Abstract
C18H17NO5, orthorhombic, P212121 (no. 19), a = 4.6793(9) Å, b = 18.118(4) Å, c = 18.380(4) Å, V = 1,558.2(5) Å3, Z = 4, R gt (F) = 0.0448, wR ref (F 2) = 0.1246, T = 173(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.24 × 0.11 × 0.09 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.10 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
Bruker Kappa Duo Apex II, 27.6°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 21,208, 3,605, 0.060 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2,887 |
| N(param)refined: | 227 |
| Programs: | Bruker, 1 , 2 X-Seed, 3 SHELX 4 , 5 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | −0.1975 (6) | 0.64458 (15) | 0.63362 (14) | 0.0259 (6) |
| H1 | −0.118021 | 0.692736 | 0.630527 | 0.031* |
| C2 | −0.4050 (6) | 0.62778 (14) | 0.68516 (14) | 0.0253 (6) |
| C3 | −0.5242 (6) | 0.55714 (15) | 0.69102 (14) | 0.0261 (6) |
| H3 | −0.664881 | 0.546774 | 0.726852 | 0.031* |
| C4 | −0.4332 (6) | 0.50291 (14) | 0.64375 (14) | 0.0247 (6) |
| C5 | −0.1295 (6) | 0.46294 (14) | 0.53830 (14) | 0.0255 (6) |
| C6 | 0.0764 (6) | 0.48697 (15) | 0.48587 (14) | 0.0271 (6) |
| H6 | 0.142710 | 0.453092 | 0.450207 | 0.032* |
| C7 | 0.1795 (6) | 0.55703 (15) | 0.48586 (14) | 0.0239 (6) |
| O8 | 0.0938 (4) | 0.60773 (10) | 0.53615 (9) | 0.0248 (4) |
| C9 | −0.1103 (6) | 0.58916 (14) | 0.58707 (13) | 0.0214 (5) |
| C10 | −0.2232 (6) | 0.51760 (15) | 0.59008 (14) | 0.0228 (5) |
| O11 | −0.4904 (5) | 0.68329 (11) | 0.72941 (11) | 0.0350 (5) |
| H11 | −0.618 (9) | 0.668 (2) | 0.766 (2) | 0.053 (11)* |
| O12 | −0.5477 (5) | 0.43424 (11) | 0.64819 (11) | 0.0345 (5) |
| H12 | −0.465 (10) | 0.410 (2) | 0.610 (2) | 0.059 (12)* |
| O13 | −0.2280 (5) | 0.39774 (10) | 0.53903 (11) | 0.0341 (5) |
| C14 | 0.3910 (6) | 0.58683 (15) | 0.43465 (14) | 0.0251 (6) |
| C15 | 0.5190 (6) | 0.65539 (15) | 0.44609 (13) | 0.0277 (6) |
| H15 | 0.466194 | 0.684041 | 0.487239 | 0.033* |
| C16 | 0.7226 (6) | 0.68217 (18) | 0.39799 (16) | 0.0337 (7) |
| H16 | 0.810140 | 0.728618 | 0.406844 | 0.040* |
| C17 | 0.7988 (7) | 0.64177 (19) | 0.33733 (16) | 0.0374 (7) |
| H17 | 0.939299 | 0.660114 | 0.304707 | 0.045* |
| C18 | 0.6687 (7) | 0.57413 (19) | 0.32429 (17) | 0.0408 (8) |
| H18 | 0.718657 | 0.546591 | 0.282179 | 0.049* |
| C19 | 0.4663 (7) | 0.54646 (17) | 0.37234 (14) | 0.0345 (7) |
| H19 | 0.378661 | 0.500121 | 0.363029 | 0.041* |
| C20 | −1.3104 (7) | 0.76011 (18) | 0.92577 (16) | 0.0346 (7) |
| H20A | −1.342695 | 0.722309 | 0.963015 | 0.052* |
| H20B | −1.490483 | 0.770621 | 0.900585 | 0.052* |
| H20C | −1.239535 | 0.805292 | 0.948869 | 0.052* |
| N21 | −1.1004 (5) | 0.73353 (13) | 0.87355 (12) | 0.0285 (5) |
| C22 | −1.0210 (8) | 0.78346 (16) | 0.81458 (16) | 0.0344 (7) |
| H22A | −0.879684 | 0.759399 | 0.783175 | 0.052* |
| H22C | −0.938909 | 0.828695 | 0.835018 | 0.052* |
| H22B | −1.191106 | 0.795797 | 0.785956 | 0.052* |
| C23 | −1.0009 (7) | 0.66530 (16) | 0.87747 (15) | 0.0354 (7) |
| H23 | −1.073683 | 0.634978 | 0.915336 | 0.042* |
| O24 | −0.8212 (6) | 0.63729 (12) | 0.83624 (13) | 0.0484 (7) |
1 Source of material
5,7-Dihydroxy-2-phenyl-4H-chromen-4-one was purchased from Sigma Aldrich and N,N-dimethylformamide (DMF) was obtained from Merck. 5,7-Dihydroxy-2-phenyl-4H-chromen-4-one (0.118 mmol, 0.03 g) was dissolved in 2.0 mL of DMF with heating. The solution was allowed to cool to room temperature. Needle crystals were obtained after 3 weeks of slow evaporation.
