Abstract
Nanotechnology is the fabrication, characterization, and potential application of various materials at the nanoscale. Over the past few decades, nanomaterials have attracted researchers from different fields because of their high surface-to-volume ratio and other unique and remarkable properties. Cobalt and cobalt oxide nanoparticles (NPs) have various biomedical applications because of their distinctive antioxidant, antimicrobial, antifungal, anticancer, larvicidal, antileishmanial, anticholinergic, wound healing, and antidiabetic properties. In addition to biomedical applications, cobalt and cobalt oxide NPs have been widely used in lithium-ion batteries, pigments and dyes, electronic thin film, capacitors, gas sensors, heterogeneous catalysis, and for environmental remediation purposes. Different chemical and physical approaches have been used to synthesize cobalt and cobalt oxide NPs; however, these methods could be associated with eco-toxicity, cost-effectiveness, high energy, and time consumption. Recently, an eco-friendly, safe, easy, and simple method has been developed by researchers, which uses biotic resources such as plant extract, microorganisms, algae, and other biomolecules such as starch and gelatin. Such biogenic cobalt and cobalt oxide NPs offer more advantages over other physicochemically synthesized methods. In this review, we have summarized the recent literature for the understanding of green synthesis of cobalt and cobalt oxide NPs, their characterization, and various biomedical applications.
Graphical abstract
1 Introduction
Nanotechnology combines various chemical and physical processes to construct nanomaterials, which are less than 100 nm in at least one dimension, and have unique properties [1]. Nanotechnology has applications across different fields, such as nanomedicines, biomaterials, nanoelectronics, environment, imaging, industries, and agriculture [2,3]. In healthcare, it has been widely used for the diagnosis and treatment of diseases, drug delivery, and novel drug formulations [2,4].
Cobalt is a transition metal that has a beneficial effect on human health [5,6]. It constitutes a part of vitamin B12, which is useful in anemia treatment as it provokes the formation of red blood cells [6]. Cobalt has unique magnetic, optical, electrical, and catalytic characteristics that make it suitable for a wide range of applications in the field of nanoelectronics and nanosensors [7,8,9]. Cobalt can exhibit variable oxidation states (Co2+, Co3+, and Co4+), which makes it attractive to be used in several industries [10]. Because of this multivalent state, cobalt has the ability to be present in different spin states in its oxide forms, i.e., low, intermediate, and high [10,11].
Recently, cobalt nanoparticles (CoNPs) have attracted considerable attention because they are more economical than the noble metal nanoparticle (NP) and show different properties, such as electrical and magnetic, due to their large surface area [12,13]. CoNPs have been explored as a therapeutic agent for the treatment of diseases, such as microbial infection, which make them attractive for biomedical applications [14,15]. CoNPs are nontoxic in the body at lower levels, have strong activities against bacteria and fungi at lower concentrations, and have fewer side effects than antibiotics [16,17].
Different types of NPs and their various applications in the area of medicine, textiles, cosmetics, electronics, optics, energy generation, and environmental science have been reported [18,19,20,21,22]. These NPs include silver NPs (AgNPs), iron NPs, CoNPs, copper NPs, gold NPs, silica NPs, platinum NPs, palladium NPs, zinc oxide NPs, magnesium oxide NPs, cerium dioxide NPs, and titanium oxide NPs [18]. Among all NPs, cobalt and cobalt oxide (Co3O4) NPs have been exploited the most because of their unique and wide range of applications [5,6]. Co3O4 is an antiferromagnetic p-type semiconductor with a direct optical band gap of 1.48 and 2.19 eV [23,24]. Co3O4 is a multifunctional material and has many applications such as biomedical applications (antibacterial, antiviral, antifungal, antileishmanial, therapeutic agents, anticancer, and drug delivery), gas sensors, solar selective absorbers, anode materials in lithium-ion batteries, energy storage, pigments and dyes, field emission materials, capacitors, heterogeneous catalysis, magneto-resistive devices, and electronic thin films [25,26,27,28,29] as shown in Figure 1. The oxides of cobalt are abundant in nature, as only the Co3O4 and CoO are stable [30], with Co3O4 possessing the highest stability. In this review, we aim to focus on the biological synthesis, characterization, and biological activities of cobalt and cobalt oxide NPs.
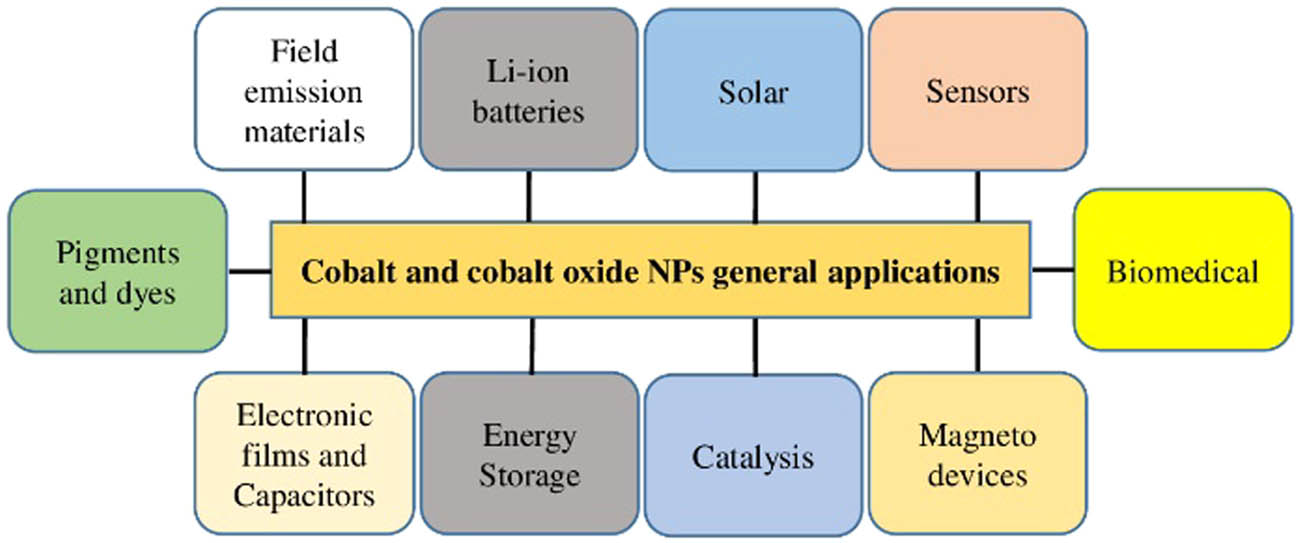
General applications of cobalt and cobalt oxide NPs.
2 Synthesis of NPs
Different approaches can be used for the synthesis of NPs. These approaches are divided into two classes: top-down approach and bottom-up approach. A top-down approach is a destructive approach in which larger molecules are decomposed into smaller ones, and these smaller molecules are then converted into suitable NPs, whereas the bottom-up is a building approach that involves the assembly of atomic size to form nano-size particles [1]. Examples of the top-down approach include chemical etching, laser ablation, mechanical milling, electro-explosion, and sputtering. In these different methods, the bulk materials are first converted into powder form and then into particular NPs. Each synthesis method has its advantages and limitations. The top-down approach has many advantages, such as the production of the desired size and large quantity of nanoparticle, whereas the fabrication of NPs using this method leads to eco-toxicity, high energy consumption and on top of that is expensive and time-consuming [2,3]. The bottom-up approach is further divided into two classes: biological and non-biological approaches. Examples of non-biological approaches include template support synthesis, flame spraying, spinning, laser pyrolysis, atomic condensation, and deposition of chemical vapors. These methods also use toxic chemicals, are expensive and time-consuming, whereas the biological approach uses various biotic resources such as plants, algae, microorganisms, and other biological molecules like starch, egg albumin, and gelatin for the production of different types of NPs. This biological approach is also known as the green approach [2,3,4,5,6,7,8,9,10]. This method is eco-friendly, simple, reliable, biocompatible, and easy for the synthesis of NPs. The classification of various methods of fabrication of NPs is shown in Figure 2. However, in this review, we only focus on the green synthesis of cobalt and cobalt oxide NPs, and their biological applications.
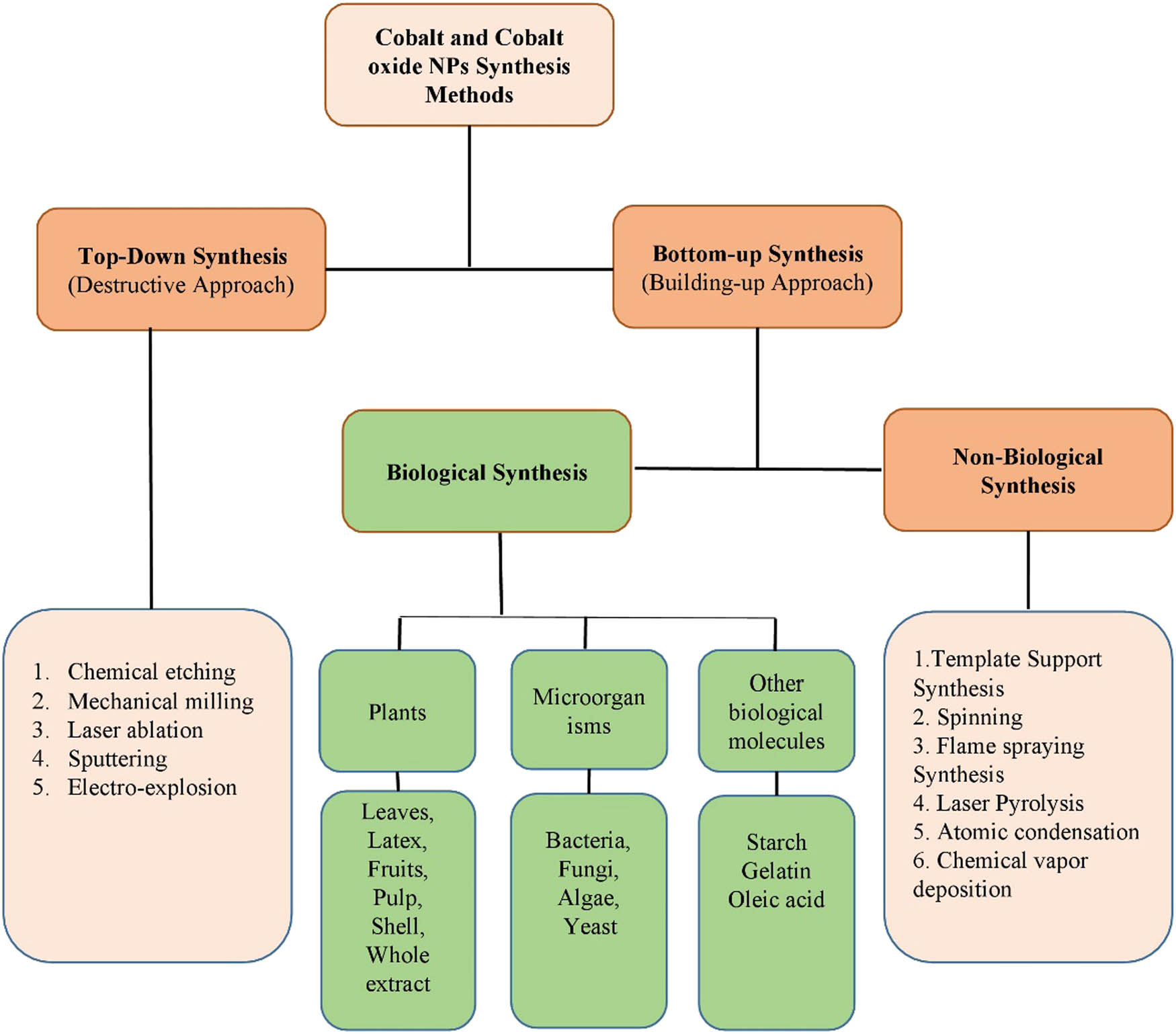
Various methods for the synthesis of cobalt and cobalt oxide NPs.
