter Gene Assay. Bioorg. Med. Chem. Lett. 2012, 22, 6433–6441; https://doi.org/10.1016/j.bmcl.2012.08.056.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