2 Experimental details
The hydroxyl hydrogen atoms were located in the difference electron density map and freely refined with isotropic temperature factors. The C–H atoms were geometrically constrained at 0.95 Å (for aromatic and 0.98 Å for methyl H atoms with U iso (H) = 1.2 for aromatic and U iso (H) = 1.5 U eq (C) for methyl H atoms).
3 Comment
5,7-Dihydroxy-2-phenyl-4H-chromen-4-one, more commonly known as chrysin is a flavanoid found in plants, honey and propolis. 6 The anti-oxidant, anti-inflammatory and other biological activities of chrysin and its derivatives have been reported. 7 The Cambridge Structural Database 8 contains eleven structures involving chrysin 9 , 10 , 11 , 12 , 13 , 14 with only one solvate. 15 The solubility of chrysin in several solvents including N,N-dimethylformamide has been studied. 16
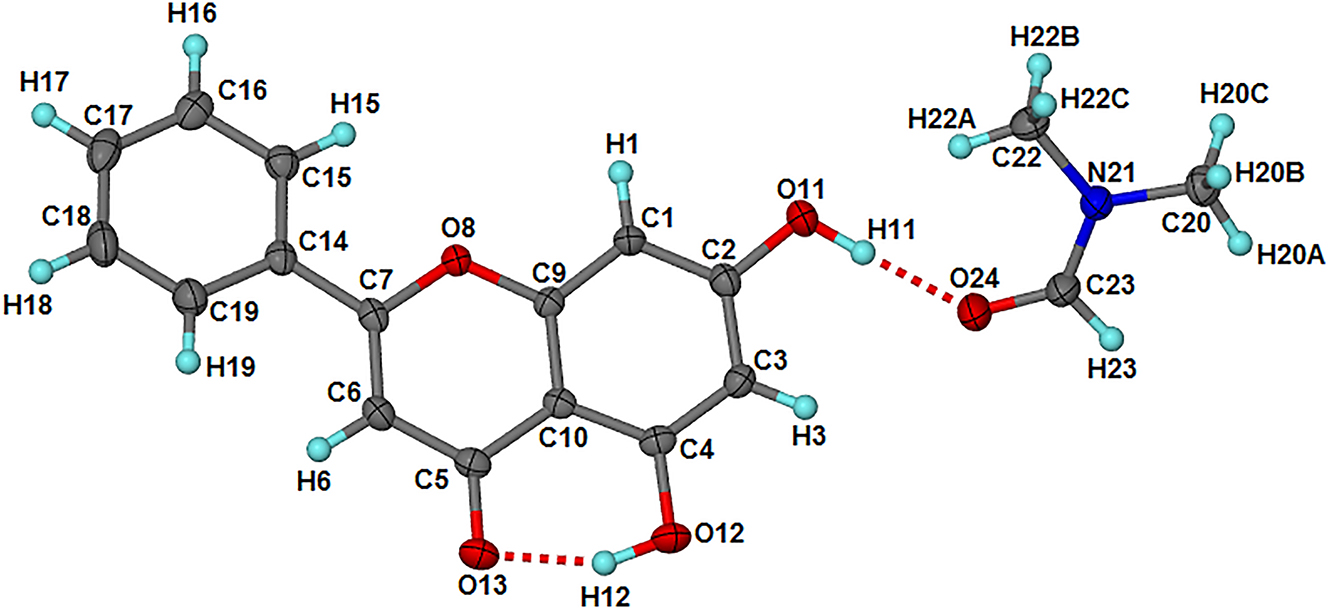
The asymmetric unit contains one chrysin molecule and one N,N-dimethylformamide molecule both in general positions. The phenyl ring of chrysin is out of plane with the fused ring system and the torsion angle τ(C15–C14–C7–O8) is 10.1(3)°.
