Abstract
C21H20N4O2, triclinic, P
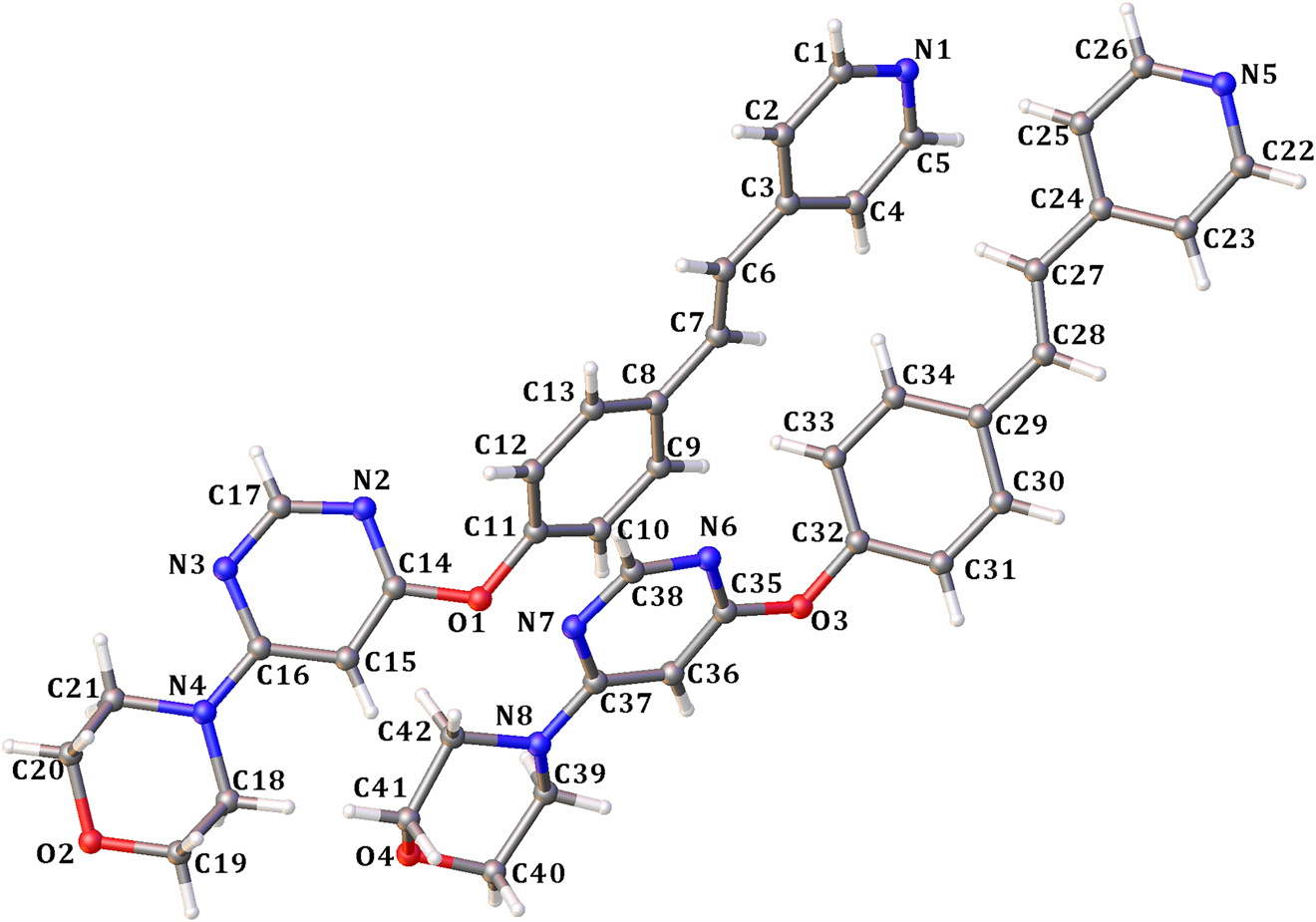
The asymmetric unit of the title crystal structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.18 × 0.16 × 0.14 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.2°, 98% |
| N(hkl)measured, N(hkl)unique, Rint: | 9263, 6352, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2989 |
| N(param)refined: | 487 |
| Programs: | Bruker [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.5299 (4) | 0.62884 (16) | 0.30751 (11) | 0.0577 (7) |
| O2 | 1.0063 (4) | 0.64440 (17) | −0.05117 (11) | 0.0653 (7) |
| O3 | 0.0291 (4) | 0.85345 (16) | 0.41130 (11) | 0.0560 (7) |
| O4 | 0.4165 (4) | 0.79593 (18) | 0.07116 (12) | 0.0639 (7) |
| N1 | 0.5381 (6) | 0.6460 (2) | 0.83867 (15) | 0.0627 (9) |
| N2 | 0.8961 (5) | 0.56661 (18) | 0.31338 (13) | 0.0500 (7) |
| N3 | 1.0908 (4) | 0.55214 (18) | 0.20679 (14) | 0.0469 (7) |
| N4 | 0.9242 (4) | 0.59575 (18) | 0.10022 (13) | 0.0475 (7) |
| N5 | 0.0921 (8) | 0.9195 (2) | 0.92154 (17) | 0.0829 (11) |
| N6 | 0.3983 (5) | 0.90779 (18) | 0.36990 (14) | 0.0493 (7) |
| N7 | 0.5769 (5) | 0.90094 (19) | 0.25852 (14) | 0.0499 (7) |
| N8 | 0.3838 (4) | 0.85246 (17) | 0.18781 (13) | 0.0444 (7) |
| C1 | 0.7242 (7) | 0.6753 (2) | 0.79246 (19) | 0.0614 (10) |
| H1 | 0.845922 | 0.693583 | 0.808712 | 0.074* |
| C2 | 0.7491 (6) | 0.6803 (2) | 0.72236 (17) | 0.0529 (9) |
| H2 | 0.883826 | 0.702357 | 0.692853 | 0.063* |
| C3 | 0.5771 (6) | 0.6532 (2) | 0.69539 (16) | 0.0429 (8) |
| C4 | 0.3817 (6) | 0.6221 (2) | 0.74314 (17) | 0.0526 (9) |
| H4 | 0.258953 | 0.602480 | 0.728277 | 0.063* |
| C5 | 0.3690 (6) | 0.6201 (2) | 0.81203 (18) | 0.0607 (10) |
| H5 | 0.234769 | 0.599600 | 0.842341 | 0.073* |
| C6 | 0.6064 (6) | 0.6565 (2) | 0.62072 (16) | 0.0486 (9) |
| H6 | 0.740367 | 0.681911 | 0.592455 | 0.058* |
| C7 | 0.4625 (6) | 0.6273 (2) | 0.58992 (16) | 0.0478 (9) |
| H7 | 0.329677 | 0.601653 | 0.618782 | 0.057* |
| C8 | 0.4853 (6) | 0.6302 (2) | 0.51631 (16) | 0.0439 (8) |
| C9 | 0.3167 (6) | 0.5944 (2) | 0.49335 (17) | 0.0541 (10) |
| H9 | 0.186883 | 0.570678 | 0.524956 | 0.065* |
| C10 | 0.3368 (6) | 0.5931 (2) | 0.42456 (17) | 0.0549 (10) |
| H10 | 0.222363 | 0.567991 | 0.410732 | 0.066* |
| C11 | 0.5238 (6) | 0.6284 (2) | 0.37732 (16) | 0.0466 (9) |
