Abstract
The deep desulfurization method of sintering flue gas based on the low-temperature oxidation method is studied. Based on the analysis of the main principle of deep desulfurization of sintering flue gas, a deep desulfurization system of sintering flue gas is constructed, which is composed of an absorption washing unit and a washing solution treatment unit. Sodium hydroxide solution is used as the desulfurizing absorbent to mix with the sintering flue gas entering the reaction tower. Sulfur dioxide in the sintering flue gas reacts with sodium hydroxide to generate sodium sulfite, and sodium sulfite is oxidized to produce sodium sulfate; ozone is produced by ozone generator, nitrogen oxide compounds are oxidized by ozone to generate oxyacid, which is easy to be removed by sodium hydroxide washing solution, and the detergent is the same as that used to remove sulfur dioxide and dust. The experimental results show that the highest desulfurization rate and denitrification rate of the proposed method are 90% and over 22%, and the reaction efficiency and economy are significantly better than that of the comparative method, which shows that the method is reasonable and effective.
1 Introduction
The iron and steel industry is not only an important basic industry of the country but also the source of high-energy consumption, high emission, and increased environmental load. It consumes on a large scale not only raw materials but also energy and creates pollution Nearly half of the materials are put into the final output in the form of waste gas, solid waste, or byproducts [1,2]. Iron and steel production consumes a lot of fuel and ore in the process of hot processing and discharges a lot of air pollutants at the same time. In 1996, the emission of sulfur dioxide (SO2) in the iron and steel industry was 9,78,000 tons, accounting for 7.5% of the national industrial sulfur dioxide emission, ranking third only after the production and supply of electric power, gas, hot water, and the manufacture of chemical raw materials and chemicals [3,4]. The sulfur dioxide emission from the sintering process accounts for 40–60% of the annual emission of iron and steel enterprises, which is the key point of sulfur dioxide pollution control in iron and steel enterprises. With the increase of sinter output and the large-scale development of sintering machine, the amount of exhaust gas and sulfur dioxide emission of single machine will increase, so it is imperative to control the sulfur dioxide pollution of sintering machine flue gas. To control the emission of sulfur dioxide in the production process of sintering machine is the key point of sulfur dioxide pollution control in iron and steel works, and one of the key links to maintain sustainable development in iron and steel works. Sintering flue gas is the dust-containing waste gas produced in the process of high-temperature sintering and forming after ignition of sintering mixture and running with trolley [5,6]. Its main characteristics are large emission, about 4,000–6,000 m3 of flue gas produced by 1-t sinter; high dust concentration, mainly iron and its compounds, and also containing silicon, calcium, and other iron-ore-associated components; high temperature [7], generally around 150°C; large moisture content, about 10% (volume ratio); corrosive gases, such as hydrogen sulfide, nitrogen oxide, and sulfur oxide; the concentration of sulfur dioxide is low, the concentration changes greatly, generally in 1,000–3,000 mg/m3.
At present, the commonly used flue gas desulfurization methods are as follows: Duan et al. used multi-layer packing cross flow rotating packed bed for deep purification of sintering flue gas, in view of the characteristics of sintering flue gas in the iron and steel smelting industry and the difficulties faced in the current industrial production, carried out the experimental research on the influence of high gravity factor β, liquid gas ratio L/G, and different inlet SO2 concentrations on the desulfurization rate η by using the multilayer packing cross flow rotating bed as the absorption equipment and NaOH as the absorbent. The results show that when β = 55, L/G = 2.0 L/m3, CSO2, in = 1,680 mg/m3, the desulfurization rate of the double-layer liquid inlet is 1.7 times of that of the single-layer liquid inlet, and the outlet concentration is lower than 35 mg/m3, which can realize deep purification. When the concentration is increased to CSO2, in = 4,396 mg/m3, the desulfurization rate is only reduced by 0.75%, which indicates that the multilayer cross flow bed is suitable for a wide range of SO2. At the same time, the fine particles can be removed, and the outlet concentration of low concentration dust is lower than 10 mg/m3, which can realize deep desulfurization and dust removal at the same time and reach the ultra-low-emission standard [8]. Wang et al. studied the main influence factors of oxygen on the NO oxidation rate in the simulated flue gas and the removal rate of NOx in the simulated flue gas after oxidation by sodium hydroxide solution absorption method. In the experiment, the effects of NO concentration, reaction temperature, oxygen concentration, and SO2 concentration in flue gas on NO oxidation rate were studied first. On this basis, NO oxidation rate was also studied. The experimental results show that at low temperature, the increase of NO concentration, oxygen concentration, and oxidation reaction time is beneficial to that of NO oxidation rate, and the NO oxidation rate initially increases significantly with the increase of the aforementioned parameters, and the increased trend slows down after reaching a certain value, while SO2 in flue gas has little effect on NO oxidation rate. When the mass concentration of sodium hydroxide solution is 6% and pH = 9, increasing the NO oxidation rate can promote the NOx removal rate of simulated flue gas [9]. Yang et al. prepared mixed steel-slag-activated carbon adsorbent with steel slag and activated carbon by mixed method, carried out simulated sintering flue gas desulfurization and denitrification experiment in programmable electric heating fixed-bed reactor, and inspected reaction temperature and SO2 concentration. The experimental results show that the highest desulfurization and denitrification rates were 79% and 34%, respectively, at 15 vol% and 120℃ [10]. Although the aforementioned methods can achieve the goal of flue gas desulfurization to a certain extent, it is difficult to fully promote the application due to the limitation of raw material sources. At the same time, there are many disadvantages, such as high investment in equipment, large floor area, large water consumption, secondary pollution caused by a large amount of byproduct gypsum, and difficulty to match with the existing industrial boilers, so the development of practical and effective alternative technology is still in constant exploration.
In view of the problems existing in the aforementioned methods, the deep desulfurization method of sintering flue gas in iron and steel plant based on the low-temperature oxidation method is studied. The method is to use metal oxide catalysts such as alumina, titanium dioxide, and sodium hydroxide to develop highly efficient low-temperature oxidation selective reduction catalyst as an absorbent to realize the deep desulfurization of sintering flue gas in iron and steel plant.
2 Materials and methods
2.1 Principle of flue gas desulfurization
Deep desulfurization of sintering flue gas based on low-temperature oxidation is a new technology developed on the basis of catalytic oxidation desulfurization. Its main principle is to add absorbent [11,12] in the liquid phase, in the presence of oxygen, oxidize sulfur dioxide to sulfuric acid, nitric oxide to nitrogen dioxide, and obtain compound fertilizer of ammonium sulfate and ammonium nitrate in the alkaline condition. At present, the most studied absorbents are transition metal ions such as iron, manganese, and sodium, and organic compounds such as ethylenediamine cobalt [13,14]. Take sodium hydroxide as an example to illustrate the principle of sintering flue gas deep desulfurization method based on low-temperature oxidation, as shown in Figure 1.