3 Green synthesis of cobalt and cobalt oxide NPs (biological methods)
The synthesis of NPs by physical and chemical methods (traditional approaches) has some detrimental effects such as emission of highly expensive and toxic chemicals that carry many threats to the ecosystem, as well as require high energy consumptions, high cost, and time-consuming processes. To overcome these problems, green synthesis of NPs is being implemented. Green-mediated approach emerged as an eco-friendly, biocompatible, and most fascinating, offering more advantages as compared to other traditional approaches [31,32]. In the green-mediated synthesis of NPs, different plants/parts of plants, fruits, bacteria, algae, fungi, and other biological molecules such as starch and egg albumin have been used as a reducing/capping/oxidizing agent [33,34,35]. Different parts of plants, microorganisms, and other biological molecules have been exploited for the fabrication of cobalt and cobalt oxide NPs as shown in Figure 3. These biological resources contain different biomolecules and metabolites that are responsible for the oxidation/reduction, stabilization, and production of particular NPs. Cobalt and cobalt oxide NPs have been synthesized using different methods such as chemical, physical, and biological methods. However, biological approach resulted in less contaminated, safer, cost-effective, and large scale production of NPs [6,31,32].
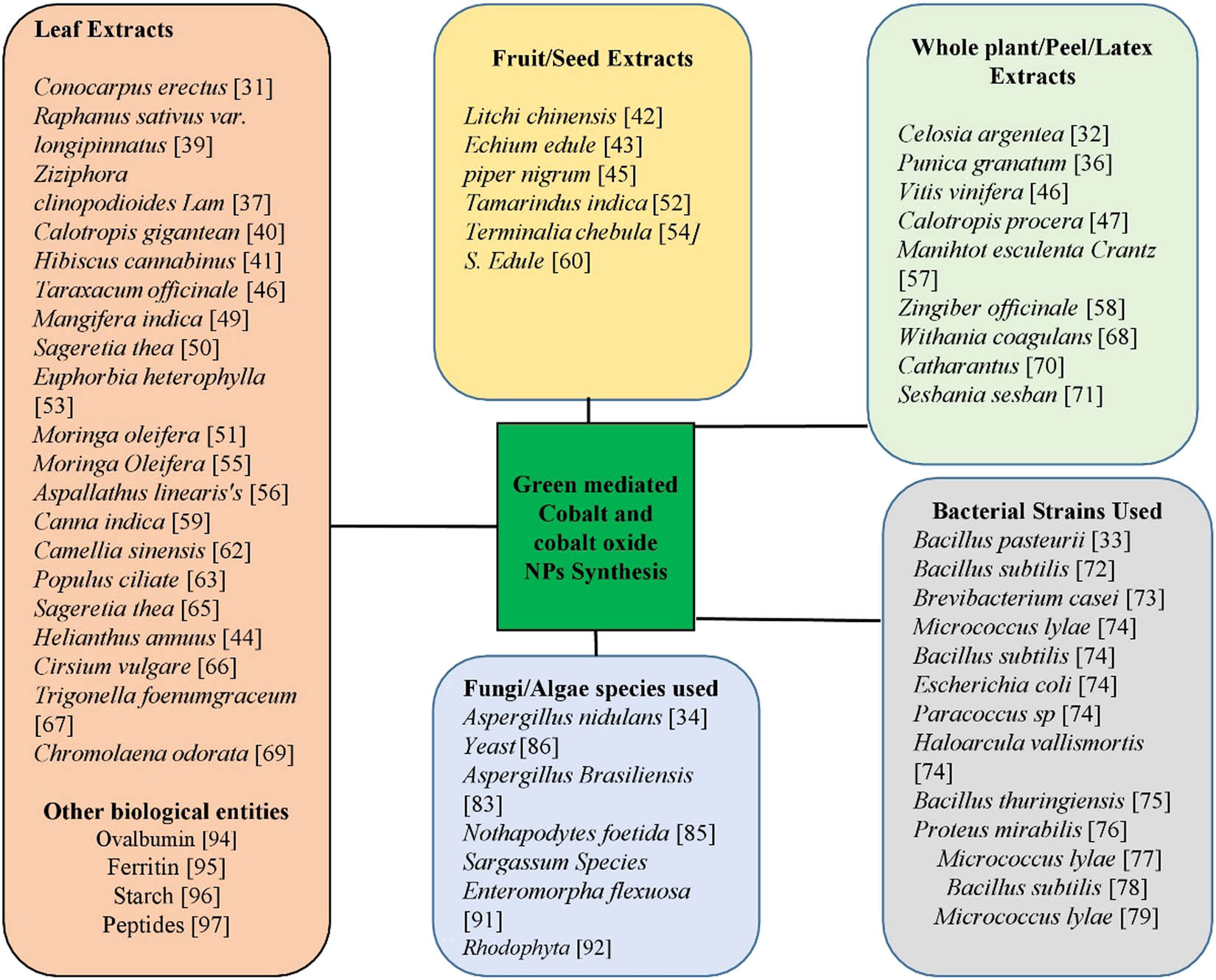
Use of different plant/parts of plant, microorganism, and other biological molecules for the synthesis of cobalt and cobalt oxide NPs.
3.1 Green synthesis using plant extracts
In contrast to bacteria, algae, and fungi, plants have been extensively used to synthesize cobalt and cobalt oxide NPs. This is because of their abundance, safe nature, and also the greater stabilization and reduction of plant phytochemicals. This method has been considered as an alternative to complex and costly physicochemical processes because of the following properties: viability, commercial feasibility, eco-friendliness, reliability, no waste generation, and simplicity [36,37]. Different parts of the plant such as leaves, roots, stem, fruits, seeds, latex, inner parts of the plant, shells, and peels have been used to synthesize Co and Co3O4 NPs as shown in Figure 3. The plant extracts have a variety of flavonoids, polysaccharides, amino acids, polyphenols, phenolic acids, ferulic acid, gentisic acid, terpenoids, thymol, tryptophan, and alkaloids, which act as a stabilizing, reducing, and chelating agent as presented in Table 1. These metabolites act as oxygen quenchers, reducing and stabilizing agents, metal chelating agents, and hydrogen donors. The reduction of metal ions by these substances within the plant extracts leads to the formation of respective nanomaterials [38]. Because of a rich source of metabolites, leaf extracts have been widely used for the synthesis of CoNPs. Plant-mediated synthesis of Co and Co3O4 NPs is a simple approach and there is no need for special requirements [6]. The metal salts have been mixed with the whole plant/part of plant extracts, and the reaction completes in a few hours under normal lab conditions [33,34,35,36,37,38].
