Abstract
β2-Agonists (β2-adrenergic agonists, bronchodilatants, and sympathomimetic drugs) are a group of drugs that are mainly used in asthma and obstructive pulmonary diseases. In practice, the substances used to contain one or more stereogenic centers in their structure and their enantiomers exhibit different pharmacological properties. In terms of bronchodilatory activity, (R)-enantiomers showed higher activity. The investigation of stereoselectivity in action and disposition of chiral drugs together with the preparation of pure enantiomer drugs calls for efficient stereoselective analytical methods. The overview focuses on the stereoselectivity in pharmacodynamics and pharmacokinetics of β2-agonists and summarizes the stereoselective analytical methods for the enantioseparation of racemic beta-agonists (HPLC, LC-MS, GC, TLC, CE). Some methods of the stereoselective synthesis for β2-agonists preparation are also presented.
1 Introduction
β2-Agonists (sympathomimetic drugs, adrenergic drugs, and adrenomimetic drugs) produce effects similar to the stimulation of sympathetic nerves. They induce stimulation of the sympathetic nervous system either by direct action on adrenergic receptors or by indirect action, causing flushing of endogenous catecholamines, with subsequent limitation of their backflushing. Natural transmitters are noradrenaline and, to a lesser extent, adrenaline. Isoprenaline (isoproterenol) was prepared as one of the first synthetic sympathomimetic amines, which is structurally related to adrenaline and acts almost exclusively on β-adrenergic receptors.
A key role in inducing β-responses is considered the activation of adenylyl cyclase with subsequent increased production of cyclic adenosine monophosphate (AMP). The mechanism of cAMP’s action consists of activating protein kinases. Activated protein kinases transfer gamma-phosphate from AMP to various proteins, phosphorylating them by binding them to serine or threonine, while changing their activity. Binding to the β1-receptors of these substances is manifested by narrowing the peripheral vessels in the skin and mucous membranes, acceleration of heart activity, and increase in blood pressure. Binding to the β2-receptors is associated with bronchodilation, uterine relaxation, and skeletal muscle glycogenolysis [1,2,3].
The presented overview focuses on stereochemical aspects of β2-agonists which are nowadays, along with corticoids, one of the most effective drugs in asthma therapy. In the case of β2-agonists, bronchospasm is being released in contrast to corticosteroids, in the case of which the inflammation process is suppressed in the asthma therapy [4,5].
According to the action of the bronchodilatory effect, they are divided into β2-agonists that are as follows:
short-acting – salbutamol, levosalbutamol, terbutaline, pirbuterol, procaterol, fenoterol
long-acting – salmeterol, formoterol, bambuterol, mabuterol, clenbuterol;
ultra-long-acting – indacaterol, olodaterol, vilanterol, carmoterol, abediterol [6,7].
In addition to these β2-agonists, naturally occurring β-agonists such as adrenaline (epinephrine), the suprarenal gland hormone, and ephedrine, an alkaloid, are also used in the treatment of asthma [8]. The overview focuses on β2-agonists from the aspect of their stereogenic configuration, especially with regard to their optically active stereoisomers. Chemical IUPAC names of presented β2-agonists are listed in Table 1.
Chemical IUPAC names of selected β2-agonists
| Albuterol | 4-[2-(tert-Butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol |
| Abediterol | 5-{(1R)-2-[6-(2,2-Difluoro-2-phenylethoxy)hexylamino]-1-hydroxy-ethyl}-8-hydroxy-1H-quinolin-2-one |
| Bambuterol | 5-[2-(tert-Butylamino)-1-hydroxyethyl]benzene-1,3-diyl-bis(dimethylcarbamate) |
| Carmoterol | 8-Hydroxy-5-{(1R)-1-hydroxy-2-[[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl}-1H-quinolin-2-one |
| Clenbuterol | 1-(4-Amino-3,5-dichlorophenyl)-2-(tert-butylamino)ethanol |
| Fenoterol | 5-{1-Hydroxy-2-[1-(4-hydroxyphenyl)propan-2-ylamino]ethyl}benzene-1,3-diol |
| Formoterol | N-{2-Hydroxy-5-[1-hydroxy-2-[1-(4-methoxyphenyl)propan-2-ylamino]-ethyl]phenyl}formamide |
| Indacaterol | 5-[(1R)-2-[(5,6-Dietyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one |
| Isoxsuprine | 4-[1-Hydroxy-2-(1-phenoxypropan-2-ylamino)propyl]phenol |
| Mabuterol | 1-[4-Amino-3-chloro-5-(trifluoromethyl)phenyl]-2-(tert-butylamino)-ethanol |
| Olodaterol | 6-Hydroxy-8-{(1R)-1-hydroxy-2-[1-(4-methoxyphenyl)-2-methylpropan-2-ylamino]ethyl}-3,4-dihydro-2H-1,4-benzoxazin-3-one |
| Orciprenaline | 5-[1-Hydroxy-2-(propan-2-ylamino)ethyl]benzene-1,3-diol |
| Ritodrine | 4-{2-[1-Hydroxy-1-(4-hydroxyphenyl)propan-2-ylamino]ethyl}phenol |
| Salmeterol | 2-(Hydroxymethyl)-4-{1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]-ethyl}phenol |
| Vilanterol | 4-{(1 R)-2-[6-[2-[(2,6-dichlorophenyl)methoxy]ethoxy]hexylamino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol |
| Trantinterol | 2-[4-Amino-3-chloro-5-(trifluoromethyl)phenyl]-2-(tert-butylamino)-ethanol |
| Terbutaline | 5-[2-(tert-Butylamino)-1-hydroxyethyl]benzene-1,3-diol |
Chemically, β2-agonists represent a single group with a side aminoethanol chain. The aromatic nucleus is substituted with hydroxyl groups that are either in the 3,4-positions (isoprenaline and hexoprenaline) or in the 3,5-positions (fenoterol, reproterol, orciprenaline, and terbutaline). For some β2-agonists, the phenolic OH group is substituted with hydroxymethyl (salbutamol and salmeterol).
There is one stereocenter in the structure of these drugs, with two optically active forms of (R)-(−) and (S)-(+) in individual drugs that differ in pharmacological and pharmacokinetic properties.
The differences in the structure of the two enantiomers can be seen in the orciprenaline structure (Figure 1).
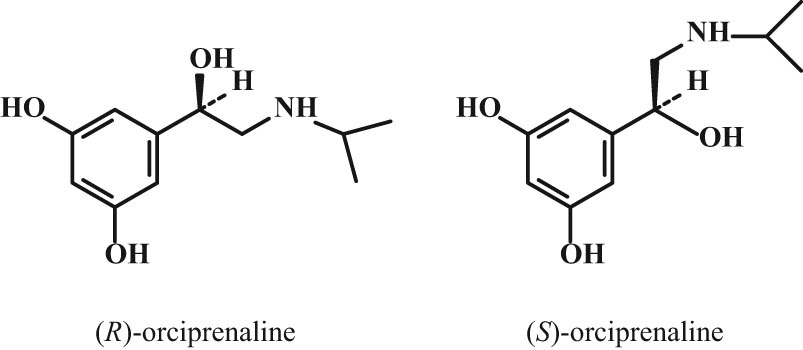
Two stereoisomers of orciprenaline.
In the case of multiple stereocenters, several stereoisomers can be distinguished, e.g., ritodrine or fenoterol with two stereocenters form four stereoisomers, and they cannot all be nonsuperimposable mirror images of each other (Figure 2). These stereoisomers (diastereoisomers) differ in their physicochemical properties unlike enantiomers.
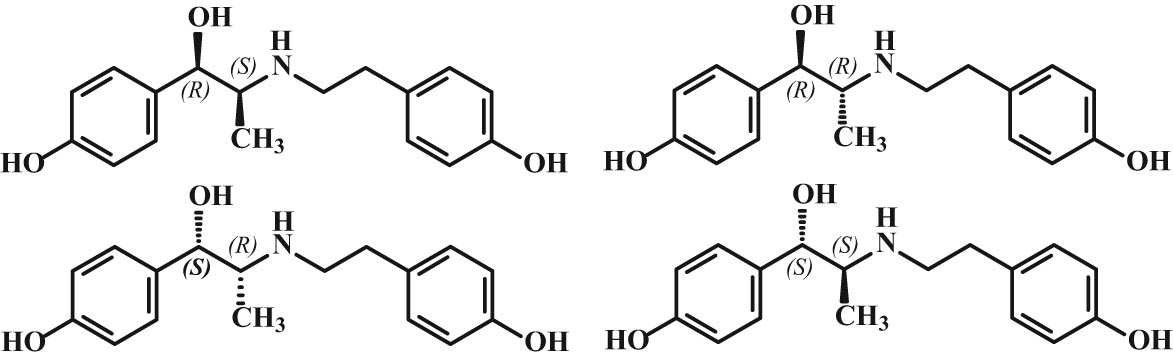
Chemical structures of ritodrine stereoisomers.
The mechanism of action of the individual enantiomers is explained through a stronger binding to the β2-receptor upon the interaction of the more efficient isomer at the receptor sites under precisely defined stereochemical conditions. By using the X-ray structural analysis, the interpretation of the Easson–Stedman hypothesis [9] was confirmed in many β2-agonists. The crucial binding sites are mainly linked to three functional groups, such as the amino group, the hydroxyl group of the side chain, and the substituted aromatic nucleus.
Mesecar and Koshland in 2000 proposed a four-point model that is suitable for the receptor binding site [10,11].
The receptor to which β2-agonists bind is a protein macromolecule composed of seven transmembrane helixes with three intracellular and three extracellular loops. A schematic structural model of the β-adrenoreceptor, highlighting the agonist and antagonist binding regions, is shown in Figure 3. The β-adrenoreceptor agonists bind to a hydrophobic pocket or active site located approximately 30–40% into the depth of the receptor. This location corresponds to the predicted location of the several amino acid residues that are critical in the binding of adrenaline [12,13]. Aspartate 306 in helix 3 forms a salt bridge with the amine of adrenaline. Serine 204 and 205 in helix 5 interact with the two hydroxyl groups of an aromatic ring. Phenylalanine 517 and 508 interact with the aromatic nucleus of adrenaline through van der Waals forces. In terms of efficacy, the hydroxyl group on the stereogenic carbon in the side chain is important. It forms a hydrogen bond with serine 413 on helix 4.
![Figure 3 Schematic diagram of the β-adrenoreceptor and its binding domain for agonist (☆) and antagonist (✪). Helices are individually numbered, and the approximate dimension of the receptor is marked in Angstroms (Å). (a) (Side-view) It shows a cut-away view of the seven-transmembrane α-helices of the β-adrenoreceptor seen from the plane of the membrane lipid bilayer. (b) (Top view) It shows the same beta-adrenoreceptor viewed from the extracellular space. (c) It shows an expanded detail of the ligand-binding site for agonists formed by helices 3, 4, 5, and 6, during binding of adrenaline. Reproduced with the permission of the © ERS 2020: European Respiratory Journal 7(3):569–78; Published 1 March 1994 [12].](/document/doi/10.1515/chem-2020-0056/asset/graphic/j_chem-2020-0056_fig_003.jpg)
Schematic diagram of the β-adrenoreceptor and its binding domain for agonist (☆) and antagonist (✪). Helices are individually numbered, and the approximate dimension of the receptor is marked in Angstroms (Å). (a) (Side-view) It shows a cut-away view of the seven-transmembrane α-helices of the β-adrenoreceptor seen from the plane of the membrane lipid bilayer. (b) (Top view) It shows the same beta-adrenoreceptor viewed from the extracellular space. (c) It shows an expanded detail of the ligand-binding site for agonists formed by helices 3, 4, 5, and 6, during binding of adrenaline. Reproduced with the permission of the © ERS 2020: European Respiratory Journal 7(3):569–78; Published 1 March 1994 [12].
Binding interactions to individual amino acids in the β2-receptor were also described in the case of noradrenaline and other β2-agonists [14,15].
A deeper study of bindings on the β-receptor enabled the resolution of the β2-receptor’s by X-ray structural analysis [16,17]. Molecular interactions between fenoterol stereoisomers and derivatives and the β2-adrenergic receptor binding site were studied by docking and molecular dynamics simulations [18,19,20]. Docking studies indicate that the hydroxyl group at the first chiral center of the ligand creates hydrogen bonds with Asp113 or/and Asn312 in the case of (R,*′) stereoisomers mainly. Molecular dynamics simulations confirm the existence of the stereoselective effects accompanying the ligand–receptor interactions, namely, different stereoisomers exhibit diverse conformational behaviors and distances between characteristic ligand–receptor atom–atom pairs. Stereochemistry of fenoprofen molecule also affects the coupling of the receptor to different G proteins. In a rat cardiomyocyte contractility model, (R,R′)-fenoprofen was shown to selectively activate Gs protein signaling while the (S,R′)-isomer activated both Gi and Gs protein. The overall data demonstrate that the chirality at the two chiral centers of the fenoprofen molecule influences the magnitude of binding affinity, thermodynamics of local interactions within the binding site, and the global mechanism of β2-adrenergic receptor activation. The marketed product is the racemic mixture of the (R,R′;S,S′)-fenoterol (Figure 4) which was selected after initial development studies, demonstrating that this racemic mixture was 9–20-fold more active than the (R,S′;S,R′)-fenoterol racemate [20].
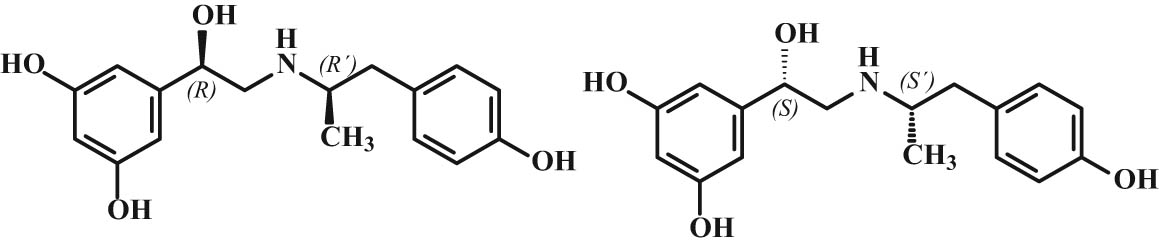
Chemical structures of more active isomers of fenoterol.
2 Stereoselective pharmacological effects of β2-agonists
β2-Agonists have been used in treating asthma and obstructive pulmonary disease (COPD) for a long time. Long-acting β2-agonists are effective in the prevention of COPD and bronchial asthma exacerbation induced by a viral infection, including infection with coronaviruses (HCoV-229E). Recently, it has been found that formoterol inhibited HCoV-229E replication partly by inhibiting receptor expression and/or endosomal function, and it could modulate infection-induced inflammation in the airway [21].