| C12 | 0.6907 (6) | 0.6679 (2) | 0.39679 (17) | 0.0547 (10) |
| H12 | 0.816655 | 0.693599 | 0.364019 | 0.066* |
| C13 | 0.6691 (6) | 0.6691 (2) | 0.46550 (17) | 0.0545 (10) |
| H13 | 0.780851 | 0.696784 | 0.478084 | 0.065* |
| C14 | 0.7183 (6) | 0.6018 (2) | 0.27506 (16) | 0.0447 (8) |
| C15 | 0.7092 (5) | 0.6116 (2) | 0.20517 (15) | 0.0434 (8) |
| H15 | 0.579583 | 0.634376 | 0.181411 | 0.052* |
| C16 | 0.9034 (5) | 0.5860 (2) | 0.17059 (16) | 0.0405 (8) |
| C17 | 1.0709 (6) | 0.5442 (2) | 0.27502 (17) | 0.0493 (9) |
| H17 | 1.197056 | 0.519446 | 0.300015 | 0.059* |
| C18 | 0.7348 (5) | 0.6230 (2) | 0.05496 (15) | 0.0528 (10) |
| H18A | 0.619510 | 0.653879 | 0.074943 | 0.063* |
| H18B | 0.664353 | 0.571627 | 0.054180 | 0.063* |
| C19 | 0.8224 (6) | 0.6818 (3) | −0.02057 (17) | 0.0600 (10) |
| H19A | 0.697878 | 0.695368 | −0.050990 | 0.072* |
| H19B | 0.872330 | 0.736505 | −0.020025 | 0.072* |
| C20 | 1.1932 (6) | 0.6233 (3) | −0.00672 (17) | 0.0635 (11) |
| H20A | 1.247410 | 0.676542 | −0.004640 | 0.076* |
| H20B | 1.318893 | 0.597930 | −0.028235 | 0.076* |
| C21 | 1.1200 (6) | 0.5599 (2) | 0.06813 (17) | 0.0545 (10) |
| H21A | 1.077780 | 0.504715 | 0.066611 | 0.065* |
| H21B | 1.246191 | 0.548514 | 0.097682 | 0.065* |
| C22 | −0.0776 (8) | 0.9434 (3) | 0.8785 (2) | 0.0799 (13) |
| H22 | −0.204368 | 0.972142 | 0.891800 | 0.096* |
| C23 | −0.0767 (7) | 0.9285 (2) | 0.81633 (18) | 0.0634 (11) |
| H23 | −0.199303 | 0.947950 | 0.788544 | 0.076* |
| C24 | 0.1047 (6) | 0.8846 (2) | 0.79436 (17) | 0.0496 (9) |
| C25 | 0.2794 (7) | 0.8582 (2) | 0.83933 (18) | 0.0644 (11) |
| H25 | 0.406764 | 0.828357 | 0.827838 | 0.077* |
| C26 | 0.2642 (8) | 0.8760 (3) | 0.9004 (2) | 0.0762 (12) |
| H26 | 0.383350 | 0.856288 | 0.929661 | 0.091* |
| C27 | 0.1235 (6) | 0.8684 (2) | 0.72777 (17) | 0.0562 (10) |
| H27 | 0.249662 | 0.835502 | 0.719988 | 0.067* |
| C28 | −0.0204 (6) | 0.8956 (2) | 0.67702 (17) | 0.0514 (9) |
| H28 | −0.149375 | 0.926434 | 0.686060 | 0.062* |
| C29 | −0.0008 (6) | 0.8830 (2) | 0.60926 (16) | 0.0443 (8) |
| C30 | −0.1779 (6) | 0.9112 (2) | 0.56425 (17) | 0.0509 (9) |
| H30 | −0.308912 | 0.936674 | 0.578915 | 0.061* |
| C31 | −0.1624 (6) | 0.9021 (2) | 0.49902 (17) | 0.0516 (9) |
| H31 | −0.281980 | 0.921612 | 0.469955 | 0.062* |
| C32 | 0.0286 (6) | 0.8644 (2) | 0.47657 (16) | 0.0454 (9) |