Plant-mediated synthesis of Co and Co3O4 NPs
| Plant scientific name/common name | Part | NPs | Characterization | Phytoconstituents present in plant | Size (nm) | Shape | References |
|---|---|---|---|---|---|---|---|
| Conocarpus erectus/buttonwood or button mangrove | Leaf extract | Cobalt | SEM and XRD | Tannins, flavonoids, and phenolic acids | 20–60 | Spherical | [31] |
| Celosia argentea/plumed cockscomb or Silver cock’s comb | Whole plant extract | Cobalt | XRD, SEM, and EDX | Flavonoids, tannins, and phenolic acids | 27.42 | [32] | |
| Punica granatum/pomegranate | Peel extract | Cobalt oxide | XRD, SEM, EDX, AFM, FTIR, and UV | Gallic acid, puni87 calagins A and B, ellagic acid, and gallotannins | 40–80 | Spherical | [36] |
| Ziziphora clinopodioides Lam/kakuti-e kuhi | Leaf extract | Cobalt | UV-vis, XRD, EDS, SEM, TEM, and FTIR | Flavonoids, α and β pinen, terpenoids, thymol, piperitenone, sis-isopulegone, pulegone, and cineol | 28.19 | Crystal | [37] |
| Raphanus sativus var. longipinnatus/Radish | Leaf extract | Cobalt | UV-vis, FTIR, SEM, and EDX | Ferulic acid, gentisic acid, raphanusin, erucic acid, sinapate, raphanin, and sulforaphen | 80 | Spherical | [39] |
| Calotropis gigantea/giant milkweed, crown flower, giant calotrope, swallow-wort | Leaf extract | Cobalt oxide | XRD, UV-vis, SEM, TEM, and EDX | Triterpenoids, flavonoids (polyphenols), steroids, cardenolides, and alkaloids | 50 | Spherical | [40] |
| Hibiscus cannabinus/Deccan hemp and Java jute | Leaf extract | Cobalt | XUV-vis, XRD, SEM, TEM, and FTIR | Phytosterols, flavonoids, polyphenols, tannins, steroids, alkaloids, saponins, lignans, essential oils, and glucosides | 20.88 | Crystalline | [41] |
| Litchi chinensis/litchi fruits | Fruits extract | Cobalt oxide | XRD, SEM, TEM, and FTIR | Phenolic acid, flavonoids condensed tannins, luteolin, anthocyanin, and proanthocyanidins. | NA | Rod like | [42] |
| Sechium edule/perennial climber | Fruit extract | Cobalt oxide | XRD, FTIR, TEM, AFM, SEM, and VSM | Ascorbic acid | 3.79 | Irregular | [43] |
| Helianthus annuus/sunflower | Leaf extract | Cobalt oxide | XRD, TGA, SEM | NA | NA | Plate | [44] |
| Piper nigrum | Seeds | Cobalt oxide | UV-vis, AFM, FTIR | NA | 30–60 | Spongy triangular | [45] |
| Vitis vinifera/common grape vine | Whole plant extract | Cobalt oxide | XRD, FT-IR, Raman, TEM, SAED, EDX, DRS, PL, and VSM | Phenolic, stilbenoids, anthocyanin, and acetylated anthocyanin | 10–20 | Rod shape | [46] |
| Calotropis procera/Sodom apple | Latex | Cobalt oxide | XRD, DSC, TEM, EDX, FTIR, and UV-vis | Tryptophan, alkaloids, resins, tannins calotropin, calactin, and calotoxin | 10 | Spherical | [47] |
| Taraxacum officinale/common dandelion | Leaf extract | Cobalt oxide | UV-vis, FT-IR, SEM, and TEM | Flavonoids and phenolic | 50–100 | Spherical | [48] |
| Mangifera indica/mango | Leaf extract | Cobalt | UV-vis, XRD, FT-IR, and SEM | Polyphenolics, flavonoids, and triterpenoids | 25–40 | Irregular shape | [49] |
| Sageretia thea/Osbeck. | Leaf extracts | Cobalt oxide | XRD, ATR-FTIR, HR-SEM, HR-TEM, SAED, and EDS | Friedeline, syringic acid, beta-sitosterol, daucosterol, gluco-syringic acid, and taraxerol | 20.03 | [50] | |
| Moringa oleifera/drumstick tree, horseradish tree, and ben oil tree or benzolive tree | Leaf extract | Cobalt oxide | HR-TEM, EDS, FTIR, and AXS | α-Maltose, adenosine, catechin, chlorogenic acid, rutin, quercetin, kaempferol, caffeic acid, etc. | 20–50 | Symmetric | [51] |
| Tamarindus indica/tamarind fruit | Fruit pulp | Cobalt aluminate | FTIR, UV-vis, XRD, SEM, and RS | Tartaric acid, malic acid, and amino acids | 71 | Rectangular like | [52] |
| Euphorbia heterophylla/fire plant, painted euphorbia, Japanese poinsettia, desert poinsettia, and wild poinsettia | Leaf extract | Cobalt oxide | FTIR, XRD, Malvern Zetasizer Particle Size Analyzer, TEM, and UV-vis DRS | Alkaloid and saponin | 69.75 | Spherical | [53] |
| Terminalia chebula/black- or chebulic myrobalan | Fruit | Cobalt oxide | ATR-FTIR, XRD, TEM, SEM, and EDS | Hydrolysable tannins, gallic acid, chebulic acid, chebulic ellagitannins, and gallate esters | 15–25 | Spherical | [54] |
| M. oleifera/drumstick | Leaf extract | Cobalt | SEM, FTIR, UV-vis, and EDX | Oleic acid, ascorbic acid, dihexadecanoate, octadecenoic acid, methyl ester-hexadecanoic acid, and octadecenamide | 168–295 | Crystal | [55] |
| Aspalathus linearis/Rooibos | Leaf powder | Cobalt oxide | HR-TEM, EDS, Max solid-state Silicon drift detector, XRD, XPS, FTIR, and Raman spectroscopy | Phenolic compounds, aspalalinin, flavones, and flavonols | 3.6 | Quasi-spherical | [56] |
| Manihtot esculenta Crantz/cassava | Whole extract | Cobalt oxide | SEM, EDS, TEM, FT-IR, XRD, VSM, TGA, and EDX | Malonaldehyde, superoxide dismutase, reduced glutathione, and catalase | N/A | Prism like-anchored octahedron | [57] |
| Zingiber officinale/ginger | Whole plant | Cobalt | XRD, SEM, FTIR, VSM, and EDS | Gingerol, zingerone, shagaols, paradole, and starch | 20–50 | Crystal | [58] |
| Canna indica/Indian shot, African arrowroot, edible canna | Leaf extract | Bimetallic cobalt | UV-vis, TEM, EDX, and Thermo Electron LED | Glycosides, alkaloids, and terpenoids | 450–550 | Polydispersed | [59] |
| S. edule/chayote | Fruit extract | Bimetallic cobalt | AAS, SEM, SAED, TEM, XRD, and EPR | Trans-cinnamic acid, phenylacetic acid, trilinolenin, etc. | 47.3 | Prismoidal | [60] |
| Cinnamomum verum/true cinnamon tree or Ceylon cinnamon tree | Bark | Cobalt aluminate | MCM, XRD, SEM, TEM, XPS, IR, and UV-vis | Polysaccharides, polyphenols, flavonoids, and amino acids | 50–60 | Polyhedral | [61] |
| Camellia sinensis/tea plant, tea shrub, and tea tree | Leaf extract | Cobalt oxide | XRD, FESEM, EDX, HR-TEM, PL, FTIR, and UV-visible | Catechins, alkaloids, flavonoids, proteins, enzymes, vitamins, carbohydrates, polyphenols, lipids, and minerals | 39.13 | Quasi-rectangular | [62] |
| Populus ciliata/safaida | Leaf extract | Cobalt oxide | FTIR, TEM, SEM, and XRD | Alcohol-benzene, lignin, holocellulose, and alphacellulose | 15–35 | Square shape | [63] |
| Tamarindus indica/Indian tamarind | Fruit extract | Cobalt aluminate | FTIR, UV-vis, XRD, SEM, and TEM | Tartaric acid, malic and citric acid, potassium ditartrate, amino acids, and vitamin B | 71.3 | Cubic spinel | [64] |
| S. thea/Osbeck. | Leaf extract | Cobalt oxide | XRD, TEM, SEM, SAED, and EDS | Acids/base | 20.03 | Cubic | [65] |
| Cirsium vulgare | Leaf extract | Cobalt oxide | XRD, SEM, and TEM | NA | 20 | [66] | |
| Trigonella foenumgraceum/fenugreek | Leaf extract | Cobalt oxide | FTIR, XPS, XRD, EDS, UV-vis, and TEM | Flavonoids, polyphenols, and different glycosides | 13.2 | Quasi-spherical | [67] |
| Withania coagulans | Whole plant extract | Cobalt oxide | UV-vis and XRD | Withanolide, withaferin, and withacoagin | NA | Cube shape | [68] |
| Chromolaena odorata | Leaf extract | Cobalt | UV-vis, XRD, FT-IR, and SEM | NA | 20–49 | Irregular, cubic, and hexagonal shapes | [69] |
| Catharanthus roseus/periwinkle | Whole plant extract | Cobalt | XRD, FESEM, and EDX | Alkaloids, tannins, flavonoids, polyphenols, and carbohydrates | 27.08 | Spherical shape | [70] |
| Sesbania sesban | Whole extract | Cobalt oxide | HR-TEM, EDAX, and XRD | NA | 15–30 | Spherical | [71] |
Abbreviations: NPs, nanoparticles; NA, not available; SAED, selected area electron diffraction; UV-vis, ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; AFM, atomic force microscopy; HR-SEM, high-resolution scanning electron microscopy; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; NA, not available; UV-DRS, ultraviolet differential reflectance spectroscopy; TGA, thermo gravimetric analysis; VSM, vibrating sample magnetometer; PL, photoluminescence spectroscopy; PDXL, integrated X-ray powder diffraction software; EDS, energy-dispersive X-ray spectroscopy.
Raphanus sativus var. longipinnatus leaf extract was used to fabricate CoNPs with a spherical structure that represents the presence of ferulic acid, gentisic acid, raphanusin, erucic acid, sinapate, and sulforaphen. The green-synthesized NPs were characterized by UV-visible spectroscopy (UV-vis), X-ray diffraction (XRD), and scanning electron microscopy-energy-dispersive X-ray (spectroscopy) (SEM-EDX). These CoNPs showed potential antibacterial activities and also showed promising cytotoxic activities against the HeLa cancer cell lines in vitro [39]. Co3O4 NPs were synthesized having spherical morphologies with a diameter size of 50–60 nm, using Calotropis gigantea leaf extracts that indicate the presence of triterpenoids, flavonoids (polyphenols), steroids, cardenolides, and alkaloids [40]. Hibiscus cannabinus leaf extract was used to synthesize crystalline CoNPs having a diameter of 20.8 nm. These CoNPs were characterized by UV-vis, XRD, and Fourier-transform infrared spectroscopy (FTIR). Authors reported that the green-synthesized NPs have strong activities against Bacillus subtilis and Escherichia coli [41]. Onwudiwe et al. (2020) reported the bio-mediated synthesis of Co3O4 NPs using the fruit extract of Litchi chinensis. The characterizations of synthesized NPs were confirmed by XRD, SEM, transmission electron microscopy (TEM), and FTIR. SEM analysis revealed that the bioinspired NPs had elongate rod-like morphology. FTIR analysis was also carried out to detect the functional group of various phytochemicals that are involved in the oxidation/reduction and hence the formation of cobalt oxide NPs. The FTIR analysis confirmed the presence of phenolic and carboxylic functional groups, indicating that these groups are responsible for the formation of cobalt oxide NPs [42]. Sechium edule and Helianthus annuus fruits’ extracts [34,44] and Piper nigrum seeds [45] have also been reported for the synthesis of Co3O4 NPs. Vitis vinifera whole plant extracts were used for the synthesis of Co3O4 NPs having a pure single crystalline structure with a size of 10–20 nm in diameter. The TEM and FTIR analysis indicated that the plant extracts play an important role in the stabilization and reduction of nanorods via different types of organic compounds present in the plant extracts [46]. Latex derived from Calotropis procera was used to synthesize Co3O4 NPs with spherical morphologies and average size of 10 nm diameter. These NPs showed only a low toxicity at a very high concentration, proving that they are safe and may be used for different applications in various fields including the field of medicines [47]. Other than aforementioned, different parts of various plants, used for the synthesis of Co and Co3O4 NPs are shown in Table 1 and Figure 3.
3.2 Synthesis of Co and Co3O4 NPs using microorganisms
3.2.1 Bacteria-mediated synthesis
Similar to other synthesis routes such as those of plants and other microorganisms, bacteria also have an intrinsic ability to synthesize NPs of different sizes and morphologies. To date, different types of bacterial strains have been used to synthesize Co and Co3O4 NPs [33,72,74]. This approach also has some challenges compared to other methods that are yet to be solved, like the synthesis of complex materials with the desired phase, a full understanding of the synthesis mechanism at the molecular level to achieve better control over shape and size, and scaling up in order to obtain large amounts of nanomaterials [73]. Chemical methods are already advanced to provide greater control on the shape and size of nanomaterials, but this approach is eco-friendly and limits the drawbacks of the chemical synthesis [73]. However, lengthy procedures and contamination of cultures are the limitations of this approach. Different bacterial species have been used for the synthesis of cobalt and cobalt oxide NPs.