Based on more recent indications, β2-agonists are also used for alleviating symptoms in amyotrophic lateral sclerosis [22].
They were mostly used in the form of racemates with a 50:50 ratio of (R)- and (S)-isomer. Their therapeutical effects were described in various overviews and publications [3,4,5,6,7,8,23,24,25,26,27,28,29,30,31,32,33,34]. Due to the chirality of β2-agonists, the pharmacological properties of their single stereoisomers were also studied. Some of them are already in clinical practice as pure enantiomers (levalbuterol, arformoterol, indacaterol, olodaterol, and vilanterol). The bronchodilatory activity of most β2-agonists is higher in the (R)-isomer. Studies made on different animal models confirmed significantly higher bronchodilatory and antidepressant activity of (R)-enantiomer of clenbuterol, albuterol, formoterol, and terbutaline [23]. In some cases in treating asthma and obstructive pulmonary disease, only this stereoisomer is used (levalbuterol – (R)-form of albuterol, arformoterol –(R,R)-form of formoterol) [23,24,25].
Albuterol also known as salbutamol is largely used as bronchodilators in the management of asthma, both in control of acute symptomatic attacks and chronic, long-term prevention and management. Levalbuterol (levosalbutamol) is the (R)-isomer (Figure 5) of albuterol. Its β-adrenoreceptive activities were about 80 times more efficient than activities of (S)-enantiomer [26]. Compared with the racemate, it has a much higher effect on respiratory diseases. Its preferential use has not even been altered by several preclinical and in vitro studies in adults and children with asthma, which confirmed that the (S)-enantiomer is not inert but has a pro-inflammatory effect [27]. The racemate of albuterol is still being widely used in comparison with levalbuterol to treat acute asthma exacerbations. Levalbuterol is recommended for severe asthma diseases, although many works indicate conflicting opinions concerning its use [28,29,30].
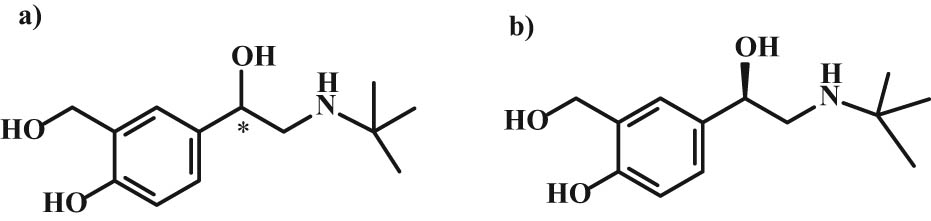
Chemical structures of (a) (RS)-(±)-albuterol (salbutamol) and (b) (R)-(−)-albuterol (salbutamol).
In formoterol that has two stereocenters, effects of the enantiomers of formoterol on inherent and induced tone in isolated human bronchi with that on guinea-pig trachea in vitro were compared. In both human bronchus and guinea-pig trachea, (S,S)-formoterol was more than 1,000 times less potent than (R,R)-formoterol. Thus, the relaxing effect of formoterol in human airways and guinea-pig trachea was shown to lie with the (R,R)-enantiomer [31].
The (R,R)-isomer of arformoterol (Figure 6), indicating a high tolerance, has recently been introduced into the clinical practice [32].
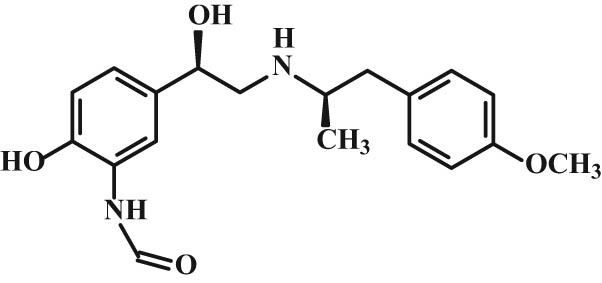
Structure of arformoterol.
Salmeterol (Figure 7) belongs to the long-acting β2-agonists used in clinical practice as a racemate [33]. Clinical studies evaluating the efficacy of this β2-agonist in the maintenance treatment of asthma demonstrated that salmeterol was more effective than albuterol (short-acting β2-agonist) in improving pulmonary function and controlling asthma symptoms [34]. For salmeterol, β2-adrenoceptor pharmacological activity (B2ADR) resides in (R)-salmeterol, while (S)-salmeterol is generally considered to be inert at this target. The difference in B2ADR activity between (R)- and (S)-salmeterol has been reported to be approximately 40-fold [35] less than for other β2-agonists.
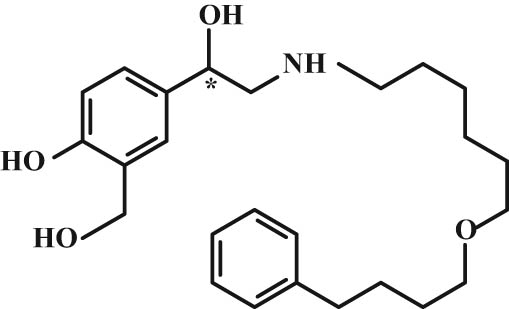
Structure of salmeterol.
Isoxsuprine has three stereogenic centers (Figure 8), and theoretically, eight stereoisomers can be created. It stimulates β-receptors, and it is known as a vasodilator and a tocolytic with anti-inflammatory and hemorheological properties [36,37]. In the in vivo model of stroke, its neuroprotective effect has also been shown [38]. Studies of bindings confirmed that (−)-isoxsuprine has higher activity than (+)-isoxsuprine in the binding on the α-receptor, while the (+) isomer has an affinity similar to the β-blocker propranolol [39].
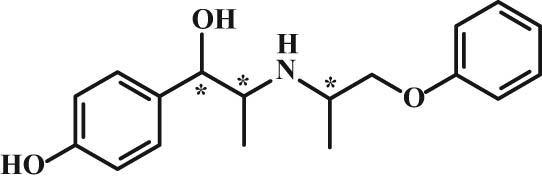
Chemical structure of isoxsuprine.
The long-acting bambuterol is used to treat asthma (Figure 9). Structurally, it is a biscarbamate which, as the form of a prodrug, can be hydrolyzed by butyrylcholinesterase and transitioned to an active form of terbutaline. The study found that (R)-bambuterol inhibited butyrylcholinesterase 5-fold faster than the (S)-isomer. The (R)-isomer is more active in response to histamine-induced asthma. Both isomers increase the heart rate [40,41,42].
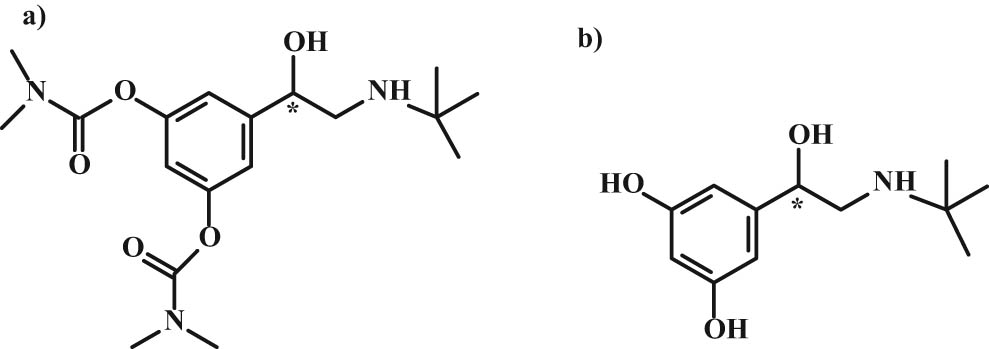
Structures of (a) bambuterol and its active form (b) terbutaline.
Many of the β2-agonists show anabolic activity, and their use is banned in certain types of sports [43]. This may concern clenbuterol (Figure 10), for which the anabolic activity of individual (R)- and (S)-stereoisomers was monitored. The activity was monitored by measuring the tissue mass and determining the protein content. The results showed that both stereoisomers had the same anabolic activity, but (S)-(+)-clenbuterol exhibited a significant increase in the mass of the heart muscle [44].
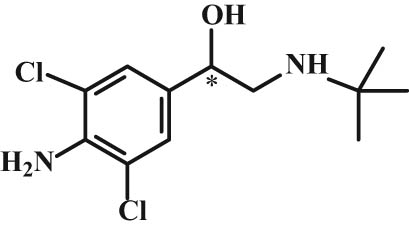
Chemical structure of clenbuterol.
Trantinterol (also known as SPFF) and mabuterol (Figure 11) were prepared as potent long-acting bronchodilators with relatively higher β2-adrenoceptor selectivity. The affinity of (−)-enantiomer of trantinterol to β2-adrenoceptor was 6- and 164-fold greater than that of (±)- and (+)-trantinterol, respectively. In addition, the isomeric difference of overall selectivity between (−)-enantiomer and (+)-enantiomer was 10.7-fold for lung versus atria [45].
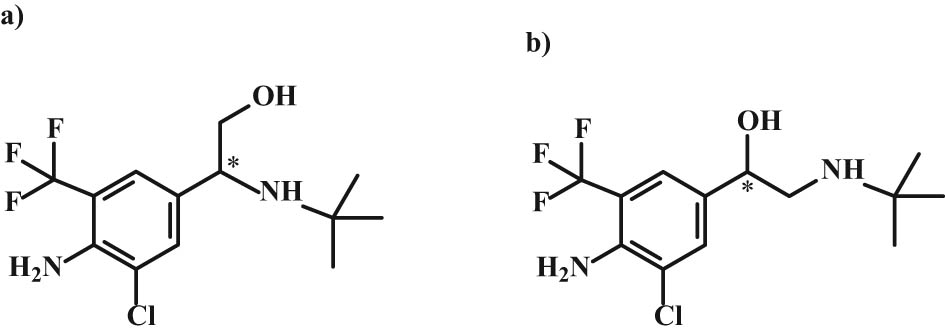
Chemical structures of (a) trantinterol and (b) mabuterol.
Many of the β2-agonists have found use in tocolytic therapy, particularly in the risk of premature birth or in miscarriage, due to the higher selectivity for inhibition of uterus contractions [46]. Ritodrine is a short-acting β2-agonist with a hydroxyphenol group on a basic nitrogen forming a hydrogen bond. Since ritodrine has a bulky N-substituent, it has high β2 selectivity. It is used as a tocolytic drug [47,48]. In animal experiments, the (−)-ritodrine showed 40 times higher contractions of the uterus than the (+)-enantiomer [49]. In terms of stereochemistry, diastereoisomers of ritodrine in pregnant women were studied, and the duration of pregnancy was compared in women expecting one child with women expecting twins. The differences were observed in the serum concentration of (−) and (+)-ritodrine [50].
Indacaterol [51,52,53] and carmoterol, containing in lipophilic part a carbostyril skeleton, are ultra-long-acting β2-agonists (Figure 12) that are well tolerated. They have a rapid onset of action, and their long duration of action allow them to be administered once a day. Indacaterol is used in therapeutic practice as pure (R)-enantiomer and carmoterol as the (R,R)-form [54,55].
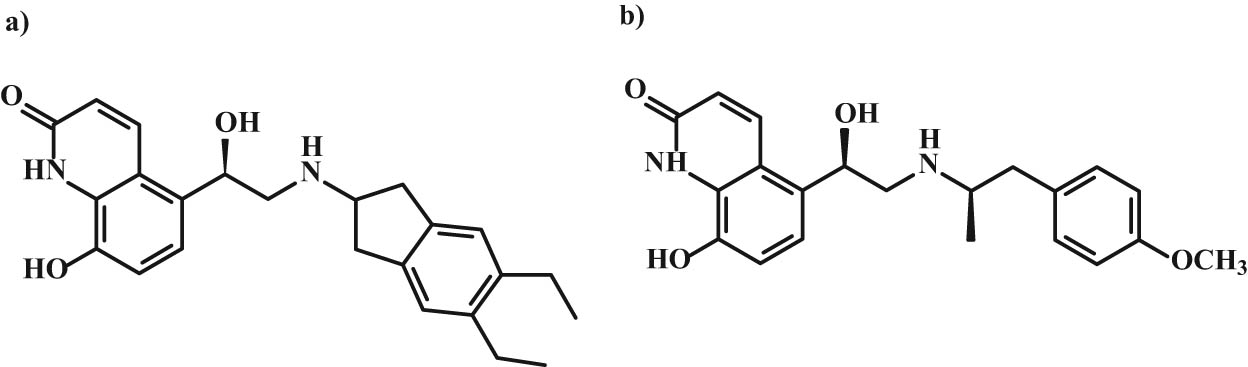
Chemical structure of (a) indacaterol and (b) carmoterol.
Abediterol (Figure 13) is a novel long-acting β2-adrenoceptor agonist currently in development for once-daily combination maintenance therapy of asthma and chronic obstructive pulmonary disease (COPD). In the preclinical stage, abediterol exhibited (5–20)-fold higher activity when compared with other bronchodilators [56].
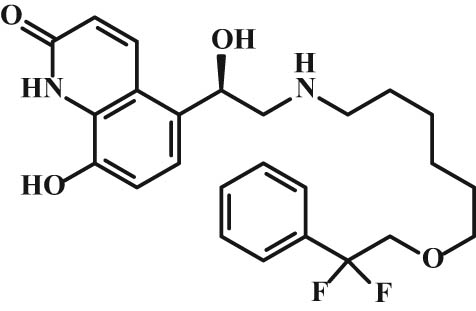
Chemical structure of abediterol.
In the case of vilanterol, the basic nitrogen contains a bulky chain with substituted phenyl and two ether oxygens, something that differentiates it from salmeterol [57]. This group also includes olodaterol with the dihydrobenzoxazine skeleton in the lipophilic and hydrophilic parts of the molecule with substituted hydrogen in 2-methylpropan-2-yl methoxyphenyl [56]. Vilanterol and olodaterol have 1 stereocenter with the (R)-configuration in their structures (Figure 14).
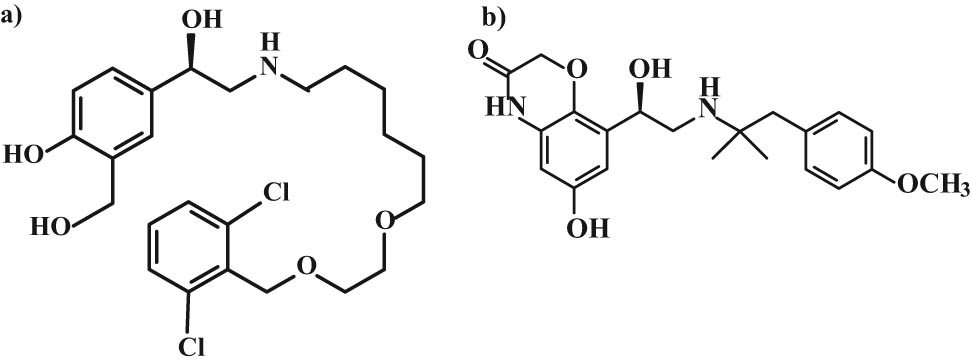
Chemical structure of (a) vilanterol and (b) olodaterol.
