Abstract
C19H18N2O4, triclinic, P21/n (no. 14), a = 11.930(6) Å, b = 7.638(4) Å, c = 18.370(4) Å, β = 97.959(9)°, V = 1657.9(13) Å3, Z = 4, R gt (F) = 0.0497, wRref (F 2) = 0.1437, T = 296(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.29 × 0.24 × 0.21 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.10 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θ max, completeness: | 28.5°, >99 % |
| N(hkl)measured, N(hkl)unique, R int: | 10,157, 4,085, 0.032 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 2,841 |
| N(param)refined: | 236 |
| Programs: | Bruker 1 , Olex2 2 , SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| C1 | 0.75682 (15) | −0.0751 (3) | 0.72892 (11) | 0.0583 (5) |
| H1A | 0.753771 | −0.201511 | 0.733417 | 0.070* |
| H1B | 0.788970 | −0.027157 | 0.776109 | 0.070* |
| C2 | 0.82699 (16) | −0.0272 (3) | 0.67285 (11) | 0.0670 (6) |
| H2A | 0.792347 | −0.069317 | 0.625826 | 0.100* |
| H2B | 0.900648 | −0.078764 | 0.684708 | 0.100* |
| H2C | 0.834075 | 0.097841 | 0.671187 | 0.100* |
| C3 | 0.58202 (14) | 0.0259 (2) | 0.76101 (9) | 0.0411 (4) |
| C4 | 0.47111 (13) | 0.0913 (2) | 0.73216 (8) | 0.0364 (3) |
| C5 | 0.39825 (14) | 0.1307 (2) | 0.77968 (8) | 0.0410 (4) |
| C6 | 0.24591 (13) | 0.1521 (2) | 0.68371 (8) | 0.0396 (4) |
| C7 | 0.31258 (12) | 0.12308 (19) | 0.63290 (8) | 0.0353 (3) |
| C8 | 0.43931 (12) | 0.12062 (19) | 0.65055 (8) | 0.0332 (3) |
| H8 | 0.467778 | 0.021457 | 0.624643 | 0.040* |
| C9 | 0.12076 (14) | 0.1603 (3) | 0.67099 (10) | 0.0513 (4) |
| H9A | 0.094995 | 0.253877 | 0.700294 | 0.062* |
| H9B | 0.089839 | 0.051048 | 0.686343 | 0.062* |
| C10 | 0.07861 (15) | 0.1922 (3) | 0.59106 (11) | 0.0631 (5) |
| H10A | −0.002223 | 0.170307 | 0.582231 | 0.076* |
| H10B | 0.090896 | 0.314071 | 0.579679 | 0.076* |
| C11 | 0.13535 (15) | 0.0801 (3) | 0.54124 (11) | 0.0618 (5) |
| H11A | 0.111713 | 0.116575 | 0.490881 | 0.074* |
| H11B | 0.111167 | −0.040157 | 0.545893 | 0.074* |
| C12 | 0.26206 (13) | 0.0881 (2) | 0.55709 (9) | 0.0420 (4) |
| C13 | 0.49243 (11) | 0.28690 (19) | 0.62538 (7) | 0.0322 (3) |
| C14 | 0.57863 (12) | 0.2782 (2) | 0.58289 (8) | 0.0383 (4) |
| H14 | 0.602990 | 0.170158 | 0.567810 | 0.046* |
| C15 | 0.62905 (13) | 0.4305 (2) | 0.56262 (8) | 0.0455 (4) |
| C16 | 0.59375 (16) | 0.5921 (2) | 0.58354 (9) | 0.0521 (5) |
| H16 | 0.627905 | 0.693882 | 0.569587 | 0.062* |
| C17 | 0.50723 (16) | 0.6001 (2) | 0.62537 (10) | 0.0512 (4) |
| H17 | 0.482213 | 0.708271 | 0.639887 | 0.061* |
| C18 | 0.45719 (13) | 0.4493 (2) | 0.64599 (9) | 0.0406 (4) |
| H18 | 0.398553 | 0.456894 | 0.674343 | 0.049* |
| C19 | 0.71870 (16) | 0.4175 (3) | 0.51764 (10) | 0.0605 (5) |
| N1 | 0.41997 (17) | 0.1357 (2) | 0.85303 (8) | 0.0561 (4) |
| H1C | 0.359 (2) | 0.140 (3) | 0.8794 (12) | 0.071 (6)* |
| H1D | 0.490 (2) | 0.097 (3) | 0.8713 (13) | 0.079 (7)* |
| N2 | 0.78706 (18) | 0.4054 (3) | 0.48128 (11) | 0.0896 (7) |
| O1 | 0.64441 (9) | −0.00630 (16) | 0.70772 (6) | 0.0448 (3) |
| O2 | 0.61719 (11) | 0.0012 (2) | 0.82548 (7) | 0.0634 (4) |
| O3 | 0.28786 (9) | 0.17702 (16) | 0.75678 (6) | 0.0478 (3) |
| O4 | 0.32137 (10) | 0.06027 (18) | 0.50958 (6) | 0.0543 (3) |
1 Source of materials
The title compound, ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, was obtained by a three-component reaction using 4-(dimethylamino)pyridine (DMAP) as the catalyst. A 50 mL ethanol solution of 3-cyanobenzaldehyde (10 mmol), ethyl 2-cyanoacetate (10 mmol), and cyclohexane-1,3-dione (10 mmol), DMAP (1 mmol) was heated at 353 K for 5 h. The solid was filtered and recrystallized from an ethanol solution to give crystals of the title compound, yield 67.6 % (based on 3-cyanobenzaldehyde). Colorless block crystals were obtained by recrystallization.