A gram-positive bacterium Bacillus thuringiensis was used to synthesize Co NPs having face-cubic morphology with an average size of 85.3 nm diameter. These green-synthesized NPs were investigated against malarial and dengue vectors (Aedes aegypti and Anopheles subpictus) and showed potential activities against these vectors [75]. Co3O4 NPs synthesized using bacterial strains Micrococcus lylae and B. subtilis have globular and rod shape morphology, respectively [74]. Gram-negative bacterium Proteus mirabilis was used to synthesize CoNPs with an average size of 57 nm and quasi-spherical morphology. The authors evaluated the antibacterial activity of these NPs, and the result showed that these NPs were promising antimicrobial potential against Salmonella typhi, E. coli, Clostridium perfringens, Staphylococcus aureus, and Bacillus cereus [76]. Table 2 provides some examples of bacterial-mediated synthesized Co and Co3O4 NPs.
Bacterial-mediated synthesis of Co and Co3O4 NPs
| Bacterial strain | Gram+/Gram− | NPs | Characterization | Size (nm) | Shape | References |
|---|---|---|---|---|---|---|
| Bacillus pasteurii | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 10–31 | Irregular | [33] |
| Bacillus subtilis | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 6.6 | Hollow rod | [72] |
| Brevibacterium casei | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 6 | Quasi-spherical | [73] |
| Micrococcus lylae | Gram+ | Cobalt oxide | XRD, SEM, FE SEM, TEM, and HR-TEM | 8 | Flower like | [77] |
| B. subtilis | Gram+ | Cobalt oxide | FESE, M ELS-Z2, FESEM, XPS, and EPMA | 3–5 | Rods | [78] |
| M. lylae | Gram+ | Cobalt oxide | XRD, TGA, FE-SEM, TEM, EDS, SAED, and HAADF-STEM | 40 | Spherical | [79] |
| M. lylae | Gram+ | Cobalt | Zetasizer Nano system and FESEM | 356 ± 55 | Globular | [74] |
| B. subtilis | Gram+ | Cobalt | Zetasizer Nano system and FESEM | NA | Rod shape | [74] |
| Escherichia coli | Gram− | Cobalt | Zetasizer Nano system and FESEM | 473 ± 54 | Rod shape | [74] |
| Paracoccus sp. | Gram− | Cobalt | Zetasizer Nano system and FESEM | NA | Biconcave | [74] |
| Haloarcula vallismortis | Gram− | Cobalt | Zetasizer Nano system and FESEM | NA | Globular | [74] |
| Proteus mirabilis | Gram− | Combine Cobalt | UV-vis, EDX, XRD, TEM, DLS, and PDI | 57 | Quasi-spherical | [76] |
| Bacillus thuringiensis | Gram+ | Cobalt | XRD, TEM, FESEM, FTIR, and EDX | 85.3 | Face-cubic | [75] |
Abbreviations: NPs, nanoparticles; NA, not available; SAED, selected area electron Diffraction; UV-vis, Ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; TGA, thermo gravimetric analysis; ATR-FTIR, attenuated total reflectance-Fourier transform infrared spectroscopy; FESEM, field emission scanning electron microscopy; EPMA, electron probe microanalyzer; HAAD-STEM, high-angle annular dark field scanning TEM; EDS, energy-dispersive X-ray spectroscopy.
3.2.2 Fungi-mediated synthesis of Co3O4 NPs
The fungi-mediated approach exhibits unique advantages, as the growth process of fungi is easy to handle and isolate, with the large amount of biomass and high yield of proteins [34]. The entophytic fungi also secrete large amounts of bioactive substances that are necessary for the synthesis of NPs in the presence of precursor substance [34,80]. During the fabrication of NPs from the precursor solution, biomass (fungi) along with the supernatant act as a reduction medium [34,81].
Using fungi, NPs can be synthesized by two pathways: intracellular and extracellular synthesis. The extracellular approach is more commonly used because of the facile separation, high mass harvesting, and easy culturing, which consequently leads to the scaling up of the process at the commercial level [82]. Fungi can also tolerate agitation, flow pressure, and other conditions in the bioreactor for commercial production. Filamentous fungi are highly resistant toward metals as compared to bacteria [83,84]. Different fungal and yeast strains have been used to synthesize cobalt oxide NPs. Aspergillus nidulans was used to synthesize spherical shape Co3O4 NPs with an average size of 20.29 nm diameter [34]. Some examples of yeast and fungi-mediated synthesis of Co3O4 NPs are presented in Table 3.
Fungal-mediated synthesis of Co and Co3O4 NPs
| Fungal species used | NPs | Characterization | Size (nm) | Shape | References |
|---|---|---|---|---|---|
| Aspergillus nidulans | Cobalt oxide | XRD, TEM, FTIR, and EDX | 20.29 | Spherical | [34] |
| Aspergillus brasiliensis | Cobalt oxide | DLS, EDX, XRD, FT-IR, HR-TEM, and FESEM | 20–27 | Quasi-spherical | [83] |
| Nothapodytes foetida | Cobalt oxide | UV-vis | N/A | H/A | [85] |
| Yeast | Cobalt oxide | SEM, XRD, and FT-IR | 24 | Hollow spheres | [86] |
Abbreviations: NPs, nanoparticles; NA, not available; UV-vis, ultraviolet visible spectroscopy; XPS, X-ray photoelectron spectroscopy; TEM, transmission electron microscopy; XRD, X-ray diffraction; HR-TEM, high-resolution transmission electron microscopy; EDX, energy-dispersive X-ray(spectroscopy); FTIR, Fourier-transform infrared spectroscopy; VSM, vibrating-sample magnetometer; FESEM, field emission scanning electron microscopy.
3.3 Algal-mediated approach for the synthesis
Algae are aquatic microorganisms that are used to a great extent for the synthesis of NPs. Algae are also called bionanofactories because they fabricate nanomaterials with high stability, are easy to handle, and do not need cell maintenance [87]. Algae are the key origin of bioactive metabolites that are involved in the fabrication of NPs [88,89]. They contain many bioactive metabolites such as proteins, polysaccharides, and various types of other phytochemicals that are comprised of amino, hydroxyl, and carboxyl functional groups, which are responsible for the fabrication of NPs [87,90]. Algae differ in size and can vary from microalgae to macroalgae [87,88,89,90]. A green-type macroalgae Enteromorpha flexuosa was used to synthesize cobalt–ferrite NPs with an average size of 5–15 nm. These bioinspired cobalt ferrite NPs were characterized by various advanced techniques such as XRD, SEM, TEM, energy-dispersive X-ray spectroscopy (EDS), and XPS [91]. Similarly, cobalt tungstate (CoWO4) NPs synthesized from red seaweeds (Rhodophyta) using agar-agar have also been reported [92].
3.4 Biological product-mediated synthesis of Co and Co3O4 NPs
The plants and microorganisms are widely used for the fabrication of NPs. Besides these, the biological derivatives are used to synthesize NPs as well. They also play a significant role in the stabilization and reduction of nanomaterials [93]. Co3O4 nanocrystals were synthesized from freshly extracted ovalbumin with 8–17 nm diameter confirmed by EPR [94]. Sun et al. (2019) also reported the synthesis of Co3O4 NPs using egg-albumin. Hosein et al. (2004) reported the synthesis of Co3O4 NPs with a uniform shape and size using ferritin [95]. Starch has also been exploited for the synthesis of carbon-encapsulated Co NPs with an average size of 20–35 nm diameter and confirmed by the nanostructure by SEM, TEM, and XRD [96]. Similarly, peptide TLVNN (threonine–leucine–valine–asparagine–asparagine) was also used as a capping agent for in situ syntheses of CoNPs for bioapplications [97].
4 Biological activities of Co and Co3O4 NPs
4.1 Antibacterial activity
Currently, throughout the globe, the emergence of bacterial resistance to the available antibiotics is a major health concern. Therefore, there is a need for the antibiotic agent that can kill pathogenic bacteria which show resistance to the available drugs [98]. The NPs have small size with high surface area compared to the bigger molecules and therefore possess strong antibacterial activities. The NPs have dose-dependent membrane permeation and inhibit the synthesis of bacterial proteins by disturbing the cell membrane [32,99]. Different metallic NPs such as gold, iron, silver, and metal oxide NPs such as iron oxide, cobalt oxide, and copper oxide showed significant antibacterial activities. The AgNPs are the main interest not only in biomedical industries but also in food industries because of its potential antimicrobial behavior [114]. Cobalt and cobalt oxide NPs also possess potential antibacterial activity. Varaprasad et al. (2017) measured the antibacterial activity of biogenic CoNPs against human pathogenic bacteria, and the results concluded that CoNPs have strong antibacterial activities compared to standard antibiotic drug ciprofloxacin [100]. Eltarahony et al. (2018) reported the green synthesis of combined CoNPs using gram-negative bacteria P. mirabilis. The characterization of NPs was confirmed by UV-vis, EDX, XRD, TEM, dynamic light scattering (DLS), and poly dispersity index (PDI). The antibacterial activity was observed using a well diffusion assay against S. typhi, E. coli, C. perfringens, S. aureus, and Enterococcus faecalis [76]. The result showed that the NPs have promising biocide efficiency against these bacteria [76]. Omran et al. (2019) synthesized quasi-spherical shape cobalt oxide NPs with an average size of 20–27 nm diameter using a fungal specie Aspergillus brasiliensis. The first-time mycosynthesized cobalt oxide NPs showed considerable activity against different bacteria [83]. Biogenic synthesis of CoNPs using Celosia argentea whole plant extract has been reported and studied for their antibacterial activities using the disk diffusion method. The synthesized NPs showed remarkable antibacterial activities against B. subtilis and E. coli [32]. Green-mediated cobalt oxide NPs have been synthesized using Hibiscus rosa-sinensis flower extract and their antibacterial activity was measured. These green-synthesized NPs showed promising activities against E. coli, Streptococcus mutans, S. aureus, and Klebsiella pneumonia [101]. Mainly two aspects have been suggested. First, in cobalt oxide NPs, the different positive states of cobalt ions, i.e., Co2+ and Co3+ interact with the parts of the bacterial cell that have a negative charge and cause the death of the bacterial cell. Second, there may be the excitement of electrons on the surface of cobalt oxide because of light irradiation in the conduction and valence band. In the conduction band, there is the formation of superoxide radical anion because of the reaction of excited electrons and oxygen molecules. Finally, the formation of hydrogen peroxide, which is a strong oxidant, occurs. On the surface of NPs, the reaction of water and superoxide radical anion destroys the bacterial cell. Therefore, Cobalt oxide nanoparticles can be a potent antibacterial agent at a minimal level of concentration [6,102]. The antibacterial activities of cobalt and cobalt oxide NPs synthesized from different green routes are presented in Table 4.