3 Stereoselective pharmacokinetics of β2-agonists
Although β2-agonists have been used in clinical practice as racemic mixtures for a long period, the data on stereoselective pharmacokinetics are incomplete for the β2-agonists.
β-Agonists are absorbed from the gastrointestinal tract by diffusion in which individual enantiomers behave differently. The plasma concentrations of the enantiomers of anti-asthma drugs may differ as a reflection of stereoselectivity in clearance, the volume of distribution, and the route of administration [58].
Enantioselectivity of salbutamol in plasma was also evident in experiments on horse models, where the values for the (S):(R) ratio were 1.25 and 1.14. Enantioselectivity was not observed in pulmonary lining fluid and the central lungs [59].
In the case of terbutaline, different bioavailability was detected [60]. In human studies, the bioavailability calculated from the plasma values was 7.5% for the (R)-(+)-terbutaline and 14.8% for the (S)-(−)-terbutaline. These differences indicate differences in absorption for both enantiomers of terbutaline and their first-pass metabolism. Bioavailability of the racemate was similar to the (S)-(−)-enantiomer [61,62,63].
The distribution of β2-agonists is closely related to their physical and chemical properties. For hydrophilic compounds, their binding to plasma proteins is negligible. For lipophilic β2-agonists, such as clenbuterol, the binding on plasma proteins is 97% [64].
Indacaterol is relatively highly bound to plasma proteins regardless of concentration. No stereoselective binding on plasma proteins has been reported with albuterol [65].
Interesting results were found by Boulton [66] in the study of transplacental albuterol distribution after administration to women before the caesarean section. The (R):(S) albuterol ratio in cord plasma was significantly lower than in maternal plasma. The distribution of (S)/(R)-enantiomers of albuterol in plasma and skeletal and cardiac muscle was compared in animal models using more recent analytical methods (LC-MS/MS) [67]. The results confirmed higher distribution of the (R)-enantiomer in individual muscles when compared with the plasma, which is of importance for anti-doping assessments. Jacobson et al. [68] observed an enantioselective distribution of salmeterol in the central part of the lungs. (R)-enantiomer exhibited about 30% higher distribution than (S)-enantiomer.
Metabolism of β2-agonists induces sulfation and glucuronidation, which differs from the β2-agonist type, e.g., albuterol and salbutamol are biotransformed by sulfation into an ineffective metabolite (Figure 15), whereas fenoterol induces glucuronidation in addition to sulfation, and the case of formoterol, it is only glucuronidation. Glucuronidation of formoterol occurs at the phenolic position as well as the formation of a benzyl glucuronide (Figure 16) [69,70,71].
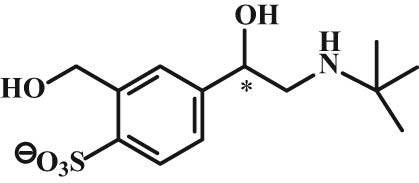
Structure of salbutamol sulfate.
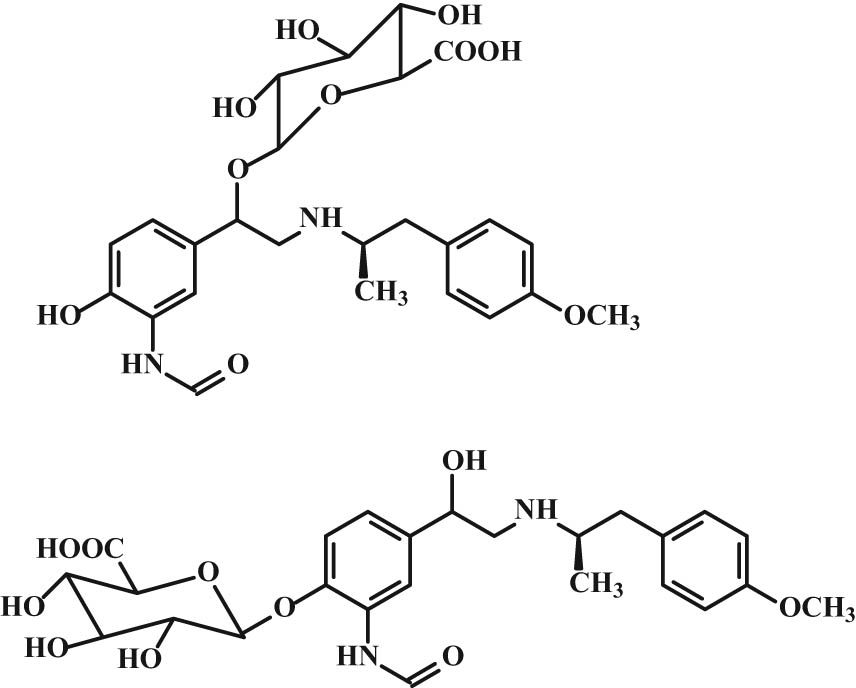
Structure of formoterol glucuronides.
The racemate of salbutamol undergoes stereoselective sulfation by sulfotransferases mainly in the intestine and liver, and it has been found that (S)-salbutamol is retained in the body longer and reaches higher plasma levels than (R)-salbutamol [71,72]. (R)-Salbutamol is metabolized up to 12 times faster than (S)-salbutamol. Following oral or inhaled administration of enantiomerically pure salbutamol, a small quantity (6%) is transferred to the second enantiomer, probably through acid-catalyzed racemization in the stomach [73]. Indacaterol also undergoes glucuronidation, where the most common metabolites are 8-O-a-N-glucuronides [74].
Some β2-agonists reported enantioselectivity in renal clearance. Following intravenous administration of albuterol in the form of the racemate and individual enantiomers, clearance exceeded creatine clearance. Renal clearance of (R)-albuterol is 2- to 3-fold higher than (S)-albuterol [75]. Differences in renal clearance were studied also in the case of terbutaline. The statistically significant value for intravenous and oral administration of (+)-terbutaline was slightly higher than for (−)-terbutaline [63].
4 Stereoselective analysis of β2-agonists
The investigation of stereoselectivity in action and disposition of chiral drugs together with the preparation of pure enantiomer calls for efficient stereoselective analytical methods.
Methods for separation and quantification of the isomers in biological samples are also desirable for pharmacological studies, pharmacokinetic studies, and therapeutic drug monitoring [76,77,78]. Recently, the enantioseparation of chiral pharmaceuticals in the environmental matrices becomes more important. Concerning biodegradation and environmental fate, the recognition of enantioselectivity is essential to provide a more realistic risk assessment of chiral compounds [79].
Several different technologies have been reported for the enantioseparation of β2-agonists enantiomers, including predominantly liquid chromatography (LC, HPLC) using chiral stationary phase (CSP) [80] or derivatizing reagents [81] as well as GC, TLC, and capillary electrophoresis (CE) using cyclodextrins or their derivatives as chiral selectors [63,82].
4.1 Stereoselective liquid chromatographic analysis of β2-agonists
4.1.1 Direct chiral chromatographic separation
Generally, chiral separation can be carried out by (a) direct separation of the enantiomers using CSPs or chiral mobile additives or (b) indirect separation by conversion of the enantiomers to diastereoisomers using a chiral reagent followed by chromatography on a chiral column [82,83].
Direct chromatographic enantioseparation on CSP has appeared as the most effective and convenient way of determining the enantiomeric composition of chiral drugs. Different structural types of CSPs including the π-donor/acceptor phases (Pirkle phase) [84], derivatized polysaccharides [85], proteins [86], β-cyclodextrin [87], and macrocyclic antibiotics [88] were utilized for the enantiomeric separation of β2-agonists (Table 2).
Direct chromatographic enantioseparation of β-agonist
| Methods | CSP | Analyte | Mobile phase | Matrix | Reference |
|---|---|---|---|---|---|
| Macrocyclic antibiotic-based CSP | |||||
| LC-MS/MS (ESI) | Chirobiotic T | Fenoterol | Methanol with 0.2% acetic acid and 0.01% triethylamine | Human plasma | 80 |
| LC-MS/MS (ESI) | Chirobiotic T | Bambuterol | Methanol–water (20:80, v/v) with 20 mM ammonium acetate and 0.005% formic acid | Human plasma | 92 |
| LC-MS/MS (APCI) | Chirobiotic T | Bambuterol, terbutaline | 20 mM ammonium acetate–methanol (10:90, v/v) | Rat plasma | 93 |
| LC-MS/MS (APCI) | Chirobiotic T | Terbutaline | 0.05% ammonium trifluoroacetate in methanol | Human plasma | 94 |
| LC-MS/MS (ESI) | Chirobiotic T | Clenbuterol | Methanol–water (95:5, v/v) with 2.5 mM ammonium formate | Rat plasma, urine and bile | 95 |
| LC-MS/MS (ESI) | Chirobiotic T | Salbutamol | 5 mM ammonium formate in methanol | Porcine urine | 96 |
| LC-MS/MS (ESI) | Chirobiotic T | Salbutamol, sulfate metabolite | Methanol–acetic acid–ammonia (994:5:1,v/v/v) | Human plasma and urine | 97 |
| LC-MS/MS (ESI) | Chirobiotic T | Salbutamol | Methanol–0.02% formic acid (60:40, v/v) with 0.1% ammonium formate in methanol | Dog plasma | 98 |
| LC-FLD (λex = 230, λem = 310) | Chirobiotic T | Salbutamol | Methanol–acetonitrile (40:60, v/v) with 0.3% glacial acetic and 0.2% diethylamine | Human plasma | 99 |
| LC-UV (λ = 227 nm) | Chirobiotic T, chirobiotic V | Salbutamol | Methanol–20 mM ammonium acetate buffer (pH 4) (98:2, v/v) | Waste water | 100 |
| LC-MS/MS (ESI) | Chirobiotic V | Salbutamol | Methanol–water with 20 mM ammonium acetate buffer (pH 4) (90:10, v/v) | Waste water | 101 |
| LC-UV (λ = 254 nm) | Chirobiotic V | Mabuterol | Methanol with 0.01% acetic acid and 0.01 % triethylamine | Rat plasma | 102 |
| LC-UV (λ = 280 nm) | Chirobiotic V | Salbutamol, terbutaline | 10 mM ammonium nitrate in ethanol, (pH 5.1) | Water | 103 |
| LC-MS/MS (ESI) | Chirobiotic V | Trantinterol | Acetonitrile–methanol (60:40, v/v) with 0.05% ammonia and 0.1% acetic acid | Rat plasma | 104 |
| LC-MS/MS (ESI) | Chirobiotic V | Trantinterol | Acetonitrile–methanol (60:40, v/v) with 0.01% ammonia and 0.02% acetic acid | Human plasma | 105 |
| Polysaccharide-based CSP | |||||
| LC-UV (λ = 254 nm) | Chiralpak AS-H | Trantinterol | Hexane–ethanol (98:2, v/v) with 0.1% triethylamine | Water | 106 |
| LC-UV (λ = 254 nm) | Chiralpak AD, Chiralcel OD Chiralcel OJ | Isoxsuprine | Hexane–propan-2-ol (80:20, v/v) with 0.1% triethylamine | Water | 107 |
| LC-UV (λ = 220 nm, λ = 225 nm) | Chiralpak AD Chiralcel OD-RH | Bambuterol salbutamol | Hexane–propan-2-ol (70:30, v/v) with 0.1% diethylamine | Water | 108 |
| LC-UV (λ = 230 nm) | Chiralpak AD | Fenoterol | Hexane–propan-2-ol (88:12, v/v) with 0.1% triethylamine, | Water | 109 |
| LC-UV (λ = 225 nm) | Chiralpak AD Chiralpak IA | Bambuterol | Hexane–propan-2-ol (100:30, v/v) with 0.1% diethylamine | Water | 110 |
| Protein-based CSP | |||||
| LC-UV (λ = 276 nm) | Chiral-AGP | Salbutamol | 0.01 M phosphate buffer, 0.13%, v/v, propan-2-ol, (pH 7.5) | Spiked plasma | 84 |
| LC- ECD (300 and 630 mV) | Chiral-AGP | Formoterol, glucuronide conjugate | 1.5% propan-2-ol, 50 μM EDTA-Na, (pH 6.8) | Urine | 112 |
| LC-ECD (700 mV) | Chiral-CBH | Salmeterol, α-hydroxysalmeterol | 5% propan-2-ol in 25 mM sodium phosphate buffer (pH 6.0), 50 mM EDTA-Na | Human liver microsomes | 113 |
| Pirkle CSP | |||||
| LC-UV (λ = 254 nm) | Chirex 3022 | Clenbuterol | Hexane-1,2-dichloroethane-ethanol-trifluoroacetic acid (80:10:10 v/v/v). | Spiked plasma, urine | 111 |
| LC-UV (λ = 276 nm) | Chirex 3022 | Salbutamol | Hexane-1,2-dichloroethane–methanol with 0.67% trifluoroacetic acid (160:93:17 v/v/v); | Spiked plasma | 84 |
APCI, atmospheric pressure chemical ionization; CSP, chiral stationary phase; ESI, electrospray ionization; ECD, electrochemical detection; FLD, fluorescence detection; UV, ultraviolet detection.
The following molecular interactions with CSP are supposed to play a role in chiral discrimination of enantiomers: H-bonding, π–π interaction, hydrophilic, dipole–dipole, and steric interactions [89]. The possible interactions between analytes depend on the type of CSP and mobile phase selection, which determines that enantiomers fit into the three-dimensional structure of sorbent [90].
In earlier applications of HPLC stereoisomers, the analysis was performed using ultraviolet (UV) and fluorescence detection (FLD). Currently, the liquid chromatography and mass spectrometry (LC-MS, LC-MS/MS) applications are utilized. Using tandem mass spectrometry assays allows for increasing the sensitivity without losing enantioselectivity [91]. Furthermore, HPLC combined with tandem mass spectrometry is particularly attractive for the simultaneous quantification of drug molecules and their metabolites in biological matrices.
Several papers have recently reported direct liquid chromatography enantioseparation methods for β2-agonists in biological fluids. The majority of enantioseparation was performed on CSP based on macrocyclic antibiotics (teicoplanin and vancomycin).