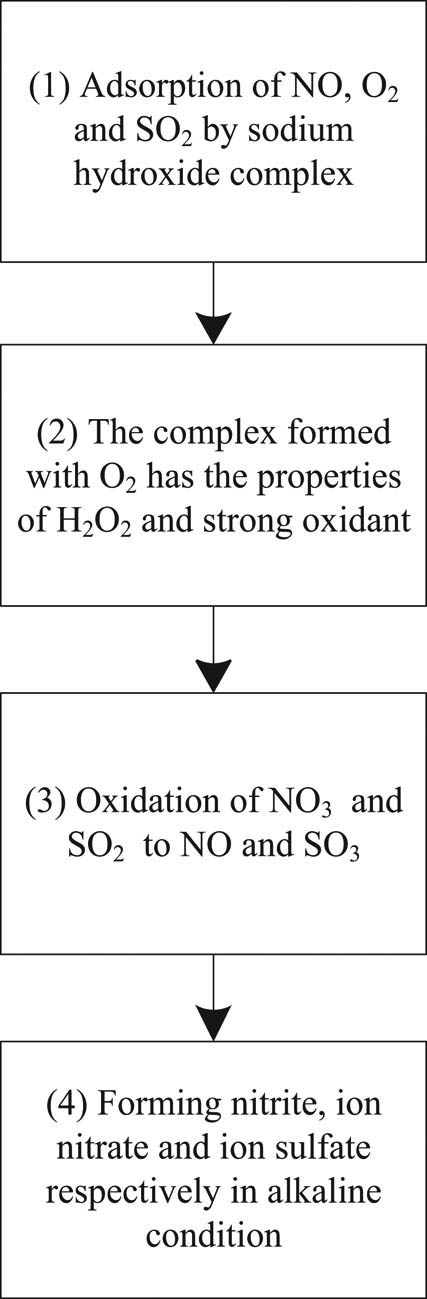
Technical principle of deep desulfurization of flue gas from low-temperature oxidation sintering of ethylenediamine cobalt.
It can be seen from Figure 1 that in the process of deep desulfurization of sintering flue gas, sodium hydroxide complex is first used to adsorb NO, O2, and SO2, and the adsorbed substance has the properties of H2O2 and strong oxidant. Then, NO3 and SO2 are converted to NO and SO3 through oxidation treatment. Finally, nitrite, nitrate, and sulfate ions can be formed under alkaline conditions.
2.2 Construction of deep desulfurization system
Based on the aforementioned principle, a deep desulfurization system for sintering flue gas of iron and steel plant based on the low-temperature oxidation method is constructed. The brief process flow of the system is shown in Figure 2. The system is divided into absorption washing unit and washing solution treatment unit [15]. The deep desulfurization process system of sintering flue gas based on low-temperature oxidation mainly consists of absorption tower system, flue gas system, oxidation system, filtration and separation system, neutralizer and catalyst supply system, catalyst recovery system, byproduct recovery system, public system, control system, etc. [16].
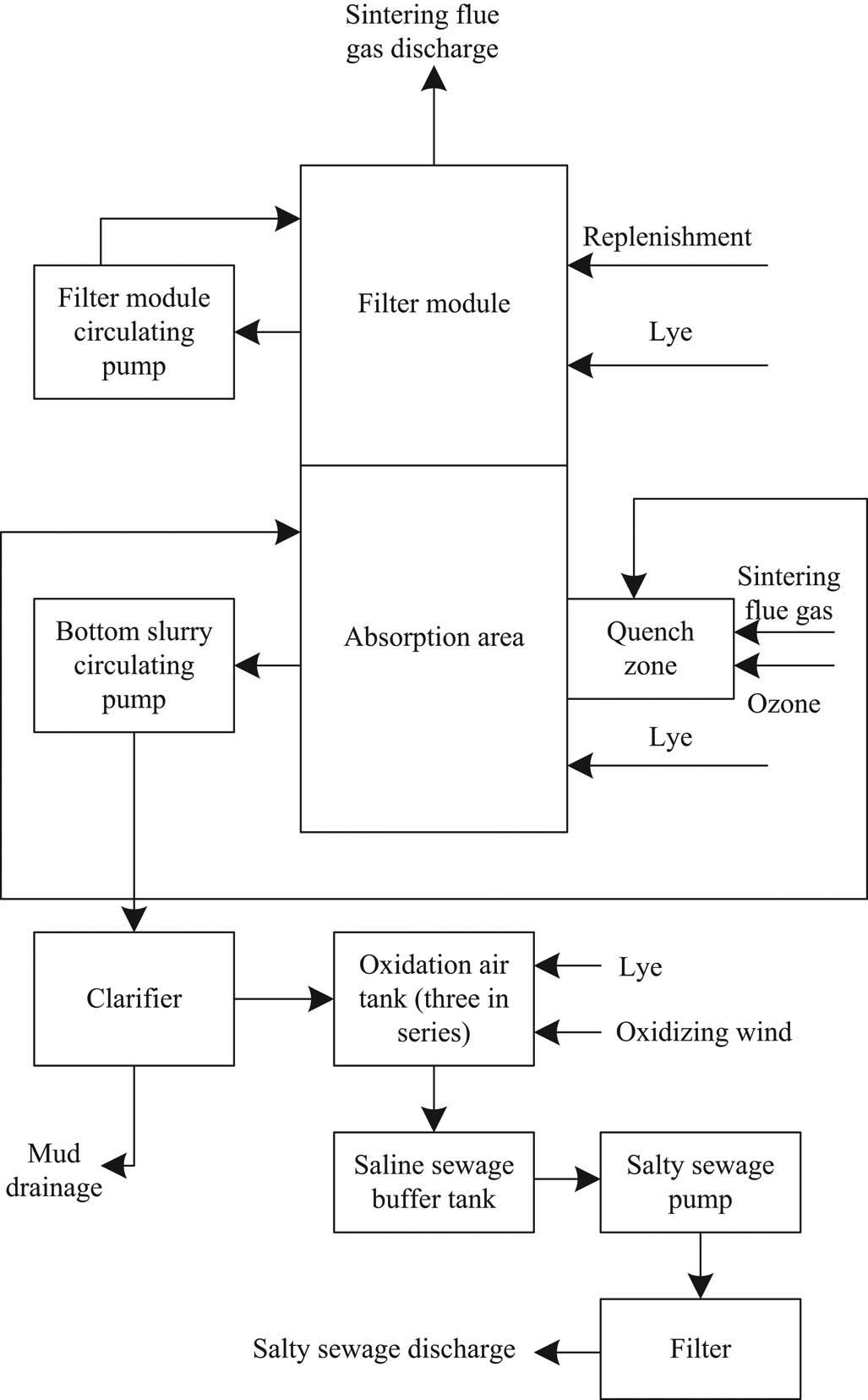
Brief process flow of sintering flue gas deep desulfurization system in iron and steel plant.