2 Experimental details
The structure was solved by Direct Methods with the SHELXL-2014 program. All H-atoms from C atoms were positioned with idealized geometry and refined isotropically (U iso(H) = 1.2 U eq(C) or U eq(N)) using a riding model with C–H = 0.930, 0.960, 0.970 and 0.980 Å, and N–H = 0.861 and 0.861 Å, respectively.
3 Comment
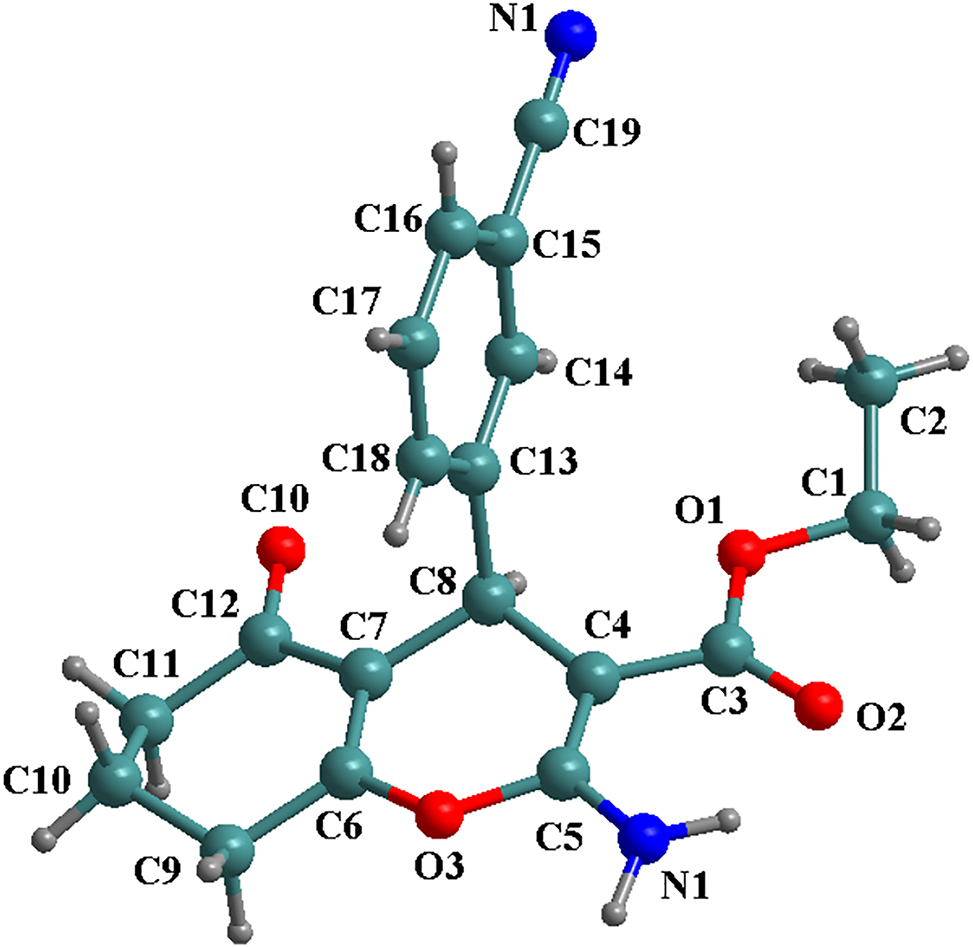
Known as pharmacological activities, 2-amino-4H-pyran-3-carboxylate derivatives, such as ethyl 2-amino-4-(4-ethoxyphenyl)-5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyran-3-carboxylate 5 and ethyl 2-amino-4-(phenyl)-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyran-3-carboxylate and their derivatives, 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 have been synthesized through a famous three-component reaction and their single crystal structures have been also reported. The title compound crystallizes in monoclinic system, P21/n group (no. 14) with the fomula of C19H18N2O4. Whereas the 3-cyanophenyl group is almost coplanar, the 4-hydropyran ring is slightly twisted from planarity. 14 The 1D chains are generated through the hydrogen bonds N1–H1A⋯N2, which are linked to form a 3D supramolecular structure by complicated hydrogen bonds C14–H14⋯O4, C16–H16⋯O4, and C2–H2A⋯O4. All the bond lengths and angles are similar to the reported results. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT v7.60A; Bruker AXS Inc: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Suche in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Sheldrick, G. Using Phases to Determine the Space Group. Acta Crystallogr. 2018, A74, A353. https://doi.org/10.1107/s0108767318096472.Suche in Google Scholar
5. Chen, H.; Yang, G.; Liao, X.; Bao, Z.; Zhan, X. Crystal Structure and Anti-Lung Cancer Activity of Novel Pyran Derivatives from Traditional Chinese Medicine. Lat. Am. J. Pharm. 2018, 37, 359–362.Suche in Google Scholar
6. Shi, D.; Zhang, S.; Wang, X.; Zhuang, Q. Ethyl 2-Amino-4-(4-Fluorophenyl)-7,7-Dimethyl 5-Oxo-5,6,7,8-Tetrahydro-4H-Benzo[b]pyran-3-carboxylate. Acta Crystallogr. 2003, E59, o1501–o1502. https://doi.org/10.1107/s1600536803019834.Suche in Google Scholar