Antibacterial activities of green-synthesized cobalt and cobalt oxide NPs
| Biological entity | NPs | Test microorganisms | Method | References |
|---|---|---|---|---|
| Celosia argentea | Cobalt | Bacillus subtilis and Escherichia coli | Disk diffusion | [32] |
| Ziziphora clinopodioides Lam | Cobalt | Salmonella typhimurium, E. coli, Streptococcus pneumonia, Pseudomonas aeruginosa, Staphylococcus aureus, and B. subtilis | Disk diffusion | [37] |
| Raphanus sativus var. longipinnatus | Cobalt | Pseudomonas putida and Klebsiella pneumonia | Disk diffusion | [39] |
| Hibiscus cannabinus | Cobalt | B. subtilis and E. coli | Agar well diffusion | [41] |
| Vitis vinifera | Cobalt oxide | P. aeruginosa, E. coli, S. aureus, and B. subtilis | Disk diffusion | [46] |
| Calotropis procera | Cobalt oxide | E. coli, Pseudomonas sp., Alcaligenes sp., and Enterococcus sp. | Disc diffusion | [47] |
| Moringa oleifera | Cobalt | E. coli and S. aureus | Agar well diffusion | [51] |
| Sechium edule | Bimetallic cobalt | B. subtilis and E. coli | Disk diffusion | [60] |
| Populus ciliata | Cobalt oxide | Bacillus licheniformis, B. subtilis, K. pneumonia, and E. coli | Well diffusion | [63] |
| Sageretia thea | Cobalt oxide | P. aeruginosa, E. coli, K. pneumonia, Staphylococcus epidermis, S. aureus, and B. subtilis | Disc diffusion | [65] |
| Chromolaena odorata | Cobalt | E. coli, K. pneumonia, S. aureus, and Streptococcus pyogenes | Agar well diffusion | [69] |
| Catharanthus roseus/Periwinkle | Cobalt | B. subtilis and E. coli | Disc diffusion | [70] |
| Sesbania sesban | Cobalt oxide | S. aureus | Disk diffusion | [71] |
| Proteus mirabilis | Cobalt | P. aeruginosa, Salmonella typhi, E. coli, Clostridium perfringens, Enterococcus faecalis, Bacillus cereus, and S. aureus | Well diffusion | [76] |
| Aspergillus brasiliensis | Cobalt oxide | B. subtilis, S. aureus, P. aeruginosa, and E. coli | Agar well diffusion | [83] |
Abbreviation: NPs, nanoparticles.
4.2 Antifungal activity
The resistance of bacteria and fungi to the available antibiotics and drugs is at an alarming rate. Therefore, there is a need for strong antifungal agents that can destroy fungi that are resistant to the drugs available [19]. Cobalt and cobalt oxide NPs have various biomedical applications because of different properties including antifungal property. Hou et al. (2020) measured the antifungal properties of green-synthesized CoNPs, and the result showed that the CoNPs have strong antifungal activities against Candida krusei, Candida guilliermondii, Candida glabrata, and Candida albicans [37]. Similarly, cobalt oxide NPs synthesis using flower extract of H. rosa-sinensis and their antifungal activities has been reported. The result showed that the synthesized NPs have strong activities against Aspergillus flavus and A. niger [62].
4.3 Larvicidal activities of CoNPs
For tropical and subtropical countries, the vector and vector-borne diseases have become a big trouble for public health [103]. For the human vector-borne infectious disease, the microorganisms (parasite, bacterium, or virus) and the vectors (mosquito, fly, or tick) are the crucial elements [104]. Mosquitos are the vector of infectious diseases such as dengue, malaria, fever, and yellow fever [104]. The mosquitoes affect humans as well as domestic animals throughout the globe. The biocontrol method is being considered because of high resistance to the chemical insecticides and no new approaches to control the mosquitos. In this approach, different microbes, e.g., B. thuringiensis, have been used in regards to their toxicity to the target vector [75,105,106].
A. subpictus and A. aegypti are the vectors that cause malaria and dengue, respectively, and are the main species of medical interest. In more than 100 countries, about 2.5 billion people are at risk of infection with dengue. Fifty million people are affected worldwide with more than 24,000 deaths per year. There is a need for development of new agents to control these vectors [75]. CoNPs have also been tested against malarial A. subpictus and A. aegypti. Marimuthu et al. (2013) tested the bacterial-mediated synthesized CoNPs using B. thuringiensis against malarial and dengue vectors in vitro. The results showed that the green-synthesized CoNPs have promising activities that exceed those of biocontrol agent (B. thuringiensis) against these vectors [75]. Therefore, it may possible to use the CoNPs in drug formulation against these parasites.
4.4 Antileishmanial activity
The World Health Organization considered the leishmaniasis as one of the uncontrolled, emerging, and neglected diseases with the second-highest prevalence rate, with malaria placing itself at the topmost among the parasitic diseases [107]. Leishmaniasis is caused by leishmania parasites that exist in two forms, promastigote (motile) and amastigote (non-motile) [108]. Leishmaniasis occurs mainly in two clinical forms: visceral leishmaniasis (also called kala-azar), which is the most severe form and - if left untreated - may lead to death, and cutaneous leishmaniasis. It is predicted that about 0.5 million cases of visceral leishmaniasis and 1.5 million cases of cutaneous leishmaniasis occur throughout the globe per year [109]. This deadly disease is endemic in 98 countries of the world. Currently, no vaccines are available against leishmaniasis. The only option available are the treatment drugs and none of the available drugs is ideal because of their cost, duration of therapy, severe side effects, high toxicity, and - most importantly - the resistance of leishmania parasites to the available treatment [107]. Therefore, there is an immediate need to develop an effective antileishmanial approach to combat this deadly infection.
Talha et al. (2017) synthesized cobalt oxide NPs using the extract of Sageretia thea (Osbeck.), a medicinal plant. The characterizations were confirmed by XRD, FTIR, EDS, selected area electron diffraction, high-resolution scanning electron microscopy, and high-resolution transmission electron microscopy. For the first time the antileishmanial activity of the green-synthesized NPs was evaluated using MTT cytotoxic assay. The results showed that the antileishmanial response was dose dependent, and the amastigote parasites were most susceptible as compared to the promastigote. Therefore, cobalt oxide NPs may be one of the possible options in nanomedicine to treat leishmania at any stage of the life cycle [50].
4.5 Antioxidant activity
Oxidative metabolism is a key process for the survival of cells. However, this process has some side effects as they produce free radicals and reactive oxygen species. When these free radicals are produced in the body in the excess amount they can inundate the enzymes such as catalases, peroxidase, and superoxide dismutase and lead to lethal cellular effects by oxidizing cellular proteins, membrane lipids, DNA enzymes, and influence signaling pathways of the cell leading to termination of cellular respiration [110]. Oxidation affects food as well, which is one of the main causes of chemical spoilage that affects flavor, texture, nutritional value, and safety of food. Different types of natural and synthetic antioxidants are available to limit the side effects of oxidation [110,111].
NPs also have strong antioxidant activities. Plant-mediated cobalt oxide NPs having cubic shape morphologies with an average size of 20.03 nm diameter have been prepared using the leaf extract of S. thea. The antioxidant assays, free radical scavenging, total antioxidant capacity, and total reducing power have been evaluated. The biogenic cobalt oxide NPs showed superior radical scavenging potential and an average total antioxidant capacity and total reducing power [50]. Shahzadi et al. (2019) also observed radical scavenging activity of bioinspired CoNPs and reported that the scavenging power and antioxidant activity are dose dependent: the increase of activity leads to the increase in the concentration of CoNPs [32]. CoNPs using the leaf extract of Ziziphora clinopodioides Lam has been synthesized and the antioxidant activities were evaluated. The green-synthesized NPs showed impressive results and have good DPPH free radical scavenging activity [37]. Similarly, DPPH radical scavenging activity of cobalt oxide NPs synthesized from the Sesbania sesban extract has been reported to have minimum activities compared to silver and copper oxide NPs [71].
4.6 Cytotoxic activity
Cytotoxicity assay is a test for the characterization and evaluation of potentially harmful and toxic effects of biomolecules or any other materials on the living organisms/cell cultures. Various types of molecules, plant extracts, and NPs have been used to check the cytotoxic effect [112]. The green-synthesized CoNPs were used in different concentrations to investigate and determine the cytotoxicity of human umbilical vein endothelial cells (HUVECs) in vitro. The cells treated with various concentrations of CoNPs were examined by the MTT test for 48 h. The absorbance rate was determined at 570 nm, which indicated good viability on (HUVECs) cell lines even up to 1,000 mg/mL of CoNPs, and the absence of such type of toxicity of CoNPs has many safe applications in nanomedicines [37]. Padigya et al. (2016) observed the cytotoxic effect of the biogenic CoNPs on HeLa cell lines. They reported that CoNPs showed potential cytotoxic effects against HeLa cancer cell lines [39]. The green-synthesized cobalt oxide NPs were also investigated for cytotoxicity through brine shrimp cytotoxicity assay. Cytotoxicity of the Co3O4 NPs was confirmed by their dose-dependent response, whereas the median lethal concentration was calculated as 19.18 μg/mL [50].
4.7 Hemolytic activities
Hemolysis is the release of hemoglobin in the blood because of the disruption of erythrocyte membranes that may lead to jaundice or anemia. That is why it is very important to check the hemolytic activity of any newly synthesized preparate that can be used for pharmacological purposes [113]. Shahzadi et al. (2019) evaluated the hemolytic activity of green-synthesized CoNPs using the plant extract of C. argentea. The results showed that the biosynthesized CoNPs have less hemolytic activity (2.95%) compared to positive control triton-X-100 which has 95.25% toxicity, whereas the negative taken has 1.02% toxicity [32]. Zaib et al. (2019) fabricated CoNPs using the Catharanthus roseus extract and evaluated their hemolytic activities. They reported that the hemolysis rate of CoNPs is approximately 1.53%, with the negative and positive control rates of 1.02% and 95.28%, respectively [70]. Therefore, CoNPs can be used in drug formation because of their safety, cost-effectiveness, and nontoxic nature. Cobalt oxide NPs were prepared using S. thea extract and evaluated in regards to their biocompatibility and various biological applications. The result presented that the median lethal dose concentration was observed as >58.55 µg/mL and 200 µg/mL for macrophages and RBCs, respectively [50]. For that reason the biosynthesized cobalt oxide NPs might be used at low concentrations for drug formulations.