It can be seen from Figure 2 that in the process of deep desulfurization of sintering flue gas, the flue gas is mainly filtered by the filter-circulating pump in the filter module, and then the treated sintering flue gas is further absorbed by the bottom slurry-circulating pump. Finally, the salty sewage is treated by the clarifier, and the salty sewage is discharged to realize deep desulfurization of sintering flue gas.
The basic principle of deep desulfurization of sintering flue gas in iron and steel plant based on the low-temperature oxidation method is to use sodium hydroxide solution as a desulfurization absorbent, mix with the sintering flue gas entering into the reaction tower, the sulfur dioxide in the sintering flue gas reacts with sodium hydroxide, to generate sodium sulfite, and the sodium sulfite is oxidized to produce sodium sulfate. The reaction formula in the above process is as follows:
For nitrogen oxide emission control in sintering flue gas of iron and steel plant, ozone is produced by ozone generator, and nitrogen oxide compounds are oxidized by ozone, generating oxyacid that can be easily removed by sodium hydroxide washing solution, which is the same as the washing solution used to remove sulfur dioxide and dust. The reaction formula in the above process is as follows:
2.2.1 Absorption washing unit
The flue gas from the carbon monoxide waste heat boiler enters the scrubber, and the flue gas is immediately cooled to the saturation temperature in the quench area at the bottom of the scrubber. The injected ozone oxidizes the nitrogen oxide to nitrogen pentoxide and further dissolves in water to generate nitric acid. The flue gas after cooling and saturation rises to the absorption area, fully contacts with the slurry droplets containing alkaline absorbent in sections, and is washed step by step to remove catalyst dust, nitric acid, and sulfur dioxide from flue gas. After washing, the flue gas rises into the filtering module area, where it is forced to pass through 13 filtering modules, with a special nozzle above each filtering channel. The saturated flue gas is depressurized and expanded through the filter module, and the small dust particles and acid droplets that are not removed in the absorption area agglomerate with water droplets, and then is filtered and captured by the high-density water curtain generated by the nozzle, so that the sintering flue gas can be further purified. The water drop separator on the upper part of the washing tower removes the liquid drop carried in the flue gas, and the purified flue gas is discharged into the atmosphere through the chimney on the top of the tower.
The system continuously injects alkali solution (30% sodium hydroxide) into the washing tower through alkali pump to maintain the pH value of the absorption solution. The circulating pump draws out the washing liquid at the bottom of the tower and sends it to the quench nozzle and the absorption nozzle for recycling the alkaline absorbent solution. To prevent the accumulation of catalyst dust and ensure the absorption efficiency and equipment safety, in normal operation, to control the solid content, total dissolved solids (TDS), chlorine element, calcium ion, etc. in the circulating absorption liquid in the scrubber, the slurry-circulating system of the scrubber needs to throw part of the slurry outside to the washing-liquid-processing unit.
2.2.2 Detergent treatment unit
The washing waste water sent by the slurry-circulating pump of the washing tower contains particles and sulfites. It is first mixed with flocculant evenly and then enters the clarifier. In the clarifier, the catalyst solid precipitates and forms the bottom layer in a centralized way. The slurry area at the bottom is stirred by the rake-type transmission mechanism to form a suitable bottom layer to prevent the solid bottom layer from hardening. The drain valve at the bottom of the clarifier is opened regularly to discharge mud. As the solid precipitates from the clarified liquid, the clarified liquid overflows from the upper part of the clarifier to the back three oxidation tanks in series for treatment. Alkali is added according to the pH value in each oxidation tank. The oxygen in the air delivered by the same fan in the oxidation tank reacts chemically to generate sulfate and reduce chemical oxygen consumption (COD). The sewage from the oxidation tank is filtered, and it is discharged to the sewage treatment plant for treatment.
2.3 Effect of different flue gas quantities on desulfurization process
In the process of flue gas denitrification, the influence of different flue gas quantities on the desulfurization process should be considered. Keep the normal sulfur dioxide concentration, oxygen content of flue gas, initial concentration of absorption solution, and reaction temperature unchanged. In the experiment of different flue gas flow rates, absorbent is no longer added in the reaction process, so the sulfur dioxide content at the inlet and outlet of flue gas is measured. According to the absorption liquid value and the concentration of each component, it is concluded that the reaction driving force in the initial stage of the desulfurization process is larger under different flue gas volumes, and SO2 can be removed rapidly, which shows that the flue gas flow has little effect on the desulfurization rate.
2.4 Calculation of removal rate
According to the analysis results in Section 2.3, the removal rate of different substances in sintering flue gas is calculated. The removal rate of sulfur dioxide and nitrogen oxide in sintering flue gas of iron and steel plant is calculated according to the following formula:
where
Ethical approval: The conducted research is not related to either human or animal use.
3 Results
To verify the effectiveness of the deep desulfurization method of sintering flue gas based on low-temperature oxidation studied in this study, a certain iron and steel plant in Chongqing, China, is taken as an example to conduct the deep desulfurization treatment of sintering flue gas with the method in this study. The operation data of three experimental objects under typical working conditions are selected below to analyze the operation effect of this method under different device loads and three working conditions. See Table 1 for the nature of regeneration sintering flue gas.
Properties of regeneration sintering flue gas
| Project | Design value | Working condition 1 | Working condition 2 | Working condition 3 | |
|---|---|---|---|---|---|
| Sulfur content of raw oil (mg/kg) | 3,900 | 5,800 | 5,700 | 2,908 | |
| Inlet flue gas flow (N m3/h) | 3,78,619(MAX) | 4,07,849 | 4,02,954 | 3,03,808 | |
| Flue gas composition of regeneration sintering | Carbon dioxide (vol%) | 12.56 | 12.22 | 12.30 | 12.27 |
| Oxygen (vol%) | 2.60 | 1.46 | 1.40 | 2.73 | |
| Nitrogen (vol%) | 72.44 | 76.15 | 76.26 | 77.4 | |
| Carbon monoxide (vol%) | 0 | 7.74 | 7.61 | 5.17 | |
| Sulfur dioxide (mg/m3) | 1603.21 | 2565.74 | 2156.25 | 1137.34 | |
| Nitrogen dioxide (mg/m3) | 209.00 | 142.00 | 135.00 | 108.00 | |
| Dust (mg/m3) | 139.00 | 113.00 | 119.00 | 130.00 | |
3.1 Operation analysis of absorption and washing unit
The main operation parameters of the scrubber are shown in Table 2 under different flue gas flow and operation conditions after the deep desulfurization of sintering flue gas by this method.