The structure is characterised by intramolecular O12–H12⋯O13 (H12⋅⋅⋅O13: 1.73(4) Å; O12⋅⋅⋅O13: 2.589(3) Å; O12–H12⋅⋅⋅O13 = 157(4)°) and intermolecular O11–H11⋯O24 (H11⋅⋅⋅O24: 1.70(4) Å; O11⋅⋅⋅O24: 2.636(3) Å; O11–H11⋅⋅⋅O24 = 175(4)°) hydrogen bonds. Furthermore, there are a few C–H⋯O contacts between chrysin and DMF. These include C19⋅⋅⋅O24: 3.462(4) Å, C20⋅⋅⋅O13: 3.542(4) Å, C22⋅⋅⋅O11: 3.451(4) Å and C23⋅⋅⋅O13: 3.425(4) Å. There are also π⋯π interactions between chrysin molecules with the shortest distance between the ring centroid of the phenyl ring (C14, C15, C16, C17, C18, C19) and that of the pyrone ring (C5, C6, C7, O8, C9, C10) of 3.6056(8) Å. The N,N-dimethylformamide molecules occupy channels parallel to [100].
Acknowledgments
We thank Sanelisiwe Mkhize for reproducing the crystallisation experiment.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Competing interests: The authors declare no conflicts of interest regarding this article.
-
Research funding: The authors acknowledge the National Research Foundation (South Africa), grant number 81245 for funding.
References
1. Bruker. Apex2; Bruker AXS Inc.: Madison, WI, USA, 2005.Search in Google Scholar
2. Bruker. SAINT-plus, Sadabs and Xprep; Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
3. Barbour, L. J. X–Seed–A Software Tool for Supramolecular Crystallography. Supramol. Chem. 2001, 1, 189–191; https://doi.org/10.1016/s1472-7862(02)00030-8.Search in Google Scholar
4. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
5. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
6. Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry 2018, 145, 187–196; https://doi.org/10.1016/j.phytochem.2017.09.016.Search in Google Scholar PubMed
7. Liu, Y.; Song, X.; He, J.; Zheng, X.; Wu, H. Synthetic Derivatives of Chrysin and their Biological Activities. Med. Chem. Res. 2014, 23, 555–563; https://doi.org/10.1007/s00044-013-0711-4.Search in Google Scholar
8. Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179; https://doi.org/10.1107/S2052520616003954.Search in Google Scholar PubMed PubMed Central
9. He, H.; Huang, Y.; Zhang, Q.; Wang, J.-R.; Mei, X. Zwitterionic Cocrystals of Flavonoids and Proline: Solid–State Characterization, Pharmaceutical Properties, and Pharmacokinetic Performance. Cryst. Growth Des. 2016, 16, 2348–2356; https://doi.org/10.1021/acs.cgd.6b00142.Search in Google Scholar
10. Halevas, E.; Mavroidi, B.; Antonoglou, O.; Hatzidimitriou, A.; Sagnou, M.; Pantazaki, A. A.; Litsardakis, G.; Pelecanou, M. Structurally Characterized Gallium-Chrysin Complexes with Anticancer Potential. Dalton Trans. 2020, 49, 2734–2746; https://doi.org/10.1039/c9dt04540f.Search in Google Scholar PubMed
11. Chadha, R.; Bhalla, Y.; Nandan, A.; Chadha, K.; Karan, M. Chrysin Cocrystals: Characterization and Evaluation. J. Pharm. Biomed. Anal. 2017, 134, 361–371; https://doi.org/10.1016/j.jpba.2016.10.020.Search in Google Scholar PubMed
12. Sa, R.; Zhang, Y.; Deng, Y.; Huang, Y.; Zhang, M.; Lou, B. Novel Salt Cocrystal of Chrysin with Berberine: Preparation, Characterization, and Oral Bioavailability. Cryst. Growth Des. 2018, 18, 4724–4730; https://doi.org/10.1021/acs.cgd.8b00696.Search in Google Scholar
13. Pang, X.; Tao, Y.; Zhang, J.; Chen, H.; Sun, A.; Ren, G.; Yang, W.; Pan, Q. New Chrysin-Based Co-crystals: Synthesis, Characterization and Dissolution Studies. J. Mol. Struct. 2023, 1271, 134079; https://doi.org/10.1016/j.molstruc.2022.134079.Search in Google Scholar
14. Zhang, Y.; Zhu, B.; Ji, W.-J.; Guo, C.-Y.; Hong, M.; Qi, M.-H.; Ren, G.-B. Insight into the Formation of Cocrystals of Flavonoids and 4,4′-Vinylenedipyridine: Heteromolecular Hydrogen Bonds, Molar Ratio, and Structural Analysis. Cryst. Growth Des. 2021, 21, 2720–2733; https://doi.org/10.1021/acs.cgd.0c01595.Search in Google Scholar
15. Fronczek, F. R. CSD Communication (Private Communication), 2019.Search in Google Scholar
16. Dong, X.; Cao, Y.; Wang, N.; Wang, P.; Li, M. Systematic Study on Solubility of Chrysin in Different Organic Solvents: The Synergistic Effect of Multiple Intermolecular Interactions on the Dissolution Process. J. Mol. Liq. 2021, 325, 115180; https://doi.org/10.1016/j.molliq.2020.115180.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