4.8 Other biological applications
Apart from antimicrobial, antioxidant, antileishmanial, cytotoxic, hemolytic, and larvicidal activities, Co and Co3O4 NPs have various biological and medical applications. After cardiovascular diseases, cancer is the second main cause of human dysphoria [19]. CoNPs showed promising anticancer activities. Kgosiemang et al. (2020) synthesized cobalt oxide NPs using the Euphorbia tirucalli extract and investigated the anti-proliferative activity using MTT assay against MCF-7 breast cancer cell lines. The result showed that the biosynthesized cobalt oxide NPs exhibit promising activities against MCF-7 breast cancer cell lines [53]. The cutaneous wound healing potential of green-synthesized CoNPs has been investigated and it was reported that the ointment of CoNPs has great potential in cutaneous wound healing [37]. The catalytic activity, enzyme inhibition, antidiabetic activity, and anticholinergic activity of biogenic cobalt and cobalt oxide NPs have been reported [37,50].
5 Conclusion
The green-synthesized cobalt and cobalt oxide NPs have various biological and biomedical applications. Traditionally, NPs are synthesized by either physical or chemical methods, which leads not only to environmental toxicity but also to costly and energy-intensive labor. Cobalt and cobalt oxide NPs are synthesized by a green route using the extracts of different plants/parts of plants, microorganisms, and other biological molecules such as gelatin, oleic acid, and starch. The biomediated cobalt and cobalt oxide NPs are environmentally friendly, facile in terms of synthesis, cost effective, and biocompatible.
-
Funding: The authors state no funding involved.
-
Author contributions: A. W. and M. A. – conceptualization and original draft preparation; A. W., A. A., A. B., M. D., S. A., and A. U. K. – review and editing. All the authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2019 Nov 1;12(7):908–31.10.1016/j.arabjc.2017.05.011Search in Google Scholar
[2] Nadeem M, Khan R, Afridi K, Nadhman A, Ullah S, Faisal S, et al. Green synthesis of cerium oxide nanoparticles (CeO2 NPs) and their antimicrobial applications: a review. Int J Nanomed. 2020;15:5951.10.2147/IJN.S255784Search in Google Scholar PubMed PubMed Central
[3] Kubik T, Bogunia-Kubik K, Sugisaka M. Nanotechnology on duty in medical applications. Curr Pharm Biotechnol. 2005;6(1):17–3310.2174/1389201053167248Search in Google Scholar PubMed
[4] Smith DM, Simon JK, Baker Jr JR. Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605.10.1038/nri3488Search in Google Scholar PubMed PubMed Central
[5] Faucon MP, Pourret O, Lange B. Element case studies: cobalt and copper. In: Agromining: farming for metals. Cham: Springer; 2018. p. 233–9.10.1007/978-3-319-61899-9_13Search in Google Scholar
[6] Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.10.1039/D0GC00885KSearch in Google Scholar
[7] Egorova KS, Ananikov VP. Toxicity of metal compounds: knowledge and myths. Organometallics. 2017 Nov 13;36(21):4071–90.10.1021/acs.organomet.7b00605Search in Google Scholar
[8] Xu Q, Li W, Ding L, Yang W, Xiao H, Ong WJ. Function-driven engineering of 1D carbon nanotubes and 0D carbon dots: mechanism, properties and applications. Nanoscale. 2019;11(4):1475–504.10.1039/C8NR08738ESearch in Google Scholar PubMed
[9] Ansari SM, Bhor RD, Pai KR, Sen D, Mazumder S, Ghosh K, et al. Cobalt nanoparticles for biomedical applications: facile synthesis, physiochemical characterization, cytotoxicity behavior and biocompatibility. Appl Surf Sci. 2017 Aug 31;414:171–87.10.1016/j.apsusc.2017.03.002Search in Google Scholar
[10] Raveau B, Seikh MM. Charge ordering in cobalt oxides: impact on structure, magnetic and transport properties. Z Anorg Allg Chem. 2015 Jul;641(8–9):1385–94.10.1002/zaac.201500085Search in Google Scholar
[11] Bersuker IB. Electronic structure and properties of transition metal compounds. New York: Wiley; 1996.Search in Google Scholar
[12] Liu J, Wang Z, Yan X, Jian P. Metallic cobalt nanoparticles imbedded into ordered mesoporous carbon: a non-precious metal catalyst with excellent hydrogenation performance. J Colloid Interface Sci. 2017 Nov 1;505:789–95.10.1016/j.jcis.2017.06.081Search in Google Scholar PubMed
[13] Su Y, Zhu Y, Jiang H, Shen J, Yang X, Zou W, et al. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale. 2014;6(24):15080–9.10.1039/C4NR04357JSearch in Google Scholar
[14] Azharuddin M, Zhu GH, Das D, Ozgur E, Uzun L, Turner AP, et al. A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun. 2019;55(49):6964–96.10.1039/C9CC01741KSearch in Google Scholar PubMed
[15] Cardoso VF, Francesko A, Ribeiro C, Bañobre‐López M, Martins P, Lanceros‐Mendez S. Advances in magnetic nanoparticles for biomedical applications. Adv Healthcare Mater. 2018 Mar;7(5):1700845.10.1002/adhm.201700845Search in Google Scholar PubMed
[16] Liakos I, Grumezescu AM, Holban AM. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules. 2014 Aug;19(8):12710–26.10.3390/molecules190812710Search in Google Scholar PubMed PubMed Central
[17] Eleraky NE, Allam A, Hassan SB, Omar MM. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics. 2020 Feb;12(2):142.10.3390/pharmaceutics12020142Search in Google Scholar PubMed PubMed Central
[18] Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9(1):1050–74.10.3762/bjnano.9.98Search in Google Scholar PubMed PubMed Central
[19] Ahmad S, Munir S, Zeb N, Ullah A, Khan B, Ali J, et al. Green nanotechnology: a review on green synthesis of silver nanoparticles – an ecofriendly approach. Int J Nanomed. 2019;14:5087.10.2147/IJN.S200254Search in Google Scholar PubMed PubMed Central
[20] Pachapur V, Brar SK, Verma M, Surampalli RY. Nanomaterials in the environment. In: Nano-ecotoxicology of natural and engineered nanomaterials for animals and humans. American society of civil engineering library; 2015. p. 421–37.10.1061/9780784414088.ch16Search in Google Scholar
[21] Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arabian J Chem. 2017. 10.1016/j.arabjc.2017.05.011.Search in Google Scholar
[22] Ranjit K, Baquee AA. Nanoparticle: an overview of preparation, characterization and application. Int Res J Pharm. 2013;12:908–31.Search in Google Scholar
[23] Anyaegbunam FN, Augustine C. A study of optical band gap and associated urbach energy tail of chemically deposited metal oxides binary thin films. Dig J Nanomater Biostruct. 2018 Jul 1;3(3):847–56.Search in Google Scholar
[24] Stella C, Soundararajan N, Ramachandran K. Structural, optical, and magnetic properties of Mn and Fe-doped Co3O4 nanoparticles. AIP Adv. 2015 Aug 4;5(8):087104.10.1063/1.4928218Search in Google Scholar
[25] Korde P, Ghotekar S, Pagar T, Pansambal S, Oza R, Mane D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles – a review on plant parts involved, characterization and their recent applications. J Chem Rev. 2020 Apr 23;2:157–68.Search in Google Scholar
[26] Li W, Jung H, Hoa ND, Kim D, Hong SK, Kim H. Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application. Sens Actuators B. 2010 Sep 21;150(1):160–6.10.1016/j.snb.2010.07.023Search in Google Scholar
[27] Li WY, Xu LN, Chen J. Co3O4 nanomaterials in lithium‐ion batteries and gas sensors. Adv Funct Mater. 2005 May;15(5):851–7.10.1002/adfm.200400429Search in Google Scholar
[28] Kumar R, Kim HJ, Park S, Srivastava A, Oh IK. Graphene-wrapped and cobalt oxide-intercalated hybrid for extremely durable super-capacitor with ultrahigh energy and power densities. Carbon. 2014 Nov 1;79:192–202.10.1016/j.carbon.2014.07.059Search in Google Scholar
[29] Adekunle AS, Oyekunle JA, Durosinmi LM, Oluwafemi OS, Olayanju DS, Akinola AS, et al. Potential of cobalt and cobalt oxide nanoparticles as nanocatalyst towards dyes degradation in wastewater. Nano-Struct Nano-Objects. 2020 Feb 1;21:100405.10.1016/j.nanoso.2019.100405Search in Google Scholar
[30] Hagelin-Weaver HA, Hoflund GB, Minahan DM, Salaita GN. Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar + bombardment. Applied Surface Science. 2004 Aug 31;235(4):420–48.10.1016/j.apsusc.2004.02.062Search in Google Scholar
[31] Ahmed K, Tariq I, Siddiqui SU, Mudassir M. Green synthesis of cobalt nanoparticles by using methanol extract of plant leaf as reducing agent. Pure Appl Biol. 2016;5(3):453.10.19045/bspab.2016.50058Search in Google Scholar
[32] Shahzadi T, Zaib M, Riaz T, Shehzadi S, Abbasi MA, Shahid M. Synthesis of eco-friendly cobalt nanoparticles using Celosia argentea plant extract and their efficacy studies as antioxidant, antibacterial, hemolytic and catalytical agent. Arabian J Sci Eng. 2019;44(7):6435–44.10.1007/s13369-019-03937-0Search in Google Scholar
[33] Hsu CM, Huang YH, Chen HJ, Lee WC, Chiu HW, Maity JP, et al. Green synthesis of nano-Co3O4 by microbial induced precipitation (MIP) process using Bacillus pasteurii and its application as supercapacitor. Mater Today Commun. 2018;1(14):302–11.10.1016/j.mtcomm.2018.02.005Search in Google Scholar
[34] Vijayanandan AS, Balakrishnan RM. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J Environ Manage. 2018;15(218):442–50.10.1016/j.jenvman.2018.04.032Search in Google Scholar PubMed