Main process parameters of washing tower unit
| Project | Design value | Working condition 1 | Working condition 2 | Working condition 3 | |
|---|---|---|---|---|---|
| Make up water volume of washing tower (T/h) | 38.20 | 52.90 | 53.00 | 48.10 | |
| Total alkali consumption (T/h) | 1.97 | 2.29 | 2.31 | 2.07 | |
| Ozone amount (N m3/h) | 1182.41 | 909.82 | 954.26 | 679.50 | |
| Discharge slurry volume (T/h) | 30.96 | 29.41 | 30.02 | 29.25 | |
| Discharge sintering flue gas temperature (°C) | 55.70 | 54.15 | 54.32 | 55.01 | |
| Composition of circulating slurry | pH (25°C) | 6.00 | 5.49 | 5.69 | 6.00 |
| TDS | 79.00 | 79.00 | 18.00 | ||
| Suspended solids | 950.00 | 1018.00 | 769.00 | ||
| COD (hash) | 7519.00 | 7449.00 | 3100.00 | ||
| Composition of discharged sintering flue gas (mg/m3) | Nitrogen dioxide | ≤39.00 | 12.00 | 49.00 | 23.00 |
| Nitric oxide | 74.00 | 77.00 | 19.00 | ||
| Sulfur dioxide | ≤239.00 | 13.00 | 14.00 | 19.00 | |
| Oxygen | 1.97 | 1.89 | 1.89 | ||
| Dust | ≤29.00 | 16.00 | 17.00 | 18.00 | |
From the data shown in Table 2, it can be seen that most of the operation parameters of the scrubber in this method meet the design requirements, and only the flow of make-up water is higher than the design value. The pH value of the absorption slurry is controlled at the set value by continuously injecting alkali solution into the tower to maintain the material balance of the absorption reaction and ensure the absorption efficiency of the washing solution for sulfur dioxide and nitric acid in the sintering flue gas. In this method, the alkali consumption of the whole system is related to the sulfur dioxide content in the sintering flue gas. When the sulfur dioxide content is reduced to the design value, the alkali consumption of this method is lower than the design value.
Under normal conditions, the decisive factor affecting the absorption effect of sulfur dioxide in sintering flue gas is the pH value of circulating slurry. The higher the pH value, the better the absorption effect. The material of the equipment in the absorption and washing unit is mainly 304L. If the circulating liquid is too acidic, it will cause stress corrosion to the equipment under the action of high concentration of chloride ion. If the circulating liquid is too alkaline, it will absorb other harmless weak acid components in the sintering flue gas, increase the alkali consumption and operation cost. Therefore, the upper limit of pH value control index of the absorbing liquid is 7.5, and the pH value of the circulating slurry in actual operation is 7.0–7.5.
Because there are no analysis data of flue gas before entering the scrubber, they can only be calculated according to the analysis data of laser particle size analyzer at the inlet of the flue gas turbine. The dust concentration of flue gas at the inlet of the flue gas turbine is generally between 100 and 150 mg/Nm3. According to this calculation, the catalyst dust carried in the regenerated flue gas is generally between 40 and 60 kg/h. According to the dust collection speed in the filter box of the washing solution treatment unit, generally, the dust concentration is below 20 mg/N m3, which meets the design value according to the regular field test data.
3.2 Validation of deep desulfurization effect of sintering flue gas
To understand the influence factors of this method on the deep desulfurization process of sintering flue gas, the influence of pH value of absorption solution and oxygen concentration on the deep desulfurization process of sintering flue gas was investigated under condition 1 in the previous experiment. The absorption solution used in this method is sodium hydroxide solution with a certain pH value, in which sulfuric acid is used as the pH regulator.
3.2.1 Effect of pH value of absorption liquid on deep desulfurization effect of sintering flue gas
Under condition 1, the influence of pH value of absorption solution on the desulfurization and denitrification of sintering flue gas is shown in Figure 3.
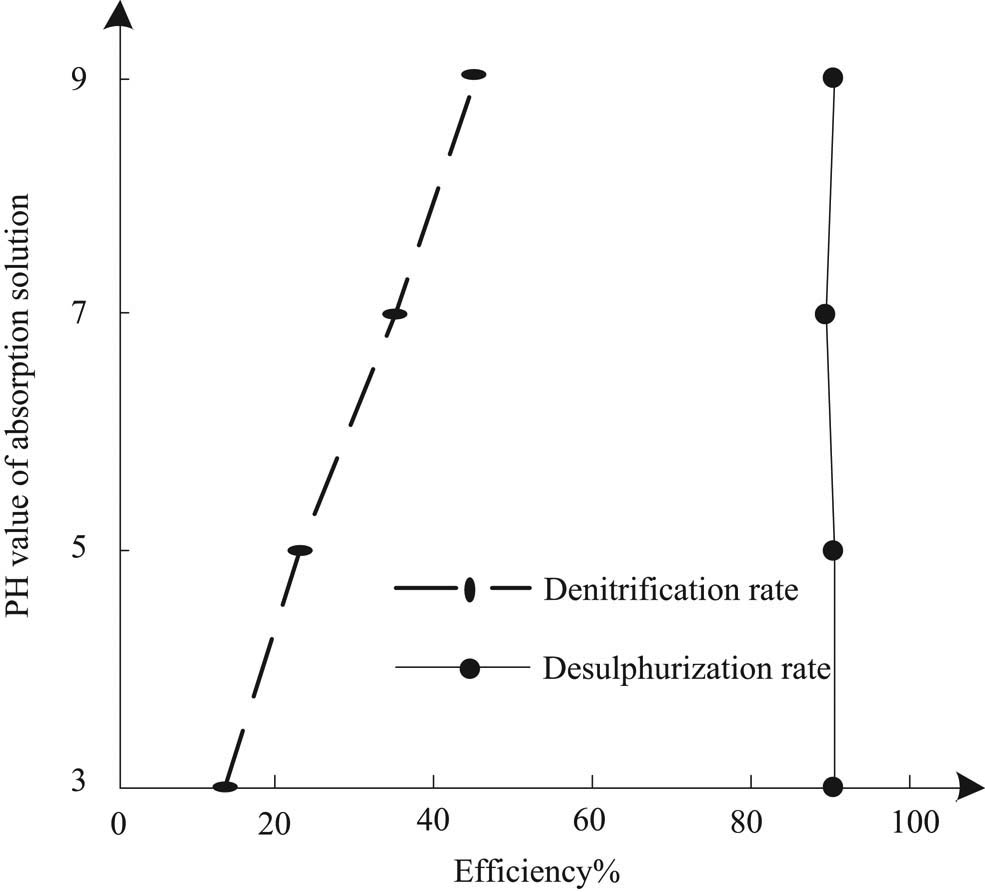
Effect of pH value of absorption solution on desulfurization and denitrification of sintering flue gas.