The fenoterol enantiomers were determined in plasma using a sensitive chiral LC-MS/MS method [80]. Enantiomers of fenoterol were separated (Rs = 1.4) on Astec Chirobiotic T analytical column containing teicoplanin as a chiral selector. The mobile phase was methanol containing 0.2% acetic acid and 0.01% triethylamine. The method was validated and applied to study the bioavailability of fenoterol after oral administration of the single enantiomer formulation and after the administration of the racemic formulation. The results showed a potential pre-systematic enantioselective interaction, in which (S,S′)-fenoterol reduces the sulfation of the active (R,R′)-fenoterol.
The LC-MS/MS method using Astec Chirobiotic T-chiral column was developed for the simultaneous determination of bambuterol (bis-dimethylcarbamate prodrug of terbutaline), its metabolite–monocarbamate bambuterol and terbutaline enantiomers in human plasma. Enantioseparation was performed under isocratic elution with a mobile phase consisting of methanol and water with the addition of 20 mM ammonium acetate and 0.005% (v/v) formic acid. The method was successfully applied to an enantioselective pharmacokinetic study of racemic bambuterol [92].
The similar LC-MS/MS method using a teicoplanin-based column for the analysis of bambuterol, and its active metabolite terbutaline enantiomers in plasma samples was described by Luo et al. and Xia et al. [93,94].
The chiral chromatography assay (LC-MS/MS with Chirobiotic column) was utilized for the study of enantioselectivity in the disposition of clenbuterol following the administration of clenbuterol racemate to rats. The results indicated that there are differences in the distribution and excretion of the clenbuterol enantiomers, and these may be predominantly due to enantioselective protein binding [95].
The enantiomers of salbutamol in porcine urine samples were assayed using the Chirobiotic T column with a mobile phase constituted of 5 mM ammonium formate in methanol. The sequential solid-phase extraction sample clean up method for the determination of salbutamol in porcine urine was developed. Enantiomers of salbutamol were detected and quantified by the LC-ESI-MS/MS method [96].
Teicoplanin as a chiral selector served for the determination of the enantiomers of salbutamol and its 4-O-sulphate metabolite in human plasma and urine [97].
Wu et al. [98] presented an automated chiral LC-MS/MS method for the determination of salbutamol in dog plasma. The method employed on-line sample extraction using turbulent flow chromatography coupled to a Chirobiotic T column with a polar organic mobile phase – methanol, 0.02% formic acid, and 0.1% ammonium formate.
Detection and determination of low levels of salbutamol enantiomers in dog plasma were also achieved on the teicoplanin CSP using HPLC analysis with fluorescence detection [99].
Chiral liquid chromatography methods with teicoplanin and vancomycin CSP were applied for the analysis of enantiomers of salbutamol and other pharmaceuticals in environmental water and wastewater [100,101].
The vancomycin-based CSP (Chirobiotic V column) with a mobile phase containing methanol with 0.01% acetic acid and 0.01% triethylamine, was used for enantiomeric HPLC separation of mabuterol during pharmacokinetics study in rats. Results indicated that enantioselective pharmacokinetics between mabuterol enantiomers occur within the metabolism phase [102].
The effect of chromatographic conditions on the chiral separation of terbutaline and salbutamol on the Chirobiotic V column was investigated. The salt concentration in the mobile phase and pH value was found to be the most important factors affecting separation. In polar mobile phase containing ammonium nitrate in 100% methanol, pH = 5.1 value was found to give the best separation [103].
Enantioseparation of a new β2-agonist trantinterol was achieved on the Chirobiotic V column with a mobile phase consisting of acetonitrile–methanol (60:40, v/v) containing 0.05% ammonia and 0.1% acetic acid [104]. The LC-MS/MS method based on this separation condition was applied to a study of stereoselective pharmacokinetics of trantinterol in rats and humans [105].
The enantioseparations of different derivatives of the same drug were also investigated on amylose-based CSP – Chiralpak AS-H column – amylose [tris(S)-α-methylbenzylcarbamate]. Trantinterol enantiomers were resolved (Rs = 2.73) within 14 min using hexane–ethanol (98:2, v/v) with 0.1% triethylamine as the mobile phase. The method was applied for the enantiomeric impurity determination of (−)-trantinterol bulk samples [106].
HPLC enantioseparation of isoxsuprine was studied on three different polysaccharide type CSPs: Chiralcel OJ –cellulose tris(4-methylbenzoate), Chiralcel OD – cellulose tris(3,5-dimethylphenylcarbamate), and Chiralpak AD – amylose tris(3,5-dimethylphenylcarbamate). The chromatographic analyses were performed in the normal phase with hexane–ethanol–triethylamine. Both of cellulose-based CSPs did not result in the acceptable enantioseparation. Baseline enantioseparation of isoxsuprine was obtained on amylose-based CSPs in the presence of triethylamine in the mobile phase [107].
Chiralpak AD and Chiralcel OD columns were also used for the enantioseparation of bambuterol and salbutamol [108] and for the separation of enantiomers of fenoterol [109] – β2-agonist with two chiral centers.
Gazić et al. [110] attempted the separation of bambuterol enantiomers on a new generation of chiral polysaccharide stationary phase Chiralpak IA, a 3,5-dimethylphenylcarbamate derivative of amylose immobilized onto silica, which is the immobilized version of Chiralpak AD. An advantage of Chiralpak IA over Chiralpak AD is that immobilization allows the use of the most common organic solvents. However, Chiralpak IA showed a much weaker enantioresolution with the mobile phase already determined as optimal for bambuterol on Chiralpak AD (hexane:propan-2-ol, 100:30 with 0.1% diethylamine).
The urea type CSP made of (S)-indoline-2-carboxylic acid and (R)-(α-naphthyl)ethylamine known as Chirex 3022 column were used for the enantioseparation of clenbuterol [111] in the normal phase condition.
The resolution of salbutamol and formoterol enantiomers was achieved also on the α1-glycoprotein chiral stationary phase (AGP) under the reverse phase condition [84,112]. Protein-based chiral column with cellobiohydrolase as a chiral selector (CBH) served for the simultaneous determination of salmeterol and its main human metabolite α-hydroxysalmeterol. Two-pair enantiomers were detected by electrochemical detectors, and the method was applied to a study of human hepatic metabolism of salmeterol in vitro [113].
4.1.2 Indirect chiral chromatographic separation
The conversion of enantiomers into diastereoisomers using chiral derivatizing agents allows the separation on a nonchiral column. This mode is sometimes simpler, less expensive than the use of CSP, and often results in better resolution, because of the more sensitive detector response. On the other hand, this method requires high optical purity derivatizing reagents and is sometimes time-consuming [114]. Various derivatizing reagents were used for the enantioseparation of β2-agonists.
Enantioseparation of fenoterol was performed by indirect separation on an octadecyl reverse phase column after chiral derivatization by (S)-(+)-1-naphthyl)ethyl isocyanate. However, better sensitivity was achieved by achiral derivatization by 1-naphtyl isocyanate followed by chiral separation on a Chiracel column. Detection was carried out fluorometrically with a detection limit in the low nanograms per milliliter. The method was adapted to the determination of fenoterol enantiomers in hat heart perfusates [82].
(S)-(−)-α-Methylbenzyl isocyanate was applied for the resolution of salbutamol enantiomers in human urine. After solid-phase extraction, the diastereoisomeric derivatives were resolved (Rs = 1.6) on the C18 column using 47% methanol as a mobile phase with fluorescence detection [115]. The same derivatizing reagents were utilized for the determination of terbutaline enantiomers by reversed-phase HPLC method (Rs = 1.4) [81].
One of the later synthesized isocyanate derivatizing agents, 2,3,4,6-tetra-O-acetyl-β-d-glucopyranosyl isothiocyanate was used for stereoanalysis of salbutamol in human urine. The salbutamol diastereoisomeric derivatives were resolved (Rs = 1.8) on a C18 column using 35% acetonitrile in 0.05 M ammonium acetate buffer as a mobile phase with electrochemical detection [116].
Chiral derivatizing reagents prepared by substitution of the fluorine atoms in 1,5-difluoro-2,4-dinitrobenzene (DFDNB) with three amino acids: l-alanine, l-valine, and (S)-benzyl-l-cysteine cysteine were used for enantioresolution of racemic β2-agonist isoxsuprine on reversed-phase HPLC and reversed-phase thin-layer chromatography [117].
Enantioseparation of salbutamol was also achieved by both TLC and RPHPLC via an indirect approach. A new chiral reagent, (S)-naproxen benzotriazole ester, was used to synthesize diastereomeric derivatives of salbutamol under microwave irradiation. Diastereoisomers were separated by both TLC and RPHPLC methods [118].
4.2 Stereoselective gas chromatographic (GC) analysis of β2-agonists
Although liquid chromatography is the most frequently used method for the enantioseparation of β2-agonists, other techniques were also occasionally described for that purpose.
Separation of enantiomers of β2-agonists by GC requires derivatization with various derivatizing agents, e.g., trimethylsilylation associated with acylation (bambuterol, salbutamol, and terbutaline) or formation of cyclic methylboronates (bambuterol, clenbuterol, formoterol, salbutamol, and salmeterol) [119,120].
Phosgene has been used to form cyclic derivatives of chiral diols, α-hydroxy acids, N-methylamino acids, and other di- and trifunctional compounds – terbutaline, salbutamol, and orciprenaline; the enantiomers were then separated by gas chromatography on the chiral polysiloxane phase XE-60 l-valine-(R)-α-phenylethylamide. In this way, phosgene can supplement the isocyanates as a versatile reagent for enantiomer separation. Factor of separation was 1.03 and (−)-enantiomer eluated before (+)-enantiomer [121]. l-α-Chloroisovaleryl chloride was used for other aminoalcohols to prepare diastereoisomers, and the samples were then dissolved in dichloromethane and N-methyl-N-trimethylsilyltrifluoro-acetamide [122]. These methods require high chemical and optical purity of the derivatizing agent and are therefore poorly used [123,124,125], and GC is currently being replaced by other separation techniques.
4.3 Stereoselective thin layer chromatography (TLC) of β2-agonists
TLC is one of the methods by which it is also possible to separate individual enantiomers in the group of β2-agonists. It is possible to use a direct and indirect enantioseparation method.
The individual methods applied to β2-agonists were likewise used with β-adrenolytic drugs. TLC served for the resolution of enantiomers of isoxsuprine using thin silica gel layers impregnated with l-glutamic acid. The limit of detection of each enantiomer was 11–13 ng/mL [117].
Enantiomers of salbutamol were resolved by adopting different modes of loading or impregnating the Cu(ii) complexes of l-proline, l-phenylalanine, l-histidine, N,N-dimethyl-l-phenylalanine, and l-tryptophan on commercial precoated normal phase plates. The detection limit was 0.18 mg for each enantiomer [126].
Direct enantiomeric resolution of salbutamol and five racemic β-adrenolytics has been achieved by thin-layer chromatography using bovine serum albumin (BSA) as a chiral additive in the stationary phase. The effect of variation in pH, temperature, amount of BSA as the additive, and composition of the mobile phase on the resolution was systematically studied [127].
4.4 Stereoselective CE method for determination of β2-agonists
Compared with HPLC, the CE analytical method is advantageous in which the chiral column is substituted by the chiral selector added to the base electrolyte using a small volume of the sample and buffer solution [128]. Recently, this method has been largely used for the enantioseparation of chiral compounds and β2-agonists. The most common chiral selectors for β2-agonists include various types of cyclodextrins (CDs) (Table 3).
Enantiomeric separation of β-agonists by CE methods
| Chiral selector | Analyte(s) | Buffer (pH) | Application | Reference |
|---|---|---|---|---|
| HE-β-CD | Clenbuterol | 200 mM phosphoric acid (3.3) | Human urine | 131 |
| HP-β-CD | Terbutaline | 0.1 M sodium phosphate (2.5, 3.5, 4.5, 5.5.) | 132 | |
| Clenbuterol | ||||
| Salbutamol | ||||
| Bambuterol | ||||
| α-CD, β-CD, HDMS-β-CD | Salbutamol | 40 mM Tris–base in water (2.5) | Tablets | 133 |
| CM-β-CD | Salbutamol | 25 mM acetate (5.0) | Syrups | 134 |
| Oral solution | ||||
| Tablets | ||||
| CM-β-CD | Clenbuterol | 50 mM monopotassium phosphate (3.5) | 135 | |
| CM-β-CD | Procaterol | 50 mM phosphoric acid (3.5) | 136 | |
| Clenbuterol | ||||
| Bambuterol | ||||
| Tulobuterol | ||||
| Salbutamol | ||||
| S-β-CD | Terbutaline | 40 mM monosodium phosphate (2.5) | 137 | |
| Orciprenaline | ||||
| S-β-CD | Terbutaline | 50 mM monosodium phosphate (2.5) | 138 | |
| Clenbuterol | ||||
| Salbutamol | ||||
| Dobutamine | ||||
| S-β-CD | Clenbuterol | 50 mM monosodium phosphate (2.4) | 139 | |
| Salbutamol | ||||
| Tulobuterol | ||||
| HDAS-β-CD | Salbutamol | 10 mM ammonium formate | Urine | 140 and 141 |
| Glu-β-CD | Terbutaline | 120 mM phosphate buffer (2.5-4.0) | 142 | |
| Clenbuterol | ||||
| α-CD, β-CD, γ-CD, CM-β-CD, HS-β-CD, SBE-β-CD, SU-β-CD, HDAS-β-CD, HDMS-β-CD, HS-β-CD, DM-β-CD, TM-β-CD | Isoxsuprine | 100 mM triethanolamine phosphate (3.0) | 107 | |
| Clarithromycin | Clenbuterol | 100 mM citric acid, 10 mM sodium hydroxide, 240–300 mM boric acid | Urine | 143 |
| Lactobionic acid/d-(+)-xylose–boric acid complex | Cycloclenbuterol | 14.4 mM triethylamine in methanol | 144 | |
| Clenbuterol | ||||
| Bambuterol | ||||
| Tulobuterol | ||||
| Terbutaline | ||||
| Clorprenaline |
CD, cyclodextrine; CM-β-CD, carboxymethyl-β-CD; DM-β-CD, dimethyl-β-CD; Glu-β-CD, glutamic acid-β-CD; HE-β-CD, hydroxyethyl-β-CD; HDAS-β-CD, heptakis(2,3-di-O-acetyl-6-O-sulfo)-β-CD; HDMS-β-CD, heptakis(2,6-O-methy-l6-O-sulfo)-β-CD; HP-β-CD, hydroxyethyl-β-CD; HS-β-CD, highly sulfated-β-CD; S-β-CD, sulfated-β-CD; SBE-β-CD, sulfobutyl-β-CD; TM-β-CD, trimethyl-β-CD.