[35] Vaya D, Das BK. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70.10.2174/2210681208666180312123055Search in Google Scholar
[36] Bibi I, Nazar N, Iqbal M, Kamal S, Nawaz H, Nouren S, et al. Green and eco-friendly synthesis of cobalt-oxide nanoparticle: characterization and photo-catalytic activity. Adv Powder Technol. 2017;28(9):2035–43.10.1016/j.apt.2017.05.008Search in Google Scholar
[37] Hou H, Mahdavi B, Paydarfard S, Zangeneh MM, Zangeneh A, Sadeghian N, et al. Novel green synthesis and antioxidant, cytotoxicity, antimicrobial, antidiabetic, anticholinergics, and wound healing properties of cobalt nanoparticles containing Ziziphora clinopodioides Lam leaves extract. Sci Rep. 2020;10(1):1–9.10.1038/s41598-018-33214-3Search in Google Scholar PubMed PubMed Central
[38] Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A. 2010 Oct 20;369(1–3):27–33.10.1016/j.colsurfa.2010.07.020Search in Google Scholar
[39] Koyyati R, Kudle KR, Padigya PR. Evaluation of antibacterial and cytotoxic activity of green synthesized cobalt nanoparticles using Raphanus sativus var. longipinnatus leaf extract. Int J PharmTech Res. 2016;9(3):466–72.Search in Google Scholar
[40] Sharma JK, Srivastava P, Singh G, Akhtar MS, Ameen S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater Sci Eng B. 2015;193:181–8.10.1016/j.mseb.2014.12.012Search in Google Scholar
[41] Kharade Suvarta D, Nikam Gurunath H, Mane Gavade Shubhangi J, Patil Sachinkumar R, Gaikwad Kishor V. Biogenic synthesis of cobalt nanoparticles using Hibiscus cannabinus leaf extract and their antibacterial activity. Res J Chem Environ. 2020;24(5):9–13.Search in Google Scholar
[42] Onwudiwe DC, Ravele MP, Elemike EE. Eco-friendly synthesis, structural properties and morphology of cobalt hydroxide and cobalt oxide nanoparticles using extract of Litchi chinensis. Nano-Struct Nano-Objects. 2020;1(23):100470.10.1016/j.nanoso.2020.100470Search in Google Scholar
[43] Das RK, Golder AK. Co3O4 spinel nanoparticles decorated graphite electrode: bio-mediated synthesis and electrochemical H2O2 sensing. Electrochim Acta. 2017;251:415–26.10.1016/j.electacta.2017.08.122Search in Google Scholar
[44] Saeed M, Akram N, Naqvi SA, Usman M, Abbas MA, Adeel M, Nisar A. Green and eco-friendly synthesis of Co3O4 and Ag-Co3O4: characterization and photo-catalytic activity. Green Proc Synth. 2019;8(1):382–90.10.1515/gps-2019-0005Search in Google Scholar
[45] Saravanakumar P, Muthukumar M, Muthuchudarkodi RR, Ramkumar P. Piper nigrum mediated green synthesis, characterization of undoped cobalt oxide and cerium ion doped cobalt oxide nanoparticles. Int J Recent Res Aspects. 2018;918–23.Search in Google Scholar
[46] Kombaiah K, Vijaya JJ, Kennedy LJ, Kaviyarasu K, Ramalingam RJ, Al-Lohedan HA. Green synthesis of Co3O4 nanorods for highly efficient catalytic, photocatalytic, and antibacterial activities. J Nanosci Nanotechnol. 2019;19(5):2590–8.10.1166/jnn.2019.15826Search in Google Scholar PubMed
[47] Dubey S, Kumar J, Kumar A, Sharma YC. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: characterization and screening of its eco-toxicity. Adv Powder Technol. 2018;29(11):2583–90.10.1016/j.apt.2018.03.009Search in Google Scholar
[48] Rasheed T, Nabeel F, Bilal M, Iqbal HM. Biogenic synthesis and characterization of cobalt oxide nanoparticles for catalytic reduction of direct yellow-142 and methyl orange dyes. Biocatal Agric Biotechnol. 2019;19:101154.10.1016/j.bcab.2019.101154Search in Google Scholar
[49] Okwunodulu FU, Chukwuemeka-Okorie HO, Okorie FC. Biological synthesis of cobalt nanoparticles from Mangifera indica leaf extract and application by detection of manganese(ii) ions present in industrial wastewater. Chem Sci Int J. 2019;10:1–8.10.9734/CSJI/2019/v27i130106Search in Google Scholar
[50] Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arabian J Chem. 2020;13(1):606–19.10.1016/j.arabjc.2017.07.004Search in Google Scholar
[51] Matinise N, Mayedwa N, Fuku XG, Mongwaketsi N, Maaza M. Green synthesis of cobalt(II, III) oxide nanoparticles using Moringa oleifera natural extract as high electrochemical electrode for supercapacitors. In AIP Conference Proceedings 2018 May 3, Vol. 1962, No. 1, p. 040005.10.1063/1.5035543Search in Google Scholar
[52] Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC, et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8.10.1016/j.mseb.2019.05.031Search in Google Scholar
[53] Oktri Mulya Dewi N, Yulizar Y, Oky Bagus Apriandanu D. Green synthesis of Co3O4 nanoparticles using Euphorbia heterophylla L. leaves extract: characterization and photocatalytic activity. MS&E. 2019;509(1):012105.10.1088/1757-899X/509/1/012105Search in Google Scholar
[54] Edison TN, Atchudan R, Sethuraman MG, Lee YR. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J Taiwan Inst Chem Eng. 2016;68:489–95.10.1016/j.jtice.2016.09.021Search in Google Scholar
[55] Younis S, Ijaz I, Nazir A, Bukhari A, Rizwan A, Gilani E. Synthesis, characterization & bacterial evaluation of cobalt nanoparticles using drumstick leaf extract via green route. EasyChair; 2020. p. 15.Search in Google Scholar
[56] Diallo A, Beye AC, Doyle TB, Park E, Maaza M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: physical properties. Green Chem Lett Rev. 2015;8(3–4):30–6.10.1080/17518253.2015.1082646Search in Google Scholar
[57] Ikhuoria EU, Omorogbe SO, Sone BT, Maaza M. Bioinspired shape controlled antiferromagnetic Co3O4 with prism like-anchored octahedron morphology: a facile green synthesis using Manihot esculenta Crantz extract. Sci Technol Mater. 2018;30(2):92–8.10.1016/j.stmat.2018.02.003Search in Google Scholar
[58] Mianai RS, Ghasemzadeh MA, Monfared MR. Green fabrication of cobalt NPs using aqueous extract of antioxidant rich zingiber and their catalytic applications for the synthesis of pyrano[2,3-c]pyrazoles. Combin Chem High Throughput Screen. 2019;22(1):18–26.10.2174/1386207322666190307160354Search in Google Scholar PubMed
[59] Abimbola Akinsiku A, Olugbenga Dare E, Oyewale Ajani O, Ayo-Ajayi J, Ademosun OT, Oluwakayode Ajayi S. Room temperature phytosynthesis of Ag/Co bimetallic nanoparticles using aqueous leaf extract of Canna indica. E&ES. 2018;173(1):012019.10.1088/1755-1315/173/1/012019Search in Google Scholar
[60] Chelli VR, Golder AK. One pot green synthesis of Pt, Co and Pt@Co core–shell nanoparticles using Sechium edule. J Chem Technol Biotechnol. 2019;94(3):911–8.10.1002/jctb.5838Search in Google Scholar
[61] Gingasu D, Mindru I, Patron L, Marinescu G, Ianculescu A, Surdu VA, et al. Synthesis of cobalt aluminate nanoparticles by combustion methods using cinnamon bark extract. Rev Roumaine Chim. 2018;63(5–6):459–66.Search in Google Scholar
[62] Anuradha CT, Raji P. Facile synthesis and characterization of Co3O4 nanoparticles for high-performance supercapacitors using Camellia sinensis. Appl Phys A. 2020;126(3):164.10.1007/s00339-020-3352-8Search in Google Scholar
[63] Hafeez M, Shaheen R, Akram B, Haq S, Mahsud S, Ali S, et al. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater Res Exp. 2020;7(2):025019.10.1088/2053-1591/ab70ddSearch in Google Scholar
[64] Mindru I, Gingasu D, Patron L, Ianculescu A, Surdu VA, Culita DC, et al. A new approach: synthesis of cobalt aluminate nanoparticles using tamarind fruit extract. Mater Sci Eng B. 2019;246:42–8.10.1016/j.mseb.2019.05.031Search in Google Scholar
[65] Khalil AT, Ovais M, Ullah I, Ali M, Shinwari ZK, Maaza M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arabian J Chem. 2020;13(1):606–19.10.1016/j.arabjc.2017.07.004Search in Google Scholar
[66] Fallahi M, Norouzi B. Synthesis of cobalt oxide nanoparticles using Cirsium vulgare leaves extract and evaluation of electrocatalytic effects on oxidation of l-cysteine. Ionics. 2020;14:1–1.10.1007/s11581-020-03451-6Search in Google Scholar
[67] Akhlaghi N, Najafpour-Darzi G, Younesi H. Facile and green synthesis of cobalt oxide nanoparticles using ethanolic extract of Trigonella foenumgraceum (Fenugreek) leaves. Adv Powder Technol. 2020;24:3562–9.10.1016/j.apt.2020.07.004Search in Google Scholar
[68] Hasan M, Zafar A, Shahzadi I, Luo F, Hassan SG, Tariq T, et al. Fractionation of biomolecules in Withania coagulans extract for bioreductive nanoparticle synthesis, antifungal and biofilm activity. Molecules. 2020;25(15):3478.10.3390/molecules25153478Search in Google Scholar PubMed PubMed Central
[69] Igwe OU, Ekebo ES. Biofabrication of cobalt Nanoparticle odorata and their potential. Res J Chem. 2018;8(1):11–7.Search in Google Scholar
[70] Zaib M, Shahzadi T, Muzammal I, Farooq U. Catharanthus roseus extract mediated synthesis of cobalt nanoparticles: evaluation of antioxidant, antibacterial, hemolytic and catalytic activities. Inorg Nano-Met Chem. 2020;9:1–0.10.1080/24701556.2020.1737819Search in Google Scholar
[71] Ghadi FE, Ghara AR, Naeimi A. Phytochemical fabrication, characterization, and antioxidant application of copper and cobalt oxides nanoparticles using Sesbania sesban plant. Chem Pap. 2018;72(11):2859–69.10.1007/s11696-018-0506-7Search in Google Scholar
[72] Shim HW, Jin YH, Seo SD, Lee SH, Kim DW. Highly reversible lithium storage in Bacillus subtilis-directed porous Co3O4 nanostructures. ACS Nano. 2011;5(1):443–9.10.1021/nn1021605Search in Google Scholar PubMed