It can be seen from Figure 3 that in the range of pH value of absorption solution from 3.0 to 9.0, with the increase of pH value, the denitration rate of sintering flue gas increases gradually, while the desulfurization rate is basically unchanged. In the desulfurization process of this method, none can react with sulfite to form sulfur nitrogen complex, and none can be reduced to nitrogen by sulfite, so as to obtain a certain denitration rate. With the increase of pH, the alkalinity of the solution increases; this is beneficial to the formation of sulfite in the desulfurization reaction of this method, so it promotes the absorption of NO. However, the excessive pH value easily causes ozone volatilization, forms aerosol, reduces the use efficiency of ozone, and improves the operation cost. Therefore, to ensure the effect of desulfurization and denitrification by this method at the same time, the pH value of the absorption solution should be controlled within the appropriate range. After many experiments, it is concluded that the best effect of desulfurization and denitrification by this method is when the pH value is between 7.0 and 9.0.
3.2.2 Effect of oxygen concentration on deep desulfurization of sintering flue gas
Under condition 1, the effect of oxygen concentration on desulfurization and denitrification of sintering flue gas is shown in Figure 4.
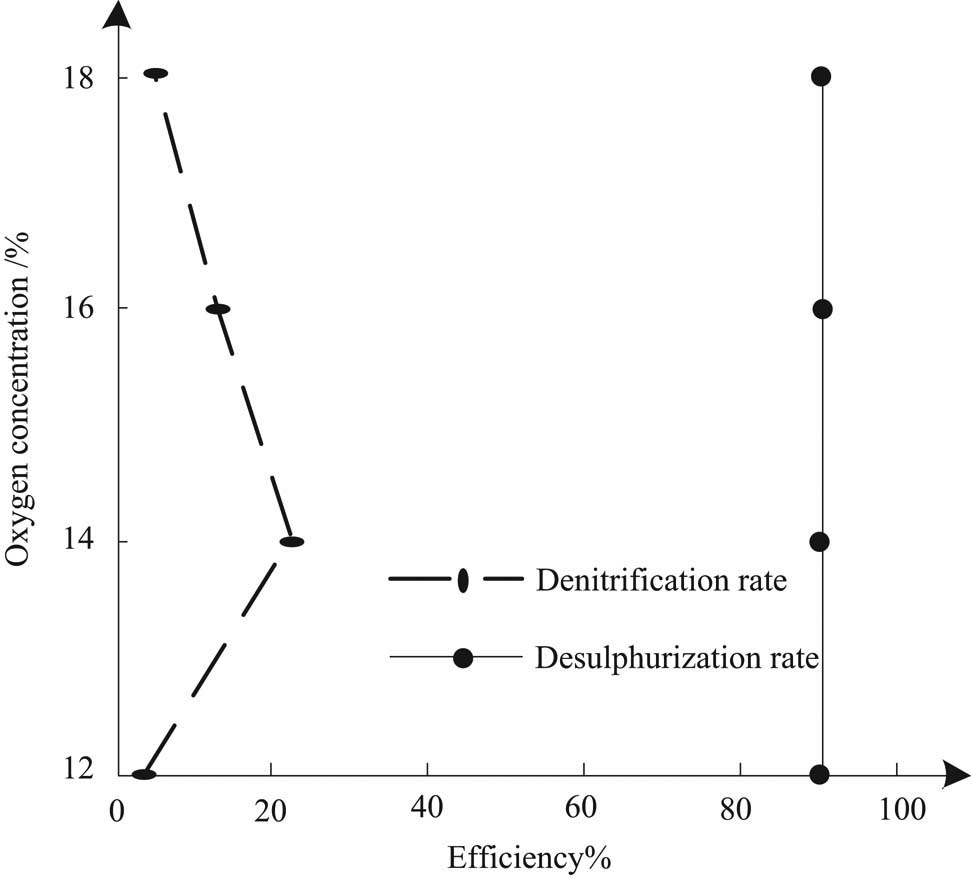
Effect of oxygen concentration on desulfurization and denitrification of sintering flue gas.
According to Figure 4, with the increase of oxygen concentration in sintering flue gas, denitration first increases and then decreases, while desulfurization rate is basically unchanged. The ratio of liquid to gas is large and the solubility of sulfur dioxide in water is good, so the desulfurization rate is close to or up to 100% under the set condition. In the setting of desulfurization conditions, too high or too low oxygen concentration in sintering flue gas is not good for the denitration of sintering flue gas. Under the aforementioned operating conditions and the oxygen concentration of 14%, the properties of sintering flue gas and absorption liquid are close to the actual situation of general desulfurization of experimental objects. At this time, the desulfurization rate and denitration rate are 90.00 and 22.48%, respectively.
3.2.3 Effect of temperature on deep desulfurization of sintering flue gas
To determine the appropriate temperature for deep desulfurization of sintering flue gas by this method, the cargo flow activity of the absorption liquid at 150, 200, and 250°C is evaluated, and the results are shown in Figure 5.
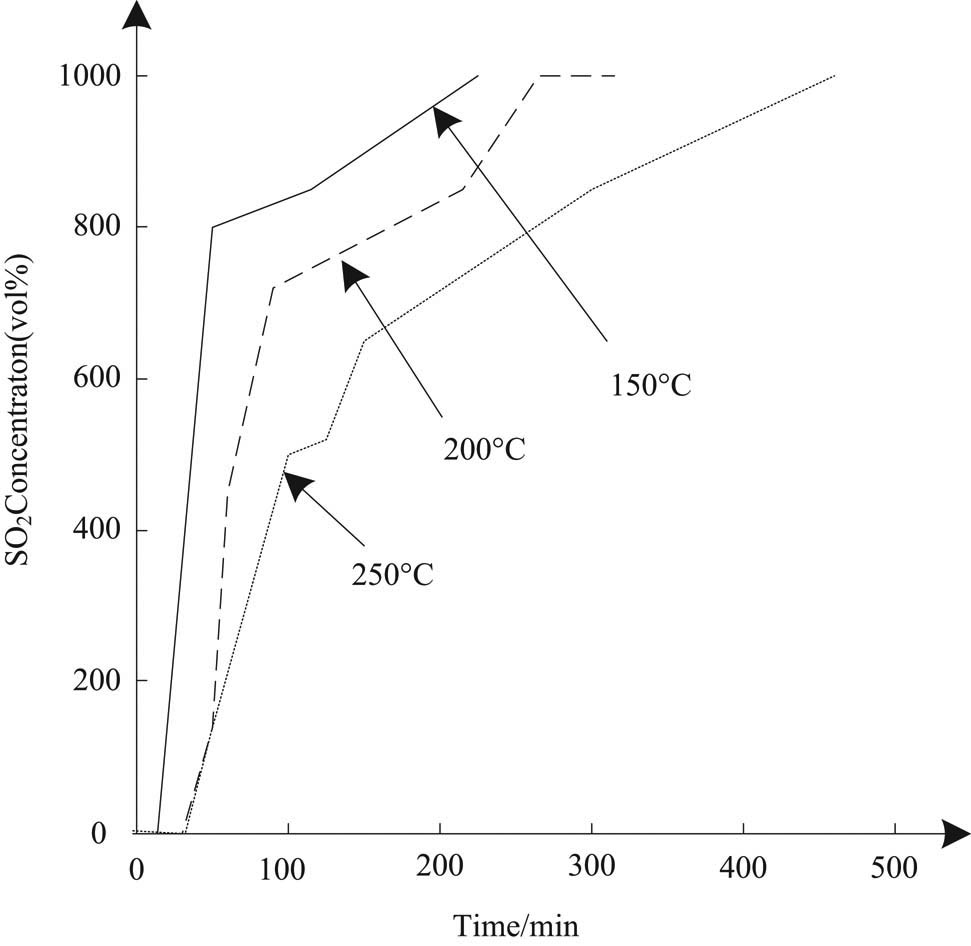
Desulfurization curve of absorption liquid at different temperatures.