CDs and their derivatives as chiral selectors are frequently used for enantioseparation due to their good solubility in aqueous buffers and wide availability [129].
Native β-cyclodextrin (β-CD) and its derivatives, namely, ethyl-β-CD, methyl-β-CD, hydroxypropyl-β-CD, and hydroxy-β-CD, as chiral selectors were used for simultaneous CE enantioseparation of trantinterol, mabuterol, clenbuterol, and bambuterol. Hydroxypropyl-β-CD was found to be the most effective complexing agent that allows excellent chiral resolution when compared with other CDs [130].
Hydroxy propyl-β-cyclodextrin was also used for stereoselective determination of clenbuterol in human urine [131] and for enantioresolution of salbutamol, terbutaline, salmeterol, and bambuterol [132].
Esquisabel et al. [133] developed the CE method using another cyclodextrin derivative – heptakis(2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD). The method was applied for the study of the release of salbutamol enantiomers from the matrix tablet.
Determination of salbutamol enantiomers in different pharmaceutical preparation – syrups, oral solutions, and tablets was performed by the CE method using carboxymethyl-β-cyclodextrin (CM-β-CD) in 25 mM acetate buffer (pH 5.0) [134].
The CM-β-CD as a chiral selector was utilized for the enantiomeric separation of clenbuterol [135] and the other five β2-agonists. The optimal separation was obtained using the following buffer: 50 mM phosphonic acid with 10 mM CD at pH 3.5 [136].
Sulfated β-cyclodextrin (HS-β-CD) was chosen for the enantioseparation of some β2-agonists (terbutalin, salbutamol, clenbuterol, dobutamine, and orciprenaline) [137,138] and for the quantitative determination of clenbuterol, salbutamol, and tulobuterol enantiomers by the CE method [139].
Other sulfo derivatives of β-cyclodextrin – heptakis(2,3-di-O-acetyl-6-O-sulfo)-β-cyclodextrin (HDAS-β-CD) were applied for the enantioselective determination of the low concentration of salbutamol in human urine by nonaqueous capillary electrophoresis (NACE) [140,141].
Satisfactory CE enantioseparation of terbutaline and clenbuterol was achieved also using glutamic acid-β-CD, which was prepared as a novel single-isomer cyclodextrin derivative [142].
Chankvetadze et al. [107] tested different native and derivatized CDs: α-CD, β-CD, γ-CD, CM-β-CD, HS-β-CD, SBE-β-CD, SU-β-CD, HDAS-β-CD, HDMS-β-CD, HS-β-CD, DM-β-CD, and TM-β-CD for the enantioseparation of isoxsuprine. β-CD exhibited the highest enantioseparation ability from native CDs and γ-CD, the lowest one. Neutral β-CD derivatives such as DM-β-CD and TM-β-CD also exhibited significant enantioseparation ability toward the enantiomers of isoxsuprine.
In nonaqueous CE, clarithromycin as a chiral selector was used for the enantioseparation of clenbuterol [143]. Two new chiral selectors, lactobionic acid–boric acid complex and D-(+)-xylose–boric acid complex, were synthesized in situ in nonaqueous background electrolytes containing methanol and triethylamine and were found to be applicable for the enantioseparation of several β2-agonists [144].
Capillary electrochromatography (CEC) has also become an important mode of CE for the separation of enantiomers. It is a hybrid of CE and HPLC and combines the high efficacy of CE and high selectivity of HPLC. Chiral separation by CEC can be performed in a wall-coated open tubular, packed, and monolithic capillary column. Enantioseparations of salbutamol were performed using a packed capillary column with CSP on-base teicoplanin [145] and vancomycin [146].
5 Stereoselective synthesis of β2-agonists
In addition to the enantiomeric separation, the individual enantiomers may also be prepared by stereoselective synthesis. For many β2-agonists, the α-chloroacetophenone or α-bromoacetophenone derivative is the base for this synthesis. Their preparation is based on the reaction with chloroacetyl chloride with a substituted aromatic nucleus, followed by stereogenic reduction with various reducing agents (Figure 17).
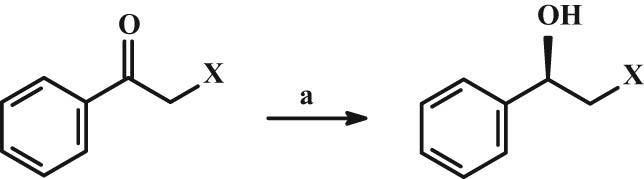
Scheme of stereogenic reduction of acetophenone derivatives X = Cl, Br. (a) BH3·S(CH3)2 in THF or NaBH4 or Rhodotorula rubra microbial culture; sodium lauryl sulfate.
In the case of salmeterol, the enantioselective reduction was performed with BH3·S(CH3)2 in THF under chiral Borodin catalysis [147] and reduction with NaBH4 [148].
For the enantioselective synthesis of (S)-salmeterol, azido ketones reduced with the aid of Pichia angusta yeast were prepared [149].
For the synthesis of (R)-(−)-salmeterol, Goswami et al. [150] used the stereoselective reduction of 4-benzyloxy-3-hydroxymethylbromoacetophenone with Rhodotorula rubra (a yeast microbe isolated from local brewery waste) in the presence of sodium lauryl sulfate, an anionic surfactant. Epoxide was regioselectively opened by nucleophilic attack with (R)-6-(4-phenylbutoxyhexyl)-1-amine in reasonable yield (94%). Catalytic hydrogenation cleaved the benzyl protecting group to give (R)-(−)-salmeterol (Figure 18).
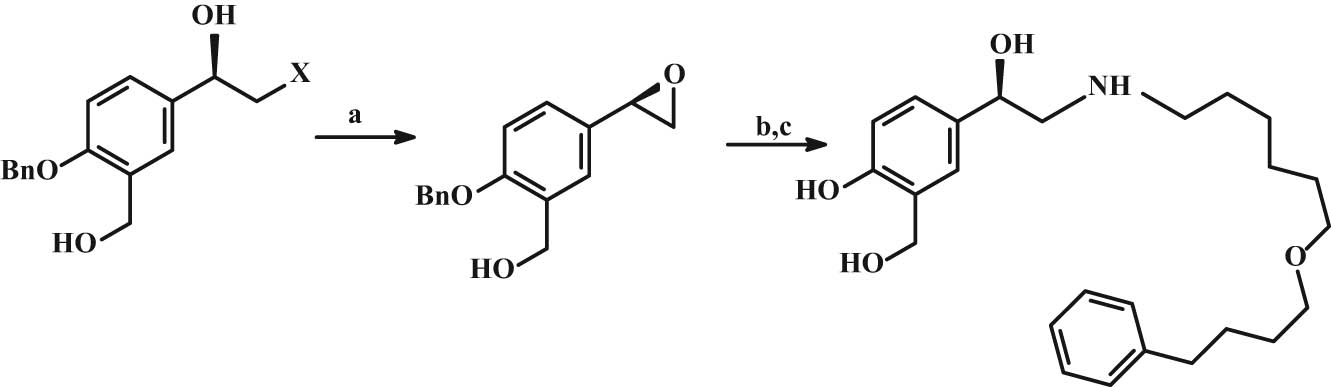
Synthesis of (R)-(−)-salmeterol. Reaction conditions: (a) K2CO3, THF/MeOH; (b) N,O-bis(trimethylsilyl)acetamide, DMSO, 3,4-dimethoxyphenylamine, (c) Pd–C/H. Note: Bn = benzyl.
Further, other enantioselective synthetic approaches of salmeterol are summarized in the published overviews [151,152].
In the case of bambuterol, to prepare the (R)-enantiomer, the (−)-DIP chloride was used for asymmetric reduction. To prepare the (S)-stereoisomer, its dextrorotatory form of (+)-DIP chloride was used. The optical purity was verified by HPLC. Optical properties were confirmed by optical rotation and the absolute configuration by circular dichroism [153].
Asami et al. [154] used whole cells of Williopsis californica JCM 3600 for asymmetric reduction of chloroketone in the synthesis (R)-bambuterolol.
In the work of Huang et al. [155], a 6-stage stereoselective synthesis of (R,R)-formoterol was developed starting from 4-hydroxy-3-nitroacetophenone (Figure 19). The key step is the reduction of the ketone group with Rh-complex as a hydrogenation catalyst.
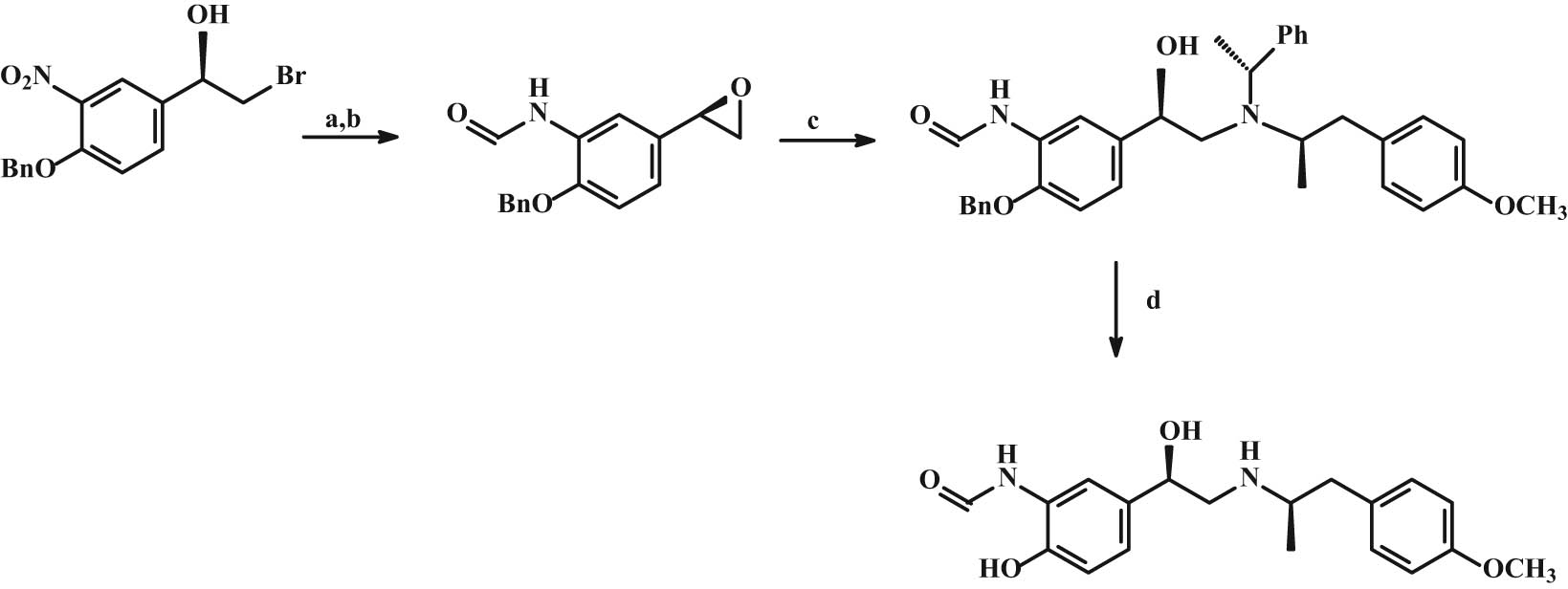
Synthesis of (R,R)-formoterol. Reaction conditions: (a) K2CO3,THF/MeOH; (b) HCOOH-Ac2O. (c) (R)-1-(4-Methoxyphenyl)-N-(R)-1-phenylethyl)propan-2-amine, 120°C; (d) Pt/C, H2, MeOH. Note: Ac = acetyl; Ph = phenyl; Bn = benzyl.
In the stereoselective synthesis of (R)-terbutaline, the starting O-protected cyanohydrins were used that are converted to amides via the Ritter N-tertiary butylation. In the next step, hydrogenation provided the amino alcohol, from which the (R)-terbutaline was obtained by the group deprotection [156].
A similar procedure was also used to prepare (R)-salbutamol. In the next step of its preparation, selective hydrogenation of protected cyanohydrin was used. A 2-aminoethanol derivative was prepared by NaBH4 hydrogenation of tert-butylamine iminoderivative. Desilylation was carried out with LiAlH4 but the hydrolytic cleavage of the protected acetal was accompanied by racemization [156].
6 Conclusion
In the presented overview, attention is focused on β2-agonists from the stereochemical point of view. Most of β2-agonists is mainly used in the treatment of asthma and obstructive pulmonary disease. In addition to the bronchodilatory activity, they also have a tocolytic and anabolic effect, and, from the more recent indications, they make it possible to monitor the amelioration of symptoms in amyotrophic lateral sclerosis. Many β2-agonists have different pharmacological and pharmacokinetic activity due to the different stereochemistry and, likewise, indicate varying degrees of toxicity. The overview focuses on β2-agonists with one stereocenter (albuterol, bambuterol, clenbuterol, salmeterol, and trantinterol) and with multiple stereocenters (formoterol, isoxsuprine, and ritodrine). From these β2-agonists, pure enantiomers of levalbuterol or levosalbutamol, such as the (R)-isomer of albuterol or salbutamol and the arformoterol (R,R)-isomer of formoterol, were introduced into the therapy by the way of the racemic switch process. More recently, long-acting drugs (indacaterol, carmoterol, abediterol, olodaterol, and vilanterol) have already been introduced into clinical practice as pure enantiomers.
Individual phases of pharmacokinetics enabled the observation of absorption, distribution, and metabolism of different β2-agonists. Nonetheless, many lack complete data on the pharmacokinetics of individual (R)- and (S)-stereoisomers.
To obtain enantioselective separation of racemates to individual enantiomers, chromatographic and electrophoretic methods were used. In the case of HPLC, various chiral phases were used for the direct methods based on CDs, macrocyclic antibiotics (vancomycin, teicoplanin) on substituted carbamates of cellulose and amylose, many of which are the basis of chiral selectors in the direct methods in CE. Besides the preparative chromatographic methods, the overview lists many methods of stereoselective synthesis enabling pure enantiomers of this group of drugs to be obtained.
Acknowledgments
This review utilizes the research results of the CEBV project, ITMS: 26240120034, and project APVV-0516-12.
Conflict of interest: Authors declare no conflict of interest.