[73] Kumar U, Shete A, Harle AS, Kasyutich O, Schwarzacher W, Pundle A, et al. Extracellular bacterial synthesis of protein-functionalized ferromagnetic Co3O4 nanocrystals and imaging of self-organization of bacterial cells under stress after exposure to metal ions. Chem Mater. 2008;20(4):1484–91.10.1021/cm702727xSearch in Google Scholar
[74] Jang E, Shim HW, Ryu BH, An DR, Yoo WK, Kim KK, et al. Preparation of cobalt nanoparticles from polymorphic bacterial templates: a novel platform for biocatalysis. Int J Biol Macromol. 2015;81:747–53.10.1016/j.ijbiomac.2015.09.009Search in Google Scholar PubMed
[75] Marimuthu S, Rahuman AA, Kirthi AV, Santhoshkumar T, Jayaseelan C, Rajakumar G. Eco-friendly microbial route to synthesize cobalt nanoparticles using Bacillus thuringiensis against malaria and dengue vectors. Parasitol Res. 2013;112(12):4105–12.10.1007/s00436-013-3601-2Search in Google Scholar PubMed
[76] Eltarahony M, Zaki S, ElKady M, Abd-El-Haleem D. Biosynthesis, characterization of some combined nanoparticles, and its biocide potency against a broad spectrum of pathogens. J Nanomater. 2018;1:2018.10.1155/2018/5263814Search in Google Scholar
[77] Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.10.1039/D0GC00885KSearch in Google Scholar
[78] Shim HW, Lee CS, Kim DW. Bacteria-mediated synthesis of free-standing cobalt oxide rods. J Nanosci Nanotechnol. 2010;10(2):1129–34.10.1166/jnn.2010.1846Search in Google Scholar PubMed
[79] Shim HW, Lim AH, Kim JC, Jang E, Seo SD, Lee GH, et al. Scalable one-pot bacteria-templating synthesis route toward hierarchical, porous-Co3O4 superstructures for supercapacitor electrodes. Sci Rep. 2013;31;2325.10.1038/srep02325Search in Google Scholar PubMed PubMed Central
[80] Baker S, Satish S. Endophytes: toward a vision in synthesis of nanoparticle for future therapeutic agents. Int J Bio-Inorg Hybd Nanomater. 2012;1(2):67–77.Search in Google Scholar
[81] Gupta S, Bector S. Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and A. flavus. Antonie Van Leeuwenhoek. 2013 May 1;103(5):1113–23.10.1007/s10482-013-9892-6Search in Google Scholar PubMed
[82] Ma L, Su W, Liu JX, Zeng XX, Huang Z, Li W, et al. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater Sci Eng C. 2017 Aug 1;77:963–71.10.1016/j.msec.2017.03.294Search in Google Scholar PubMed
[83] Omran BA, Nassar HN, Younis SA, El‐Salamony RA, Fatthallah NA, Hamdy A, et al. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404 – optimization, characterization and antimicrobial activity. J Appl Microbiol. 2020;128(2):438–57.10.1111/jam.14498Search in Google Scholar PubMed
[84] Chowdhury S, Basu A, Kundu S. Green synthesis of protein capped silver nanoparticles from phytopathogenic fungus Macrophomina phaseolina (Tassi) Goid with antimicrobial properties against multidrug-resistant bacteria. Nanoscale Res Lett. 2014 Dec;9(1):1–1.10.1186/1556-276X-9-365Search in Google Scholar PubMed PubMed Central
[85] Valappil RS, Vijayanandan AS, Balakrishnan RM. Decolorization of Reactive Blue 220 aqueous solution using fungal synthesized Co3O4 nanoparticles. J Water Supply Res Technol—AQUA. 2019;68(8):675–86.10.2166/aqua.2019.086Search in Google Scholar
[86] Yang L, Guan W, Bai B, Xu Q, Xiang Y. Synthesis of yeast-assisted Co3O4 hollow microspheres – a novel biotemplating technique. J Alloys Compd. 2010;504(1):L10–3.10.1016/j.jallcom.2010.05.072Search in Google Scholar
[87] Song JY, Kim BS. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng. 2009 Jan 1;32(1):79.10.1007/s00449-008-0224-6Search in Google Scholar PubMed
[88] Singaravelu G, Arockiamary JS, Kumar VG, Govindaraju K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B. 2007 May 15;57(1):97–101.10.1016/j.colsurfb.2007.01.010Search in Google Scholar PubMed
[89] Rajeshkumar S, Malarkodi C, Gnanajobitha G, Paulkumar K, Vanaja M, Kannan C, et al. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J Nanostruct Chem. 2013 Dec 1;3(1):44.10.1186/2193-8865-3-44Search in Google Scholar
[90] Salem DM, Ismail MM, Aly-Eldeen MA. Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea, Egypt. Egyptian J Aquat Res. 2019 Sep 1;45(3):197–204.10.1016/j.ejar.2019.07.002Search in Google Scholar
[91] Lu L, Jiao X, Fan J, Lei W, Ouyang Y, Xia X, et al. Cobalt ferrite on honeycomb-like algae-derived nitrogen-doped carbon for electrocatalytic oxygen reduction and ultra-cycle-stable lithium storage. Electrochim Acta. 2019;295:461–71.10.1016/j.electacta.2018.10.139Search in Google Scholar
[92] Azevêdo HV, Raimundo RA, Ferreira LS, Silva MM, Morales MA, Macedo DA, et al. Green synthesis of CoWO4 powders using agar-agar from red seaweed (Rhodophyta): structure, magnetic properties and battery-like behavior. Mater Chem Phys. 2020 Feb 15;242:122544.10.1016/j.matchemphys.2019.122544Search in Google Scholar
[93] Vaya D, Das BK. Green synthesis of cobalt oxide nanoparticles by a starch-assisted method. Nanosci Nanotechnol. 2019 Sep 1;9(3):362–70.10.2174/2210681208666180312123055Search in Google Scholar
[94] Ahmadov TO, Durmus Z, Baykal A, Kavas H. A simple approach for the synthesis of Co3O4 nanocrystals. Inorg Mater. 2011 Apr 1;47(4):426–30.10.1134/S0020168511040017Search in Google Scholar
[95] Hosein HA, Strongin DR, Allen M, Douglas T. Iron and cobalt oxide and metallic nanoparticles prepared from ferritin. Langmuir. 2004 Nov 9;20(23):10283–7.10.1021/la0491100Search in Google Scholar PubMed
[96] Yu C, Qiu JS. Preparation and magnetic behavior of carbon-encapsulated cobalt and nickel nanoparticles from starch. Chem Eng Res Des. 2008 Aug 1;86(8):904–8.10.1016/j.cherd.2008.02.006Search in Google Scholar
[97] Thanh NT, Puntes VF, Tung LD, Fernig DG. Peptides as capping ligands for in situ synthesis of water soluble Co nanoparticles for bioapplications. J Phys Conf Ser. 2005 Jan;17:70–6.10.1088/1742-6596/17/1/012Search in Google Scholar
[98] Kapil A. The challenge of antibiotic resistance: need to contemplate. Indian J Med Res. 2005 Feb 1;121(2):83–91.Search in Google Scholar
[99] Patil Shriniwas P. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem Biophys Rep. 2017 Jul;10:76.10.1016/j.bbrep.2017.03.002Search in Google Scholar PubMed PubMed Central
[100] Varaprasad T, Govindh B, Rao BV. Green synthesized cobalt nanoparticles using Asparagus racemosus root extract & evaluation of antibacterial activity. Int J ChemTech Res. 2017;10:339–45.Search in Google Scholar
[101] Anuradha CT, Raji P. Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus Rosa-sinensis. Mater Res Exp. 2019 Jul 17;6(9):095063.10.1088/2053-1591/ab2f9eSearch in Google Scholar
[102] Iravani S, Varma RS. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020;22(9):2643–61.10.1039/D0GC00885KSearch in Google Scholar
[103] Klempner MS, Unnasch TR, Hu LT. Taking a bite out of vector-transmitted infectious diseases. New Engl J Med. 2007 Jun 21;356(25):2567–9.10.1056/NEJMp078081Search in Google Scholar PubMed PubMed Central
[104] James AA. Mosquito molecular genetics: the hands that feed bite back. Science. 1992 Jul 3;257(5066):37–9.10.1126/science.1352413Search in Google Scholar PubMed
[105] Kaushik R, Saini P. Larvicidal activity of leaf extract of Millingtonia hortensis (Family: Bignoniaceae) against Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti. J Vector Borne Dis. 2008 Mar 1;45(1):66.Search in Google Scholar
[106] Barreto ML, Teixeira MG. Dengue no Brasil: situação epidemiológica e contribuições para uma agenda de pesquisa. Estudos Avançados. 2008 Dec;22(64):53–72.10.1590/S0103-40142008000300005Search in Google Scholar
[107] Abamor ES. Antileishmanial activities of caffeic acid phenethyl ester loaded PLGA nanoparticles against Leishmania infantum promastigotes and amastigotes in vitro. Asian Pac J Tropic Med. 2017 Jan 1;10(1):25–34.10.1016/j.apjtm.2016.12.006Search in Google Scholar
[108] Ogden GB, Melby PC. Encyclopedia of microbiology, 3rd edn. Elsevier Inc.; 2009.Search in Google Scholar
[109] Cobo F. Imported infectious diseases: the impact in developed countries. Sawston, Cambridge: Woodhead Publishing; 2014 Oct 7.Search in Google Scholar
[110] Bauer V, Sotnikova R, Machova J, Matyas S, Pucovský V, Štefek M. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life sciences. 1999 Oct 1;65(18–19):1909–17.10.1016/S0024-3205(99)00446-4Search in Google Scholar
[111] Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127(1):183–98.10.1039/b009171pSearch in Google Scholar PubMed
[112] Ballantyne B. Local and systemic ophthalmic pharmacology and toxicology of organophosphate and carbamate anticholinesterases. In Toxicology of Organophosphate & Carbamate Compounds. USA: Academic Press. 2006 Jan 1. p. 423–45.10.1016/B978-012088523-7/50032-6Search in Google Scholar
[113] Wilson DA. Gasterophilus in clinical veterinary advisor. The Horse. St. Louis, MO: Elsevier Saunders; 2012.Search in Google Scholar
[114] Mikołajczuk-Szczyrba A, Kieliszek M, Giurgiulescu L, Sokołowska B. Characteristics and application of silver nanoparticles in the food industry-review. Carpathian J Food Sci Technol. 2019;11(4):153–60.10.34302/2019.11.4.14Search in Google Scholar
© 2021 Abdul Waris et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