According to the analysis of Figure 5, the change rule of desulfurization activity of absorption liquid with temperature is 250°C > 200°C > 150°C, which indicates that the reactivity between the active component of absorption liquid and sulfur dioxide increases with the increase of temperature.
3.3 Comparison of desulfurization effect
To verify the desulfurization effect of this method, simulate the field environment of the research object and compare the desulfurization effect of this method, wet-method-based desulfurization method, and catalytic cracking-based desulfurization method under the same conditions. The desulfurization effect of three different methods is shown in Figure 6.
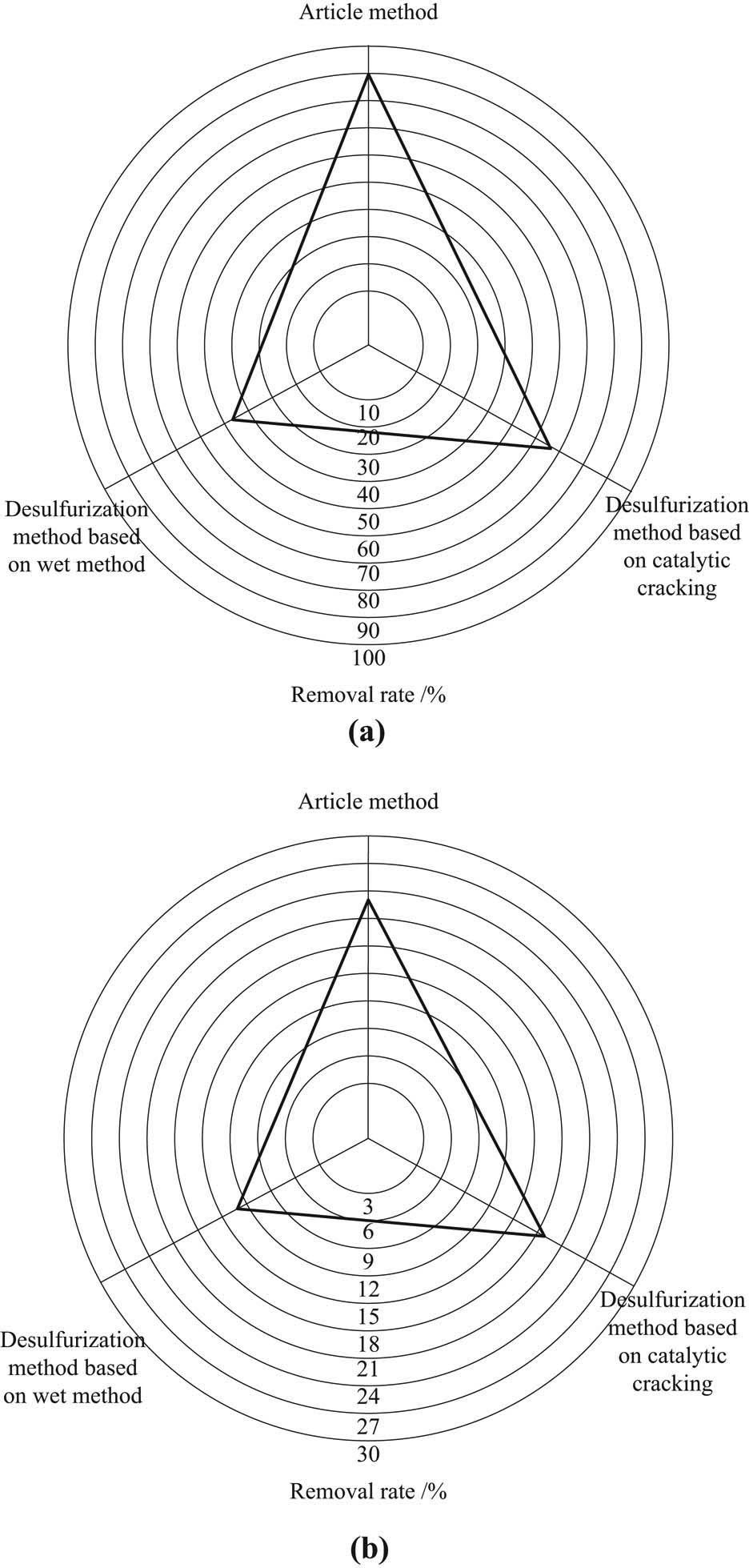
Effect comparisons of different methods. (a) Desulfurization effect. (b) Denitration effect.
It can be seen from the analysis of Figure 6 that the maximum desulfurization rate of this method is as high as 90% and the denitration rate is about 22.5% when the method is used for deep desulfurization of sintering flue gas in iron and steel plant, which has significant advantages compared with the other two methods. It can be seen that the desulfurization effect of this method is significantly better than the two comparison methods.
3.4 Reaction efficiency comparison
To verify the desulfurization effect of this method, simulate the field environment of the research object and compare the reaction efficiency of this method, wet desulfurization method, and catalytic cracking desulfurization method under the same conditions. The reaction efficiency of three different methods is shown in Figure 7.