References
[1] Mersmann HJ. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci. 1998;76:160–72.10.2527/1998.761160xSearch in Google Scholar
[2] Billington CK, Ojo OO, Penn RB, Ito S. cAMP regulation of airway smooth muscle function. Pulm Pharmacol Ther. 2013;26:112–20.10.1016/j.pupt.2012.05.007Search in Google Scholar
[3] Billington CK, Penn RB, Hall IP. β2 agonists. Handb Exp Pharmacol. 2017;237:23–40.10.1007/164_2016_64Search in Google Scholar
[4] Anderson GP. Interaction between corticosteroids and β-adrenergic agonists in astma disease induction, progression and exacerbation. Am J Respir Crit Care Med. 2000;161:188–96.10.1164/ajrccm.161.supplement_2.a1q4-9Search in Google Scholar
[5] Alangari AA. Corticosteroids in the treatment of acute asthma. Ann Thorac Med. 2014;9:187–92.10.4103/1817-1737.140120Search in Google Scholar
[6] Tashkin DP, Fabbri LM. Long-acting beta-agonists in the management of chronic obstructive pulmonary disease: current and future agents. Respir Res. 2010;11:149.10.1186/1465-9921-11-149Search in Google Scholar
[7] Fuso L, Mores N, Valente S, Malerba M, Montuschi P. Long-acting beta-agonists and their association with inhaled corticosteroids in COPD. Curr Med Chem. 2013;20:1477–95.10.2174/0929867311320120003Search in Google Scholar
[8] Waldeck B. β-Adrenoceptor agonists and astma – 100 years of development. Eur J Pharmacol. 2002;445:1–12.10.1016/S0014-2999(02)01728-4Search in Google Scholar
[9] Easson LH, Stedman E. Studies on the relationship between chemical constitution and physiological action: molecular dissymmetry and physiological activity. Biochem J. 1933;27:1257–66.10.1042/bj0271257Search in Google Scholar PubMed PubMed Central
[10] Mesecar AD, Koshland DE. A new model for protein stereospecificity. Nature. 2000;403:614–5.10.1038/35001144Search in Google Scholar PubMed
[11] Mesecar AD, Koshland Jr. DE. Sites of binding and orientation in a four-location model for protein stereospecificity. IUBMB Life. 2000;49:457–66.10.1080/152165400410326Search in Google Scholar PubMed
[12] Anderson GP, Linden A, Rabe KF. Why are longacting beta-adrenoceptor agonists long acting? Eur Respir J. 1994;7:569–78.10.1183/09031936.94.07030569Search in Google Scholar PubMed
[13] Freddolino PL, Kalani MY, Vaidehi N, Floriano WB, Hall SE, Trabanino RJ, et al. Predicted 3D structure for the human beta 2 adrenergic receptor and its binding site for agonists and antagonists. Proc Natl Acad Sci U S A. 2004;101:2736–41.10.1073/pnas.0308751101Search in Google Scholar PubMed PubMed Central
[14] Huber T, Menon S, Sakmar TP. Structural basis for ligand binding and specificity in adrenergic receptors: implications for GPCR-targeted drug discovery. Biochemistry. 2008;47:11013–23.10.1021/bi800891rSearch in Google Scholar PubMed
[15] Lemke TL, Williams DA, Roche VF, Zito SW. Foye's principles of medicinal chemistry. 7th ed. Baltimore, Philadelphia: Wolter Kluwer, Lippincott Williams & Wilkins; 2013.Search in Google Scholar
[16] Rasmussen SG, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–455.10.1038/nature10361Search in Google Scholar PubMed PubMed Central
[17] Bang I, Choi HJ. Structural features of β2 adrenergic receptor: crystal structures and beyond. Mol Cells. 2015;38:105–11.10.14348/molcells.2015.2301Search in Google Scholar PubMed PubMed Central
[18] Jozwiak K, Plazinska A, Toll L, Jimenez L, Woo AY, Xiao RP, et al. Effect of fenoterol stereochemistry on the β2 adrenergic receptor system: ligand-directed chiral recognition. Chirality. 2011;23(Suppl. 1):E1–6.10.1002/chir.20963Search in Google Scholar PubMed PubMed Central
[19] Plazinska A, Kolinski M, Wainer IW, Jozwiak K. Molecular interactions between fenoterol stereoisomers and derivatives and the β2-adrenergic receptor binding site studied by docking and molecular dynamics simulations. J Mol Model. 2013;19:4919–30.10.1007/s00894-013-1981-ySearch in Google Scholar PubMed PubMed Central
[20] Plazinska A, Plazinski W, Jozwiak K. Agonist binding by the β2-adrenergic receptor: an effect of receptor conformation on ligand association–dissociation characteristics. Eur Biophys J. 2015;44:149–63.10.1007/s00249-015-1010-4Search in Google Scholar PubMed PubMed Central
[21] Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58(3):155–68, 10.1016/j.resinv.2019.12.005.Search in Google Scholar PubMed PubMed Central
[22] Bartus RT, Bétourné A, Basile A, Peterson BL, Glass J, Boulis NM. β2-Adrenoceptor agonists as novel, safe and potentially effective therapies for Amyotrophic lateral sclerosis (ALS). Neurobiol Dis. 2016;85:11–24.10.1016/j.nbd.2015.10.006Search in Google Scholar PubMed
[23] Peepliwal AK, Bagade S, Bonde CH. A review: stereochemical consideration and eudismic ratio in chiral drug development. J Biomed Sci Res. 2010;2:29–45.Search in Google Scholar
[24] Handley DA, Anderson AJ, Koester J, Snider ME. New millennium bronchodilators for asthma: single-isomer beta agonists. Curr Opin Pulm Med. 2000;6:43–49.10.1097/00063198-200001000-00009Search in Google Scholar PubMed
[25] Handley DA. Single-isomer beta-agonists. Pharmacotherapy. 2001;21:21S–27S.10.1159/000062133Search in Google Scholar
[26] Hartley D, Middlemiss D. Absolute configuration of the optical isomers of salbutamol. J Med Chem. 1971;14:995–6.10.1021/jm00291a036Search in Google Scholar PubMed
[27] Nowak R, Emerman C, Hanrahan JP, Parsey MV, Hanania NA, Claus R, et al. A comparison of levalbuterol with racemic albuterol in the treatment of acute severe asthma exacerbations in adults. Am J Emerg Med. 2006;24:259–67.10.1016/j.ajem.2006.01.027Search in Google Scholar PubMed
[28] Ameredes BT, Calhoun WJ. Albuterol enantiomers: pre clinical and clinical value? Front Biosci. 2010;2:1081–92.10.2741/e166Search in Google Scholar PubMed
[29] Patel M, Thomson NC. (R)-Salbutamol in the treatment of asthma and chronic obstructive airways disease. Expert Opin Pharmacother. 2011;12:1133–41.10.1517/14656566.2011.571210Search in Google Scholar PubMed
[30] Jat KR, Khairwa A. Levalbuterol versus albuterol for acute astma: a systematic review and meta-analysis. Pulm Pharmacol Ther. 2013;26:239–48.10.1016/j.pupt.2012.11.003Search in Google Scholar PubMed
[31] Schmidt D, Källström B-L, Waldeck B, Branscheid D, Magnussen H, Rabe KF. The effect of the enantiomers of formoterol on inherent and induced tone in guinea-pig trachea and human bronchus. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;361:405–9.10.1007/s002109900213Search in Google Scholar PubMed
[32] Loh ChH, Donohue JF, Ohar JA. Review of drug safety and efficacy of arformoterol in chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015;14:463–72.10.1517/14740338.2015.998196Search in Google Scholar PubMed
[33] Brogden RN, Faulds D. Salmeterol xinafoate. A review of its pharmacological properties and therapeutic potential in reversible obstructive airways disease. Drugs. 1991;42:895–912.10.2165/00003495-199142050-00010Search in Google Scholar PubMed
[34] Pearlman DS. Long-acting beta 2-agonist salmeterol compared with albuterol in maintenance asthma therapy. Ann Allergy Asthma Immunol. 1995;75:180–4.Search in Google Scholar
[35] Nials AT, Coleman RA, Johnson M, Vardey C. The β-adrenoceptor pharmacology of the enantiomers of salmeterol. Am J Rev Resp Dis. 1994;149:A481.Search in Google Scholar
[36] Giorgino FL, Egan CG. Use of isoxsuprine hydrochloride as a tocolytic agent in the treatment of preterm labour: a systematic review of previous literature. Arzneimittelforschung. 2010;60:415–20.10.1055/s-0031-1296305Search in Google Scholar PubMed
[37] Belloli C, Carcano R, Arioli F, Beretta C. Affinity of isoxsuprine for adrenoreceptors in equine digital artery and implications for vasodilatory action. Equine Vet J. 2000;32:119–24.10.2746/042516400777591543Search in Google Scholar PubMed
[38] Hill JW, Thompson JF, Carter MB, Edwards BS, Sklar LA, Rosenberg GA. Identification of isoxsuprine hydrochloride as a neuroprotectant in ischemic stroke through cell-based high-throughput screening. PLoS One. 2014;9:e96761.10.1371/journal.pone.0096761Search in Google Scholar PubMed PubMed Central
[39] Belloli C, Badino P, Carcano R, Odore R, Arioli F, Caloni F, et al. Investigations on the stereoselective action of isoxsuprine on alpha- and beta-adrenoceptors in equine common digital artery. Pharmacol Res. 1999;40:177–82.10.1006/phrs.1999.0487Search in Google Scholar PubMed
[40] Bosak A, Gazić I, Vinković V, Kovarik Z. Stereoselective inhibition of human, mouse, and horse cholinesterases by bambuterol enantiomers. Chem Biol Interact. 2008;175:192–5.10.1016/j.cbi.2008.04.050Search in Google Scholar PubMed
[41] Pistolozzi M, Du H, Wei H, Tan W. Stereoselective inhibition of human butyrylcholinesterase by the enantiomers of bambuterol and their intermediates. Drug Metab Dispos. 2015;43:344–52.10.1124/dmd.114.060251Search in Google Scholar PubMed
[42] Wu J, Liu F, Wang S, Wang H, Liu Q, Song X, et al. Resolution of rac-bambuterol via diastereoisomeric salt formation with o-chloromandelic acid and differences in the enantiomers’ pharmacodynamical effects in Guinea Pigs and Beagles. Chirality. 2016;28:306–12.10.1002/chir.22573Search in Google Scholar PubMed
[43] Jacobson GA, Fawcett JP. Beta2-agonist doping control and optical isomer challenges. Sports Med. 2016;46:1787–95.10.1007/s40279-016-0547-4Search in Google Scholar PubMed
[44] Kitaura T, Suzuki S, Kraemer WJ. Effect of clenbuterol enantiomers on growth of young male rats. J Phys Fitness Sports Med. 2015;4:369–76.10.7600/jpfsm.4.369Search in Google Scholar
[45] Hao Z, Zhang Y, Pan L, Su X, Cheng M, Wang M, et al. Comparison of enantiomers of SPFF, a novel beta2-Adrenoceptor agonist, in bronchodilating effect in guinea pigs. Biol Pharm Bull. 2008;31:866–72.10.1248/bpb.31.866Search in Google Scholar
[46] Bieniarz J, Motew M, Scommegna A. Uterine and cardiovascular effects of ritodrine in premature labor. Obstet Gynecol. 1972;40:65–73.10.1097/00006250-197207000-00013Search in Google Scholar
[47] Barden TP. Effect of ritodrine on human uterine motility and cardiovascular responses in term labor and the early postpartum state. Am J Obstet Gynecol. 1972;112:645–52.10.1016/0002-9378(72)90789-2Search in Google Scholar
[48] Yamazaki N, Fukuda Y, Shibazaki Y, Niizato T, Kosugi T, Yoshioka S, (−)-Ritodrine, therapeutic composition and use, and method of preparation. US5449694, 1995.Search in Google Scholar
[49] Konda A, Ito T, Yoshida H, Toda T, Hayakawa T, Inotsume N. Pharmacokinetics of ritodrine diastereomers in patients pregnant with singletons and twins. Eur J Clin Pharmacol. 2009;65:913–7.10.1007/s00228-009-0665-0Search in Google Scholar PubMed
[50] Caughey AB, Parer JT. Tocolysis with beta-adrenergic receptor agonists. Semin Perinatol. 2001;25:248–55.10.1053/sper.2001.27166Search in Google Scholar PubMed
[51] Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, et al. In vitro and in vivo pharmacological characterization of 5-[(R)-2-(5,6-diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (indacaterol) a novel inhaled β2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317:762–70.10.1124/jpet.105.098251Search in Google Scholar PubMed
[52] Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indicaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–81.10.1183/09031936.00032806Search in Google Scholar PubMed
[53] Steiropoulos P, Archontogeorgis K, Nena E, Bouros D. New developments in the management of COPD: clinical utility of indacaterol 75 μg. Int J Chron Obstruct Pulmon Dis. 2014;9:1–7.10.2147/COPD.S24940Search in Google Scholar PubMed PubMed Central
[54] Cazzola M, Matera MG, Lötvall J. Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2005;14:775–83.10.1517/13543784.14.7.775Search in Google Scholar PubMed
[55] Matera MG, Cazzola M. Ultra-long-acting β2-adrenoceptor agonists: an emerging therapeutic option for asthma and COPD? Drug. 2007;67:503–15.10.2165/00003495-200767040-00002Search in Google Scholar PubMed
[56] Aparici M, Gavalda A, Ramos I, Carcasona C, Otal R, Fernández-Blanco JA, et al. In vitro and in vivo preclinical profile of abediterol (LAS100977), an inhaled long-acting β-adrenoceptor agonist, compared with indacaterol, olodaterol and vilanterol. Eur J Pharmacol. 2016;770:61–69.10.1016/j.ejphar.2015.11.053Search in Google Scholar PubMed
[57] Malerba M, Radaeli A, Montuschi P, Morjaria JB. Vilanterol trifenatate for the treatment of COPD. Expert Rev Respir Med. 2016;10:719–31.10.1080/17476348.2016.1184976Search in Google Scholar PubMed
[58] Vakily M, Mehvar R, Brocks D. Stereoselective pharmacokinetics and pharmacodynamics of anti-asthma agents. Ann Pharmacother. 2002;36:693–701.10.1345/aph.1A248Search in Google Scholar PubMed