| C33 | 0.2054 (6) | 0.8333 (2) | 0.52050 (17) | 0.0528 (9) |
| H33 | 0.333924 | 0.806485 | 0.505977 | 0.063* |
| C34 | 0.1878 (6) | 0.8427 (2) | 0.58560 (17) | 0.0512 (9) |
| H34 | 0.305996 | 0.821501 | 0.615011 | 0.061* |
| C35 | 0.2167 (6) | 0.8697 (2) | 0.36136 (16) | 0.0428 (8) |
| C36 | 0.1984 (6) | 0.8480 (2) | 0.30351 (16) | 0.0467 (9) |
| H36 | 0.066137 | 0.822365 | 0.299623 | 0.056* |
| C37 | 0.3830 (5) | 0.8654 (2) | 0.25067 (16) | 0.0417 (8) |
| C38 | 0.5680 (6) | 0.9195 (2) | 0.31673 (18) | 0.0550 (10) |
| H38 | 0.699830 | 0.944670 | 0.321572 | 0.066* |
| C39 | 0.2086 (6) | 0.7952 (2) | 0.18246 (16) | 0.0534 (9) |
| H39A | 0.060153 | 0.808887 | 0.202404 | 0.064* |
| H39B | 0.245130 | 0.734340 | 0.210680 | 0.064* |
| C40 | 0.1974 (6) | 0.8072 (3) | 0.10508 (17) | 0.0602 (10) |
| H40A | 0.091910 | 0.764916 | 0.103507 | 0.072* |
| H40B | 0.138941 | 0.865535 | 0.078556 | 0.072* |
| C41 | 0.5633 (6) | 0.8630 (3) | 0.06904 (17) | 0.0621 (11) |
| H41A | 0.495182 | 0.920083 | 0.043990 | 0.075* |
| H41B | 0.709438 | 0.859647 | 0.042244 | 0.075* |
| C42 | 0.6018 (6) | 0.8537 (2) | 0.14389 (16) | 0.0514 (9) |
| H42A | 0.686069 | 0.799735 | 0.166924 | 0.062* |
| H42B | 0.693383 | 0.902329 | 0.141000 | 0.062* |
Source of material
A mixture of (E)-4-(2-(pyridin-4-yl)vinyl)phenol (197 mg, 1 mmol) and 4,6-dichloropyrimidine (205 mg, 1 mmol) was suspended in DMF (5 ml), then copper powder (6.4 mg, 0.1 mmol) and cesium carbonate (815 mg, 2.5 mmol) were added. The reaction mixture was held at 110 °C under nitrogen for 6 h and then diluted with ethyl acetate (30 ml). The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by chromatography (petroleum ether: ethyl acetate = 6:1, v/v) on silica gel to give (E)-4-chloro-6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidine as a yellow solid. A mixture of (E)-4-chloro-6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidine (307 mg, 1 mmol) and morpholine (87 mg, 1 mmol) in DMF (5 ml) was heated in a microwave reactor at 130 °C and 100 W for 30 min. The reaction mixture was diluted with ethyl acetate (30 ml). The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by chromatography (petroleum ether: ethyl acetate = 10:1, v/v) on silica gel to give the title compound as a white solid [4].