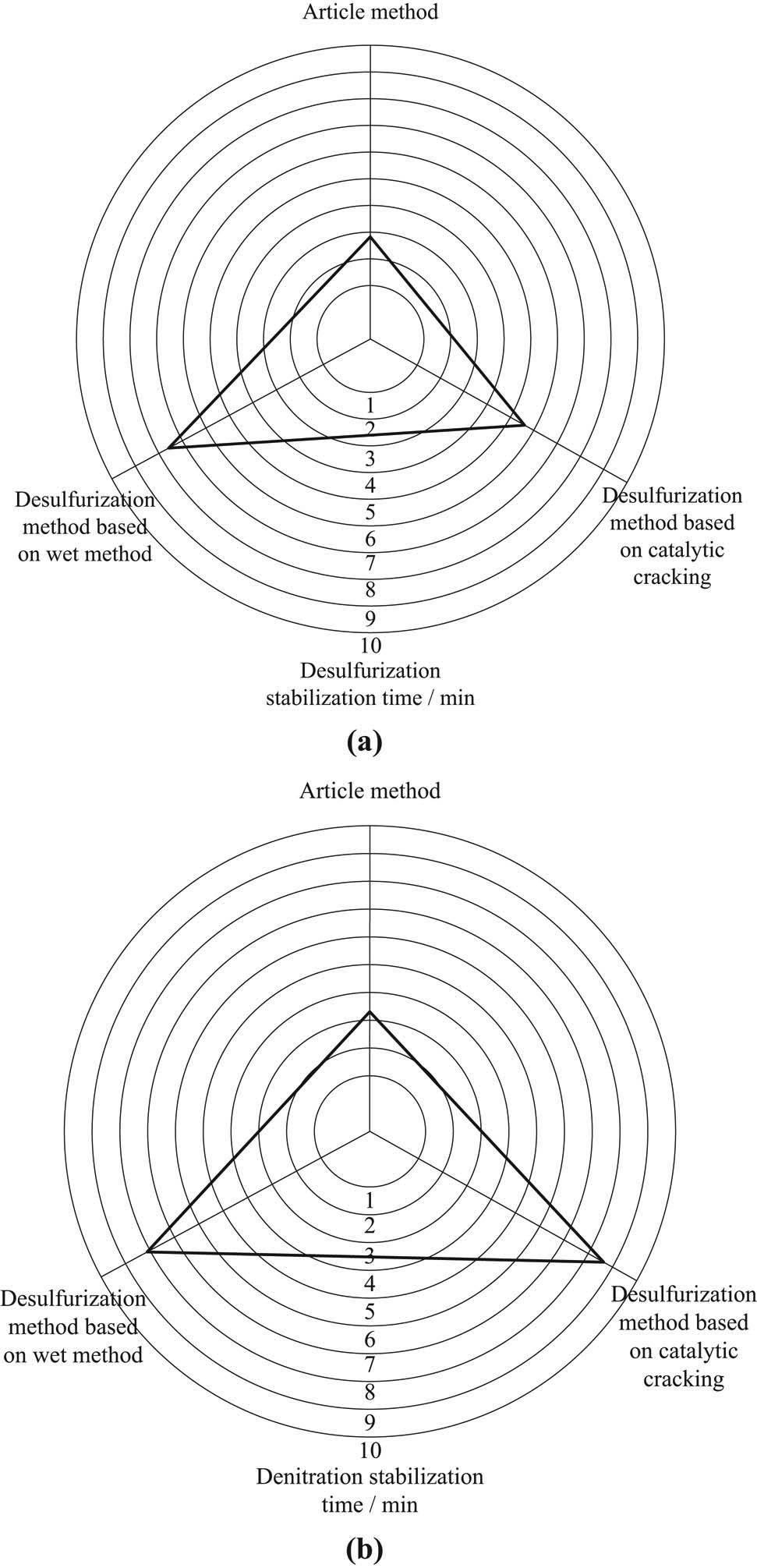
Efficiency comparison of different methods. (a) Desulfurization efficiency. (b) Denitration efficiency.
It can be seen from the analysis of Figure 7 that the advanced desulfurization treatment of sintering flue gas in the iron and steel plant is carried out by the method in this paper, and the desulfurization and denitration can enter the stable state in about 2 min, while the other two methods have a significantly longer time of stability state than the method in this study. It can be seen that this method has a significant efficiency advantage compared with the comparative method.
3.5 Economic comparison
The comparison of the analysis results of the main economic and technical indicators of the above three methods is shown in Table 3.
Economic index analysis of deep desulfurization method of sintering flue gas
| Project | Article method | Desulfurization method based on wet method | Desulfurization method based on catalytic cracking |
|---|---|---|---|
| Proportion of space occupied by equipment (%) | Less | Large | Secondary |
| Equipment cost proportion (kW) | 200 ± 25 | 225 ± 15 | 300 ± 15 |
| Proportion of operation cost (%) | 9.7 | 20.6 | 23.1 |
| By products and benefits | Agricultural fertilizer | Agricultural fertilizer | Nothing |
| Byproduct benefit | Yes | Yes | No, and there is secondary pollution |
| Flue gas reheat | No need to reheat | No need to reheat | Reheat |
| Regional restriction | Nothing | Nothing | Yes |
| Technology maturity | Industrial demonstration | Industrial demonstration application | Domestic commercialized introduction |
It can be seen from the information in Table 3 that compared with the other two comparison methods, this method integrates desulfurization, denitration, dioxin removal, dust removal, and heavy metal removal, with optimized overall investment, simple equipment required, small proportion of unit investment cost in the project. The operation cost is about 50% of the operation cost of the mature wet desulfurization and catalytic cracking desulfurization methods in the current industry, and in the operation process. Due to the high cost of activated coke caused by mechanical and chemical consumption, the process can effectively recover sulfur dioxide in flue gas for the production of sulfuric acid, liquid sulfur dioxide, or sulfur with good economic benefits, so as to realize the resource utilization of sulfur dioxide, thus reducing the overall operation cost of this method. At the same time, the method in this study basically does not consume process water, solves the problems of the byproduct gypsum produced by traditional wet desulfurization, such as difficult to digest in the market and waste water discharge, and has good economic benefits and application value.
4 Discussion
The deep desulfurization method of sintering flue gas based on the low-temperature oxidation method is an advanced new generation of comprehensive treatment and utilization technology of wet sintering flue gas, which is developed on the basis of summarizing previous technology, experience, and lessons. Using metal elements as a catalyst and oxygen as an oxidant, the operation cost is low, which overcomes the problems of low desulfurization efficiency, unstable operation, easy to block and scale, and no use value of by-products in the traditional wet process, greatly improves the stability and desulfurization efficiency of desulfurization system, and basically eliminates the secondary pollution caused by desulfurization system itself.
The desulfurization effect of this method is significantly better than that of the comparative method
The amount of desulfurizer is small and the utilization rate is high;
The water consumption is low, and the water consumption for the absorption liquid to improve its activity by humidification is very small. Generally, the water content to mass ratio of circulating desulfurizer is 3–5%;
The agitator in the tower strengthens the mass transfer process, prolongs the desulfurization reaction time, and ensures the operation effect of the system;
The adaptability of the system to the variation of flue gas and load with different sulfur dioxide concentrations is very strong, which is a remarkable advantage of the technology;
The absorption liquid is dry in the whole desulfurization process, the operating temperature is higher than the dew point, there is no corrosion or condensation, and there is no waste water;
The tower body is made of ordinary steel without special anticorrosion measures such as alloy, coating, and rubber lining;
Sintering flue gas can be discharged without reheating.