[59] Ward JK, Dow J, Dallow N, Eynott P, Milleri S, Ventresca GP. Enantiomeric disposition of inhaled, intravenous and oral racemic salbutamol in man – no evidence of enantioselective lung metabolism. Br J Clin Pharmacol. 2000;49:15–22.10.1046/j.1365-2125.2000.00102.xSearch in Google Scholar PubMed PubMed Central
[60] Jacobson GA, Raidal S, Robson K, Narkowicz ChK, Nichols DS, Walters EH. Bronchopulmonary pharmacokinetics of (R)-salbutamol and (S)-salbutamol enantiomers in pulmonary epithelial lining fluid and lung tissue of horses. Br J Clin Pharmacol. 2017;83:1436–45.10.1111/bcp.13228Search in Google Scholar PubMed PubMed Central
[61] Borgström L, Liu CX, Walhagen A. Pharmacokinetic of the enantiomers of terbutaline after repeated oral dosing with racemic terbutaline. Chirality. 1989;1:174–7.10.1002/chir.530010213Search in Google Scholar PubMed
[62] Borgström L, Kennedy BM, Nilsson B, Angelin B. Relative absorption of the two enantiomers of terbutaline after duodenal administration. Eur J Clin Pharmacol. 1990;38:621–3.10.1007/BF00278593Search in Google Scholar PubMed
[63] Borgström L, Nyberg L, Jönsson S, Lindberg C, Paulson J. Pharmacokinetic evaluation in man of terbutaline in man given as separate enantiomers and as the racemate. Br J Clin Phartmacol. 1989;27:49–56.10.1111/j.1365-2125.1989.tb05334.xSearch in Google Scholar PubMed PubMed Central
[64] Morgan DJ. Clinical pharmacokinetics of beta-agonists. Clin Pharmacokinet. 1990;18:270.10.2165/00003088-199018040-00002Search in Google Scholar PubMed
[65] Cazzola M, Calzetta L, Page CP, Matera MG. Use of indacaterol for the treatment of COPD: a pharmacokinetic evaluation. Expert Opin Drug Metab Toxicol. 2014;10:129–37.10.1517/17425255.2014.865723Search in Google Scholar PubMed
[66] Boulton DW, Fawcett JP, Fiddes TM. Transplacental distribution of salbutamol enantiomers at Caesarian section. Br J Clin Pharmacol. 1997;44:587–90.10.1046/j.1365-2125.1997.t01-1-00617.xSearch in Google Scholar PubMed PubMed Central
[67] Jacobson GA, Yee KC, Premilovac D, Rattigan S. Enantioselective disposition of (R/S)-albuterol in skeletal and cardiac muscle. Drug Test Anal. 2014;6:563–7.10.1002/dta.1575Search in Google Scholar PubMed
[68] Jacobson GA, Raidal S, Robson K, Narkowicz CK, Nichols DS, Walters EH. Salmeterol undergoes enantioselective bronchopulmory distribution with receptor localisation a likely determinant of duration of action. J Pharm Biomed Anal. 2018;154:102–7.10.1016/j.jpba.2018.02.048Search in Google Scholar PubMed
[69] Jacobson GA, Hostrup M, Narkowicz CK, Nichols DS, Walters EH. Enantioselective disposition of (R,R)-formoterol, (S,S)-formoterol and their respective glucuronides in urine following single inhaled dosing and application to doping control. Drug Test Anal. 2019;11:950–6.10.1002/dta.2587Search in Google Scholar PubMed
[70] Wilson AA, Wang J, Koch P, Walle T. Stereoselective sulphate conjugation of fenoterol by human phenolsulphotransferases. Xenobiotica. 1997;27:1147–54.10.1080/004982597239903Search in Google Scholar PubMed
[71] Pacifici GM, De Santi C, Mussi A, Ageletti CA. Interindividual variability in the rate of salbutamol sulphation in the human lung. Eur J Clin Pharmacol. 1996;49:299–303.10.1007/BF00226331Search in Google Scholar PubMed
[72] Sjöswärd KN, Josefsson M, Ahlner J, Andersson RG, Schmekel B. Metabolism of salbutamol differs between asthmatic patients and healthy volunteers. Pharmacol Toxicol. 2003;92:27–32.10.1034/j.1600-0773.2003.920105.xSearch in Google Scholar PubMed
[73] Boulton DW, Fawcett JP. The pharmacokinetics of levosalbutamol: what are the clinical implications? Clin Pharmacokinet. 2001;40:23–40.10.2165/00003088-200140010-00003Search in Google Scholar PubMed
[74] Kagan M, Dain J, Peng L, Reynolds C. Metabolism and pharmacokinetics of indacaterol in humans. Drug Metab Dispos. 2012;40:1712–22.10.1124/dmd.112.046151Search in Google Scholar PubMed
[75] Maier G, Rubino C, Hsu R, Grasela T, Baumgartner RA. Population pharmacokinetics of (R)-albuterol and (S)-albuterol in pediatric patients aged 4–11 years with astma. Pulm Pharmacol Ther. 2007;20:534–42.10.1016/j.pupt.2006.05.003Search in Google Scholar PubMed
[76] Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics: cardiovascular drugs. In: Reddy IK, Mehvar R, editors. Chirality in drug design and development. 1st ed. New York: Marcel Dekker; 2004.10.1201/9780203021811.ch6Search in Google Scholar
[77] Gumieniczek A, Berecka A. Analytical tools for determination of new oral antidiabetic drugs, glitazones, gliptins, gliflozins and glinides, in bulk materials, pharmaceuticals and biological samples. Open Chem. 2016;14:215–42.10.1515/chem-2016-0023Search in Google Scholar
[78] Nie Y, Liu X, Yang X, Zhao Z. Recent application of chiral liquid chromatography-tandem mass spectrometric methods for enantiomeric pharmaceutical and biomedical determinations. J Chromatogr Sci. 2013;51:753–63.10.1093/chromsci/bms209Search in Google Scholar
[79] Ribeiro AR, Maia AS, Cass QB, Tiritan ME. Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: an overview. J Chromatogr B. 2014;968:8–21.10.1016/j.jchromb.2014.02.049Search in Google Scholar
[80] Sanghvi M, Ramamoorthy A, Strait J, Wainer IW, Moaddel R. Development and validation of a sensitive LC-MS/MS method for the determination of fenoterol in human plasma and urine samples. J Chromatogr B. 2013;933:37–43.10.1016/j.jchromb.2013.06.020Search in Google Scholar
[81] Kim KH, Kim HJ, Kim JH, Lee JH, Lee SC. Determination of the optical purity of (R)-terbutaline by 1H-NMR and RP-LC using chiral derivatizing agent, (S)-(−)-alpha-methylbenzyl isocyanate. J Pharm Biomed Anal. 2001;25:947–56.10.1016/S0731-7085(01)00386-7Search in Google Scholar
[82] Ullrich T, Wesenberg D, Bleuel C, Krauss GJ, Schmid MG, Weiss M, et al. Chiral separation of the β2-sympathomimetic fenoterol by HPLC and capillary zone electrophoresis for pharmacokinetic studies. Biomed Chromatogr. 2010;24:1125–9.10.1002/bmc.1415Search in Google Scholar PubMed
[83] Gubitz G, Schmidt MG. Chiral separation principles: an introduction. In: Gubitz G, Schmidt MG, editors. Chiral separation: method and protocols. Totowa, New Jersey: Humana Press; 2004.Search in Google Scholar
[84] Aboul-enein HY, Serignese V. Direct separation of albuterol enantiomers in biological fluids and pharmaceutical formulations using α1-acid glycoprotein and pirkle urea type columns. Chirality. 1995;7:158–62.10.1002/chir.530070309Search in Google Scholar
[85] Siluk D, Mager DE, Kim HS, Wang Y, Furimsky AM, Ta A, et al. Pharmacokinetics and metabolism of (R,R)-methoxyfenoterol in rat. Xenobiotica. 2010;40:195–206.10.3109/00498250903434533Search in Google Scholar PubMed PubMed Central
[86] Tanaka Y, Terabe S. Separation of the enantiomers of basic drugs by affinity capillary electrophoresis using a partial filling technique and α1-acid glycoprotein as chiral selector. Chromatographia. 1997;44:119–28.10.1007/BF02466445Search in Google Scholar
[87] Boulton DW, Fawcett JP. Interaction of beta 2-adrenoceptor agonists with native cyclodextrins: application to the development of chiral assays for terbutaline. Pharm Acta Helv. 1996;71:293–6.10.1016/S0031-6865(96)00030-1Search in Google Scholar
[88] Mostafa GA, Hefnawy MM, El-Majed A. Separation and determination of clenbuterol by HPLC using a vancomycin chiral stationary phase. J AOAC Int. 2009;92:824–9.10.1093/jaoac/92.3.824Search in Google Scholar
[89] Ali I, Kumerer K, Aboul-Enein HY. Mechanistic principles in chiral Separations using liquid chromatography and capillary electrophoresis. Chromatographia. 2006;63:295–307.10.1365/s10337-006-0762-5Search in Google Scholar
[90] Ilisz I, Berkecz R, Peter A. Retention mechanism of high-performance liquid chromatographic enantioseparation on macrocyclic glycopeptide-based chiral stationary phases. J Chromatogr A. 2009;1216:1845–60.10.1016/j.chroma.2008.08.041Search in Google Scholar PubMed
[91] Chen J, Korfmacher WA, Hsieh Y. Chiral liquid chromatography-tandem mass spectrometric methods for stereoisomeric pharmaceutical determinations. J Chromatogr B. 2005;820:1–8.10.1016/j.jchromb.2005.02.012Search in Google Scholar PubMed
[92] Zhou T, Liu S, Zhao T, Zeng J, He M, Xu B, et al. Chiral analysis of bambuterol, its intermediate and active drug in human plasma by liquid chromatography-tandem mass spectrometry: application to a pharmacokinetic study. J Chromatogr B. 2015;997:38–44.10.1016/j.jchromb.2015.05.024Search in Google Scholar PubMed
[93] Luo W, Zhu L, Deng J, Liu A, Guo B, Tan W, et al. Simultaneous analysis of bambuterol and its active metabolite terbutaline enantiomers in rat plasma by chiral liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010;52:227–31.10.1016/j.jpba.2009.12.020Search in Google Scholar PubMed
[94] Xia Y-Q, Liu DQ, Bakhtiar R. Use of online-dual-column extraction in conjunction with chiral liquid chromatography tandem mass spectrometry for determination of terbutaline enantiomers in human plasma. Chirality. 2002;14:742–9.10.1002/chir.10135Search in Google Scholar PubMed
[95] Hirosawa I, Ishikawa M, Ogino M, Ito H, Hirao T, Yamada H, et al. Enantioselective disposition of clenbuterol in rats. Biopharm Drug Dispos. 2014;35:207–17.10.1002/bdd.1885Search in Google Scholar PubMed
[96] Chan SH, Lee W, Asmawi MZ, Tan SC. Chiral liquid chromatography-mass spectrometry (LC-MS/MS) method development for the detection of salbutamol in urine samples. J Chromatogr B. 2016;1025:83–91.10.1016/j.jchromb.2016.05.015Search in Google Scholar PubMed
[97] Joyce KB, Jones AE, Scott RJ, Biddlecombe RA, Pleasance S. Determination of the enantiomers of salbutamol and its 4-O-sulphate metabolites in biological matrices by chiral liquid chromatography tandem mass spectrometry. Rapid Commun Mass Spectrom. 1998;12:1899–910.10.1002/(SICI)1097-0231(19981215)12:23<1899::AID-RCM417>3.0.CO;2-ISearch in Google Scholar
[98] Wu ST, Xing J, Apedo A, Wang-Iverson DB, Olah TV, Tymiak AA, et al. High-throughput chiral analysis of albuterol enantiomers in dog plasma using on-line sample extraction/polar organic mode chiral liquid chromatography with tandem mass spectrometric detection. Rapid Commun Mass Spectrom. 2004;18:2531–6.10.1002/rcm.1655Search in Google Scholar
[99] Fried KM, Koch P, Wainer IW. Determination of the enantiomers of albuterol in human and canine plasma by enantioselective high-performance liquid chromatography on a teicoplanin-based chiral stationary phase. Chirality. 1998;10:484–91.10.1002/(SICI)1520-636X(1998)10:5<484::AID-CHIR11>3.0.CO;2-WSearch in Google Scholar
[100] Rosales-Conrado N, Dell'Aica M, de León-González ME, Pérez-Arribas LV, PoloDíez LM. Determination of salbutamol by direct chiral reversed-phase HPLC using teicoplanin as stationary phase and its application to natural water analysis. Biomed Chromatogr. 2013;27:1413–22.10.1002/bmc.2937Search in Google Scholar
[101] MacLeod SL, Sudhir P, Wong CS. Stereoisomer analysis of waste water-derived beta-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2007;1170:23–33.10.1016/j.chroma.2007.09.010Search in Google Scholar
[102] Lu X, Liu P, Chen H, Qin F, Li F. Enantioselective pharmacokinetics of mabuterol in rats studied using sequential achiral and chiral HPLC. Biomed Chromatogr. 2005;19:703–8.10.1002/bmc.584Search in Google Scholar
[103] Hashem H, Tründelberg C, Attef O, Jira T. Effect of chromatographic conditions on liquid chromatographic chiral separation of terbutaline and salbutamol on Chirobiotic V column. J Chromatogr A. 2011;1218:6727–31.10.1016/j.chroma.2011.07.090Search in Google Scholar
[104] Qin F, Wang X, Jing L, Pan L, Cheng M, Sun G, et al. Bidirectional chiral inversion of trantinterol enantiomers after separate doses to rats. Chirality. 2013;25:934–8.10.1002/chir.22236Search in Google Scholar
[105] Qin F, Wang Y, Wang L, Zhao L, Pan L, Cheng M, et al. Determination of trantinterol enantiomers in human plasma by high-performance liquid chromatography-tandem mass spectrometry using vancomycin chiral stationary phase and solid phase extraction and stereoselective pharmacokinetic application. Chirality. 2015;27:327–31.10.1002/chir.22432Search in Google Scholar
[106] Yang J, Guan J, Pan L, Jiang K, Cheng M, Li F. Enantioseparation and impurity determination of the enantiomers of novel phenyletanolamine derivatives by high performance liquid chromatography on amylose stationary phase. Anal Chim Acta. 2008;610:263–7.10.1016/j.aca.2008.01.027Search in Google Scholar
[107] Chankvetadze B, Burjanadze N, Blaschke G. Enantioseparation of chiral vasodilator drug isoxsuprine in high-performance liquid chromatography and capilary electrophoresis. J Pharm Biomed Anal. 2002;27:157–9.10.1016/S0731-7085(01)00552-0Search in Google Scholar