Experimental details
All hydrogen atoms were placed in the calculated positions and constrained to ride on their parent atoms.
Comment
Nitrogen containing heterocycles especially pyrimidines form an important class of heterocyclic compounds [5, 6]. Heterocycles containing pyrimidine ring exist as the basic structures of many natural botanicals and bioactive compounds, and are widely found in herbal medicines with therapeutic effects on diabetes [7], [8], [9].
There are two crystallographically independent molecules in the asymmetric unit (see the figure). The bond lengths and angles are in the expected ranges [10].
Funding source: Science and Technology Partnership Program, Ministry of Science and Technology of China
Award Identifier / Grant number: KY201904005
Funding source: Tangshan Science and Technology Innovation Team Training Program to Ji-an Li
Award Identifier / Grant number: 19130205C
Funding source: Science and Technology Planning Project of Hebei Province, China
Award Identifier / Grant number: 14397705D
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by Hebei Key Laboratory of Integrated Traditional Chinese and Western Medicine for Diabetes and Its Complications, College of Traditional Chinese Medicine, North China University of Science and Technology, 21 Bohai Road, Tangshan 063210, China. This work was supported by Science and Technology Partnership Program, Ministry of Science and Technology of China (KY201904005), Tangshan Science and Technology Innovation Team Training Program (19130205C) to Ji-an Li, Science and Technology Planning Project of Hebei Province, China (No. 14397705D).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT. Version 8.23B; Bruker AXS Inc.: Madison, Wisconsin, USA, 2013.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Sun, W. J., Hu, S. Q., Fang, S. B., Yan, H. Design, synthesis and biological evaluation of pyrimidine-based derivatives as VEGFR-2 tyrosine kinase inhibitors. Bioorg. Chem. 2018, 22, 393–405; https://doi.org/10.1016/j.bioorg.2018.04.005.Search in Google Scholar PubMed
5. Elderfield, R. C. Heterocyclic compounds: six-membered heterocycles containing two hetero atoms and their benzo derivatives. Heterocycl. Compd. 1957, 6, 564–600.Search in Google Scholar
6. Lagoja, I. M. Pyrimidine as constituent of natural biologically active compounds. Chem. Biodivers. 2005, 2, 1–50; https://doi.org/10.1002/cbdv.200490173.Search in Google Scholar PubMed
7. Vorbruggen, H. Advances in amination of nitrogen heterocycles. Adv. Heterocycl. Chem. 1990, 49, 117–192; https://doi.org/10.1016/s0065-2725(08)60554-1.Search in Google Scholar
8. Turck, A., Ple, N., Mongin, F., Queguiner, G. Advances in the directed metallation of azines and diazines (pyridines, pyrimidines, pyrazines, pyridazines, quinolines, benzodiazines and carbolines). Part 2: metallation of pyrimidines, pyrazines, pyridazines and benzodiazines. Tetrahedron 2001, 57, 4489–4505; https://doi.org/10.1016/s0040-4020(01)00225-3.Search in Google Scholar
9. Schomaker, J. M., Delia, T. J. Arylation of halogenated pyrimidines via a Suzuki coupling reaction. J. Org. Chem. 2001, 66, 7125–7128; https://doi.org/10.1021/jo010573+.10.1021/jo010573+Search in Google Scholar PubMed
10. Sun, W., Fang, S., Yan, H. Discovery of novel picolinamide-based derivatives as novel VEGFR-2 kinase inhibitors: synthesis, in vitro biological evaluation and molecular docking. MedChemComm 2018, 9, 1054–1058; https://doi.org/10.1039/c8md00057c.Search in Google Scholar PubMed PubMed Central
© 2022 Su-Fen Bai et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