At present, there are many processes of sintering flue gas desulfurization. For the desulfurization treatment of sintering flue gas, we should make a reasonable choice according to the characteristics of flue gas and the situation of the site.
The process selection shall adhere to the following principles: advanced and mature technology, in line with the technical and economic environment of the enterprise itself, simple and reliable equipment, simple operation, high automation, low investment, high and stable desulfurization rate, low operation cost and energy consumption, wide source of absorption liquid, easy treatment of byproducts, and no secondary pollution.
The dense coherent tower flue gas desulfurization process belongs to the semidry desulfurization process, which fully conforms to the above process selection principle, and is suitable for the desulfurization of sintering flue gas.
In the sintering process, the concentration of sulfur dioxide in the flue gas is variable, sometimes with large range and high frequency. The concentration of sulfur dioxide in the head and tail flue gas is low and that in the middle flue gas is high. To reduce the scale of the desulfurization unit, only the flue gas with high concentration of sulfur dioxide can be introduced into the desulfurization unit, which can save most of the funds.
Accelerate the industrial application of sintering flue gas desulfurization technology, gradually eliminate the negative impact of sulfur dioxide and acid rain pollution on economic development, and promote the sustainable development of iron and steel enterprises.
5 Conclusions
In this study, the deep desulfurization method of sintering flue gas based on low-temperature oxidation developed iron and steel plant is proposed. The method has good desulfurization and denitrification effect on sintering flue gas and dust removal effect, which is lower than the national environmental protection emission standard, greatly reducing the emission of harmful pollutants, stable and reliable operation of equipment, and achieves the expected effect. The effective improvement of the surrounding environment provides a strong guarantee for the sustainable development of the whole petrochemical enterprise.
Acknowledgments
This research had been financed by The Chongqing Foundation and Frontier Plan in 2016 “Research on Energy Supply Chain Integrated Scheduling Based on Gas System in Iron and Steel Enterprises” (cstc2016jcyjA0341).
Conflict of interest: The authors declare no conflict of interest.
References
[1] Xu Z, Zheng C, Zhang Y. Operation optimization of flue gas desulfurization system in power plant based on an improved association rules algorithm. Proc Csee. 2017;37(15):4408–14. 10.13334/j.0258-8013.pcsee.161942.Search in Google Scholar
[2] Zhao L, Li XL, Zhang G. Test and evaluation of dust emissions during sintering process in an iron and steel enterprise. J Northeastern Univ. 2017;38(3):356–60. 10.3969/j.issn.1005-3026.2017.03.011.Search in Google Scholar
[3] Yu ZF, Wang XZ, Hou YN. Facile preparation of nitrogen-doped porous carbons via salt melt synthesis with efficient catalytic desulfurization performance. J Inorg Mater. 2017;32(7):770. 10.15541/jim20160632.Search in Google Scholar
[4] Norberto JSM, Igor B, Jeppe VL. Single-layer MoS2 formation by sulfidation of molybdenum oxides in different oxidation states on Au(111). Phys Chem Chem Phys. 2017;19(21):14020. 10.1039/C7CP00958E.Search in Google Scholar
[5] Yang L, Xiao JQ, Shen Y. The efficient removal of thallium from sintering flue gas desulfurization wastewater in ferrous metallurgy using emulsion liquid membrane. Environ Sci Pollut Res Int. 2017;24(31):1–9. 10.1007/s11356-017-0040-0.Search in Google Scholar PubMed
[6] Zhang X, Mwamulima T, Wang Y. Preparation of porous pellets based on nano-zero valent iron-enhanced fly ash and their application for crystal violet removal. Res Environ Sci. 2017;30(8):1295–302. 10.13198/j.issn.1001-6929.2017.02.48.Search in Google Scholar
[7] Li ZQ, He WX, Zhang YQ. Preparation and characterization of graphene by different reductant. Chin J Power Sources. 2018;42(6):865–7. 10.3969/j.issn.1002-087X.2018.06.034.Search in Google Scholar
[8] Duan SS, Yuan ZG, Gao YJ, Zhang CY, Li HT. Deep purification of sintering flue gas by multi-layer packing cross-flow rotating packed bed. Mod Chem Ind. 2019;39(5):207–10. 10.16606/j.cnki.issn.0253-4320.2019.05.046.Search in Google Scholar
[9] Wang Y, Li B, Zhao GM, Gao L, Zhou ZZ. Experimental investigation on denitration of coal-fired flue gas with wet absorption combined with oxidation using pure oxygen. Sci Technol Eng. 2019;19(02):267–71. CNKI:SUN:KXJS.0.2019-02-043.Search in Google Scholar
[10] Yang XB, Han YL, Li YG, Zhang H, Qian FP, Hu YM. Experimental study on desulfurization and denitration of sintering flue gas by activated carbon mixed with steel slag. Chinese J Proc Eng. 2019;19(2):440–6. 10.12034/j.issn.1009-606X.218257.Search in Google Scholar
[11] Nasser HS, Samia AH, Salah AH. Can mesoporous TiO2–Al2O3-supported NiMoS OR CoMoS effectively perform in ultra-deep desulfurization of gas oil. Pet Chem. 2018;58(5):387–94. 10.1134/S0965544118050158.Search in Google Scholar
[12] Edgar MMV, Carlos OCA, Sonia AG. Kinetic Assessment of the simultaneous hydrodesulfurization of dibenzothiophene and the hydrogenation of diverse polyaromatic structures. ACS Catal. 2018;8(5):3926–42. 10.1021/acscatal.8b00629.Search in Google Scholar
[13] Liang ZY, Yu M, Xu ZW. Influence of low-temperature pre-oxidation by ozone on corrosion resistance of T91 steel in steam at 600°C. Oxid Met. 2018;90(3–4):355–64. 10.1007/s11085-018-9841-x.Search in Google Scholar
[14] Liberman EY, Naumkin AV, Tsodikov MV. Synthesis structure, and properties of a Au/MnOx–CeO2 nanocatalyst for low-temperature oxidation of carbon monoxide. Inorg Mater. 2017;53(4):406–12. 10.1134/S0020168517040112.Search in Google Scholar
[15] Zhang W, Li X, Wang H. Deep desulfurization of model oil by photocatalytic air oxidation and adsorption using Ti(1−x)MxO2, (M = Zr, Ce). Korean J Chem Eng. 2017;34(12):3132–41. 10.1007/s11814-017-0229-4.Search in Google Scholar
[16] Gao W, Ghanbari B, Baskonus HM. New numerical simulations for some real world problems with Atangana-Baleanu fractional derivative. Chaos Solitons Fractals. 2019;128:34–43.10.1016/j.chaos.2019.07.037Search in Google Scholar
© 2020 Hua Meng, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