[108] Bartolinčić A, Drušković V, Šiporec A, Vinković V. Development and validation of HPLC methods for the enantioselective analysis of bambuterol and albuterol. J Pharm Biomed Anal. 2005;36:1003–10.10.1016/j.jpba.2004.09.006Search in Google Scholar
[109] Beigi F, Bertucci C, Zhu W, Chakir K, Wainer IW, Xiao R, et al. Enantioselective separation and online affinity chromatographic characterization of R,R- and S,S-fenoterol. Chirality. 2006;18:822–7.10.1002/chir.20317Search in Google Scholar
[110] Gazić I, Bosak A, Šinko G, Vinković V, Kovarik Z. Preparative HPLC separation of bambuterol enantiomers and stereoselective inhibition of human cholinesterases. Anal Bioanal Chem. 2006;385:1513–9.10.1007/s00216-006-0566-3Search in Google Scholar
[111] Abou-Basha LI, Aboul-Enein HY. Direct enantioselective separation of clenbuterol by chiral HPLC in biological fluids. Biomed Chromatogr. 1996;10:69–72.10.1002/(SICI)1099-0801(199603)10:2<69::AID-BMC554>3.0.CO;2-HSearch in Google Scholar
[112] Zhang M, Fawcett JP, Shaw JP. Stereoselective urinary excretion of formoterol and its glucuronide conjugate in human. Br J Clin Pharmacol. 2002;54:246–50.10.1046/j.1365-2125.2002.01641.xSearch in Google Scholar
[113] Zhang M, Fawcett JP, Shaw JP. Rapid chiral high-performance liquid chromatographic assay for salmeterol and alpha-hydroxysalmeterol. Application to in vitro metabolism studies. J Chromatogr B Biomed Sci Appl. 1999;729:225–30.10.1016/S0378-4347(99)00156-5Search in Google Scholar
[114] Bojarski J. Stereoselective chromatography of cardiovascular drugs: an update. J Biochem Biophys Methods. 2002;54:197–220.10.1016/S0165-022X(02)00143-4Search in Google Scholar
[115] Kim KH, Kim TK, Kwon YH, Sohn YT. Resolution of salbutamol enantiomers in human urine by reversed-phase high performance liquid chromatography after derivatization with (S)-(−)-alpha-methylbenzyl isocyanate. Arch Pharm Res. 1997;20:486–90.10.1007/BF02973945Search in Google Scholar
[116] Kim KH, Kim TK. Resolution of salbutamol enantiomers in human urine by reversed-phase high performance liquid chromatography after derivatization with 2,3,4,6-tetra-O-acetyl-beta-d-glucopyranosyl isothiocyanate. Arch Pharm Res. 1998;21:217–22.10.1007/BF02974031Search in Google Scholar
[117] Bhushan R, Nagar H. Resolution and isolation of enantiomers of (±)-isoxsuprine using thin silica gel layers impregnated with l-glutamic acid, comparison of separation of its diastereomers prepared with chiral derivatizing reagents having l-amino acids as chiral auxiliaries. Biomed Chromatogr. 2015;29:357–65.10.1002/bmc.3284Search in Google Scholar
[118] Malik P, Bhushan R. Development of liquid chromatographic methods for enantioseparation and sensitive detection of β-adrenolytics/β2-agonists in human plasma using a single enantiomer reagent. J Chromatogr B. 2017;1061–2:117–22.10.1016/j.jchromb.2017.06.041Search in Google Scholar
[119] Damasceno L, Ventura R, Ortuño J, Segura J. Derivatization procedures for the detection of beta(2)-agonists by gas chromatographic/mass spectrometric analysis. J Mass Spectrom. 2000;35:1285–94.10.1002/1096-9888(200011)35:11<1285::AID-JMS61>3.0.CO;2-LSearch in Google Scholar
[120] Damasceno L, Ventura R, Cardoso J, Segura J. Diagnostic evidence for the presence of beta-agonists using two consecutive derivatization procedures and gas chromatography-mass spectrometric analysis. J Chromatogr B. 2002;780:61–71.10.1016/S1570-0232(02)00414-2Search in Google Scholar
[121] König WA, Gyllenhaal O, Vessman J. Enantiomer separation of phenolic α- and β-receptor active drugs by chiral capillary gas chromatography after derivatization with diazomethane and phosgene. J Chromatogr A. 1986;356:354–8.10.1016/S0021-9673(00)91499-7Search in Google Scholar
[122] Köenig WA, Stoelting K, Kruse K. Gas chromatographic separation of optically active compounds in glass capillaries. Chromatographia. 1977;10:444–8.10.1007/BF02257358Search in Google Scholar
[123] Caban M, Migowska N, Stepnowski P, Kwiatkowski M, Kumirska J. Matrix effects and recovery calculations in analyses of pharmaceuticals based on the determination of β-blockers and β-agonists in environmental samples. J Chromatogr A. 2012;1258:117–27.10.1016/j.chroma.2012.08.029Search in Google Scholar
[124] Caban M, Mioduszewska K, Stepnowski P, Kwiatkowski M, Kumirska J. Dimethyl(3,3,3-trifluoropropyl)silyldiethylamine – a new silylating agent for the derivatization of β-blockers and β-agonists in environmental samples. Anal Chim Acta. 2013;782:75–88.10.1016/j.aca.2013.04.018Search in Google Scholar
[125] Segura J, Ventura R, Jurado C. Derivatization procedures for gas chromatographic-mass spectrometric determination of xenobiotics in biological samples, with special attention to drugs of abuse and doping agents. J Chromatogr B Biomed Sci Appl. 1998;713:61–90.10.1016/S0378-4347(98)00089-9Search in Google Scholar
[126] Bhushan R, Tanwar S. Different approaches of impregnation for resolution of enantiomers of atenolol, propranolol and salbutamol using Cu(ii)-l-amino acid complexes for ligand exchange on commertial thin layer chromatographic plates. J Chromatogr A. 2010;1217:1395–8.10.1016/j.chroma.2009.12.071Search in Google Scholar
[127] Malik P, Bhushan R. Thin layer chromatographic resolution of some β-adrenolytics and a β2-agonist using bovine serum albumin as chiral additive in stationary phase. J Chromatogr Sci. 2018;56:92–98.10.1093/chromsci/bmx082Search in Google Scholar
[128] Mikuš P, Valášková I, Havránek E. Determination of salbutamol in pharmaceuticals by capillary electrophoresis. Arch Pharm Chem Life Sci. 2005;338:498–501.10.1002/ardp.200500135Search in Google Scholar
[129] Scriba GK. Cyclodextrins in capillary electrophoresis enantioseparations – recent developments and applications. J Sep Sci. 2008;31:1991–2011.10.1002/jssc.200800095Search in Google Scholar
[130] Yang J, Lu X, Pan L, Jiang K, Cheng M, Li F. Simultaneous enantioseparation of four beta2-agonists by capillary electrophoresis with cyclodextrin additives. Study of the enantioselective mechanism. J Sep Sci. 2008;31:3749–54.10.1002/jssc.200800311Search in Google Scholar
[131] Gausepohl C, Blaschke G. Stereoselective determination of clenbuterol in human urine by capillary electrophoresis. J Chromatogr B Biomed Sci Appl. 1998;713:443–6.10.1016/S0378-4347(98)00178-9Search in Google Scholar
[132] Kim KH, Kim HJ, Jeun EY, Seo SH, Hong SP, Kang JS, et al. Chiral separation of beta2-agonists by capillary electrophoresis using hydroxypropyl-alpha-cyclodextrin as a chiral selector. Arch Pharm Res. 2001;24:281–5.10.1007/BF02975092Search in Google Scholar
[133] Esquisabel A, Hernández RM, Gascón AR, Igartua M, Calvo B, Pedraz JL. Determination of salbutamol enantiomers by high-performance capillary electrophoresis and its application to dissolution assays. J Pharm Biomed Anal. 1997;16:357–66.10.1016/S0731-7085(97)00047-2Search in Google Scholar
[134] Ekiert E, García-Ruiz C, García MA, Marina ML. Rapid determination of salbutamol in pharmaceutical preparations by chiral capillary electrophoresis. Electrophoresis. 2003;24:2680–6.10.1002/elps.200305490Search in Google Scholar PubMed
[135] Zhou J, Li Y, Liu Q, Fu G, Zhang Z. Capillary electrophoresis of clenbuterol enantiomers and NMR investigation of the clenbuterol/carboxymethyl-β-cyclodextrin complex. J Chromatogr Sci. 2013;51:237–41.10.1093/chromsci/bms133Search in Google Scholar PubMed
[136] Zhou J, Yao H, Shao H, Li Y, Zhang Z. Enantioseparation of β-agonists with carboxymethyl-β-cyclodextrin by CE. J Liquid Chromatogr Rel Technol. 2012;35:50–58.10.1080/10826076.2011.593387Search in Google Scholar
[137] Yang GS, Chen DM, Yang Y, Tang B, Gao JJ, Aboul-Enein HY, et al. Enantioseparation of some clinically used drugs by capillary electrophoresis using sulfated β-cyclodextrin as a chiral selector. Chromatographia. 2005;62:441–5.10.1365/s10337-005-0632-6Search in Google Scholar
[138] Aboul-Enein HY, Efstatiade MD, Baiulescu GE. Cyclodextrins as chiral selectors in capillary electrophoresis: a comparative study for the enantiomeric separation of some beta-agonists. Electrophoresis. 1999;20:2686–90.10.1002/(SICI)1522-2683(19990901)20:13<2686::AID-ELPS2686>3.0.CO;2-0Search in Google Scholar
[139] Vela J, Yanes EG, Stalcup AM. Quantitative determination of clenbuterol, salbutamol and tulobuterol enantiomers by capillary electrophoresis. Fresenius J Anal Chem. 2001;369:212–9.10.1007/s002160000584Search in Google Scholar
[140] Servais A-C, Fillet M, Mol R, Somsen GW, Chiap P, de Jong GJ, et al. On-line coupling of cyclodextrin mediated nonaqueous capillary electrophoresis to mass spectrometry for the determination of salbutamol enantiomers in urine. J Pharm Biomed Anal. 2006;40:752–7.10.1016/j.jpba.2005.08.004Search in Google Scholar
[141] Servais A-C, Chiap P, Hubert P, Crommen J, Fillet M. Determination of salbutamol enantiomers in human urine using heptakis(2,3-di-O-acetyl-6-O-sulfo)-beta-cyclodextrin in nonaqueous capillary electrophoresis. Electrophoresis. 2004;25:1632–40.10.1002/elps.200405854Search in Google Scholar
[142] Liu Y, Deng M, Yu J, Jiang Z, Guo X. Capillary electrophoretic enantioseparation of basic drugs using a new single-isomer cyclodextrin derivative and theoretical study of the chiral recognition mechanism. J Sep Sci. 2016;39:1766–75.10.1002/jssc.201501026Search in Google Scholar
[143] Lebedeva MV, Prokhorova AF, Shapovalova EN, Shpigun OA. Clarithromycin as a chiral selector for enantioseparation of basic compounds in nonaqueous capillary electrophoresis. Electrophoresis. 2014;35:2759–64.10.1002/elps.201400135Search in Google Scholar
[144] An N, Wang L, Zhao J, Lv L, Wang N, Guo H. Enantioseparations of fourteen amino alcohols by nonaqueous capillary electrophoresis using the lactobionic acid/d-(+)-xylose-boric acid complexes as chiral selectors. Anal Methods. 2016;8:1127–34.10.1039/C5AY02686ESearch in Google Scholar
[145] Catarcini P, Fanali S, Presutti C, D'Acquarica I, Gasparrini F. Evaluation of teicoplanin chiral stationary phases of 3.5 and 5 µm inside diameter silica microparticles by polar-organic mode capillary electrochromatography. Electrophoresis. 2003;24:3000–5.10.1002/elps.200305504Search in Google Scholar
[146] Yao C, Tang S, Gao R, Jiang C, Yan C. Enantiomer separations on a vancomycin stationary phase and retention mechanism of pressurized capillary electrochromatography. J Sep Sci. 2004;27:1109–14.10.1002/jssc.200401875Search in Google Scholar
[147] Hett R, Helquist P. Enantioselective synthesis of salmeterol via asymmetric borane reduction. Tetrahedron Lett. 1994;35:9375–8.10.1016/S0040-4039(00)78546-7Search in Google Scholar
[148] Jiang L, Lin Ch, Oiu Y, Quan X, Zhu J, Shi H. A highly enantioselective synthesis of (R)-salmeterol using a chiral auxilliary. J Chem Res. 2016;40:564–6.10.3184/174751916X14719559894647Search in Google Scholar
[149] Procopiou PA, Morton GE, Todd M, Webb G. Enantioselective synthesis of (S)-salmeterol via asymmetric reduction of azidoketone by Pichia angusta. Tetrahedron Asymmetry. 2001;12:2005–8.10.1016/S0957-4166(01)00350-0Search in Google Scholar
[150] Goswani J, Berbaruah RL, Goswami A, Borthakur N. A convenient stereoselective synthesis of (R)-(−)-denopamine and (R)-(−)-salmeterol. Tetrahedron Asymmetry. 2001;12:3343–8.10.1016/S0957-4166(02)00010-1Search in Google Scholar
[151] Anwar MM, El-Haggar RS, Zaghary WA. Salmeterol xinafoate. In: Brittain H, editor. Profiles drug substances, excipients and related methodology. vol. 40, 1st ed. New York: Elsevier Academic Press; 2015.10.1016/bs.podrm.2015.02.002Search in Google Scholar PubMed
[152] Buchanan DJ, Dixon DJ, Looker BE. A short stereoselective synthesis of (R)-salmeterol. Synlett. 2005;12:1948–50.10.1002/chin.200550078Search in Google Scholar
[153] Cao G, Hu A-X, Zou K-S, Xu L, Chen J-L, Tan W. Highly enantioselective synthesis, crystal structure, and circular dichroism spectroscopy of (R)-bambuterol hydrochloride. Chirality. 2008;20:856–62.10.1002/chir.20558Search in Google Scholar PubMed
[154] Asami K, Machida T, Jung S, Hanaya K, Shoji M, Sugai T. Synthesis of (R)-bambuterol based on asymmetric reduction of 1-[3,5-bis(dimethylcarbamoyloxy)phenyl]-2-chloroethane with incubated whole cell of Williopsis californica JCM3600. J Mol Catal B Enzym. 2013;97:106–9.10.1016/j.molcatb.2013.08.003Search in Google Scholar
[155] Huang L, Liu J, Shan W, Liu B, Shi A, Li X. The asymmetric synthesis of (R,R)-formoterol via transfer hydrogenation with polyethylene glycol bound Rh catalyst in PEG2000 and water. Chirality. 2010;22:206–11.Search in Google Scholar
[156] Effenberger F, Jäger J. Synthesis of the adrenergic bronchodilators (R)-terbutaline and (R)-salbutamol from (R)-cyanohydrins. J Org Chem. 1997;62:3867–73.10.1021/jo970032dSearch in Google Scholar
© 2020 Čižmáriková Ružena et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