7. Khan, A. T.; Lal, M.; Ali, S.; Khan, M. M. One-Pot Three Component Reaction for the Synthesis of Pyran Annulated Heterocyclic Compounds Using DMAP as a Catalyst. Tetrahedron Lett. 2011, 52, 5327–5332. https://doi.org/10.1016/j.tetlet.2011.08.019.Suche in Google Scholar
8. Xiao, Y.-S.; Zhang, L.; Hu, W.-L.; Zhao, Y.-B.; Hu, H. In Vitro Activity of Synthesized Pyran Derivatives-Inhibitor Against Prostate Cancer. Lat. Am. J. Pharm. 2017, 36, 609–612.Suche in Google Scholar
9. Nongrum, R.; Nongthombam, G. R.; Kharkongor, M.; Rani, J. W. S.; Rahman, N.; Kathing, C.; Myrboh, B.; Nongkhlaw, R. A Nano-Organo Catalyzed Route towards the Efficient Synthesis of Benzo[b]pyran Derivatives Under Ultrasonic Irradiation. RSC Adv. 2016, 6, 108384–108392. https://doi.org/10.1039/c6ra24108e.Suche in Google Scholar
10. Yang, D.-Q.; Li, Z.-M. Crystal Structure of Ethyl 2-Amino-4-(3-Cyanophenyl)-7,7-Dimethyl-5-Oxo-5,6,7,8-Tetrahydro-4H-Chromene-3-Carboxylate, C21H22N2O4. Z. Kristallogr. N. Cryst. Struct. 2018, 233, 903–904. https://doi.org/10.1515/ncrs-2018-0089.Suche in Google Scholar
11. Ramireddy, N.; Abbaraju, S.; Ding, D.; Arman, H.; Zhao, J. C.-G. Organocatalyzed Enantioselective Synthesis of 2-Amino-4H-Chromene Derivatives. J. Heterocycl. Chem. 2017, 54, 677–691. https://doi.org/10.1002/jhet.2641.Suche in Google Scholar
12. Shin, S. Y.; Yoo, M.; Koh, D. The Crystal Structure of Ethyl 2-Amino-4-(3,5-Difluorophenyl)-7,7-Dimethyl-5-Oxo-5,6,7,8-Tetrahydro-4H-Chromene-3-Carboxylate, C20H21F2NO4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 307–309. https://doi.org/10.1515/ncrs-2020-0566.Suche in Google Scholar
13. Wang, X.-S.; Shi, D.-Q.; Tu, S.-J.; Yao, C.-S. A Convenient Synthesis of 5-Oxo-5,6,7,8-Tetrahydro-4H-Benzo-[b]-Pyran Derivatives Catalyzed by KF–Alumina. Synth. Commun. 2003, 33, 119–126. https://doi.org/10.1002/chin.200323120.Suche in Google Scholar
14. Fan, X.-X.; Shen, P.; Zhou, X.-H.; Khrustalev, V. N.; Rivera, D. G.; Nenajdenko, V. G. Three-Component Synthesis and Crystal Structure of 2-Amino-3-Cyano-4H-Pyran and -Thiopyran Derivatives. Russ. J. Org. Chem. 2022, 58, 1786–1796. https://doi.org/10.1134/s1070428022120077.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3

