Abstract
C26H21N5O8Cu, monoclinic, P21/c (no. 14), a = 17.2262(10) Å, b = 7.3474(3) Å, c = 21.1086(11) Å, β = 111.498(6)°, V = 2485.8(2) Å3, Z = 4, Rgt(F) = 0.0629, wRref(F2) = 0.1433, T = 293(2) K.
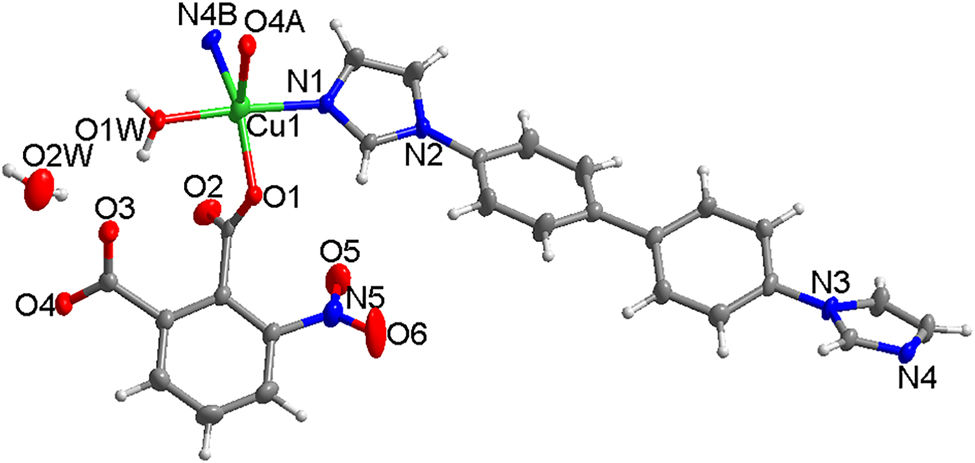
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Blue block |
| Size: | 0.37 × 0.34 × 0.33 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.94 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 25.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 25925, 4609, 0.089 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3869 |
| N(param)refined: | 361 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cu1 | 0.15767 (3) | 0.40843 (6) | 0.49484 (2) | 0.02090 (17) |
| C1 | 0.2736 (2) | −0.0474 (5) | 0.60176 (18) | 0.0205 (8) |
| C2 | 0.2237 (3) | −0.1863 (5) | 0.61101 (19) | 0.0237 (9) |
| C3 | 0.2571 (3) | −0.3136 (6) | 0.6623 (2) | 0.0350 (11) |
| H3 | 0.2235 | −0.4082 | 0.6666 | 0.042* |
| C4 | 0.3384 (3) | −0.3035 (7) | 0.7068 (2) | 0.0423 (12) |
| H4 | 0.3589 | −0.3874 | 0.7420 | 0.051* |
| C5 | 0.3894 (3) | −0.1681 (6) | 0.6988 (2) | 0.0347 (11) |
| H5 | 0.4447 | −0.1590 | 0.7285 | 0.042* |
| C6 | 0.3567 (3) | −0.0458 (5) | 0.64573 (19) | 0.0244 (9) |
| C7 | 0.2357 (2) | 0.0982 (5) | 0.54786 (19) | 0.0230 (9) |
| C8 | 0.1312 (3) | −0.1947 (5) | 0.56855 (19) | 0.0240 (9) |
| C9 | 0.2643 (3) | 0.6549 (7) | 0.4432 (3) | 0.0517 (15) |
| H9 | 0.2205 | 0.6909 | 0.4041 | 0.062* |
| C10 | 0.3444 (3) | 0.7116 (8) | 0.4613 (3) | 0.0556 (17) |
| H10 | 0.3654 | 0.7905 | 0.4371 | 0.067* |
| C11 | 0.3334 (3) | 0.5279 (6) | 0.5374 (2) | 0.0257 (9) |
| H11 | 0.3470 | 0.4577 | 0.5767 | 0.031* |
| C12 | 0.4744 (2) | 0.6604 (5) | 0.56325 (19) | 0.0227 (9) |
| C13 | 0.5267 (3) | 0.7401 (7) | 0.5354 (2) | 0.0378 (11) |
| H13 | 0.5069 | 0.7679 | 0.4892 | 0.045* |
| C14 | 0.6091 (3) | 0.7794 (7) | 0.5758 (2) | 0.0372 (11) |
| H14 | 0.6441 | 0.8298 | 0.5558 | 0.045* |
| C15 | 0.6400 (3) | 0.7448 (5) | 0.64522 (19) | 0.0236 (9) |
| C16 | 0.5877 (3) | 0.6597 (7) | 0.6713 (2) | 0.0425 (13) |
| H16 | 0.6076 | 0.6305 | 0.7173 | 0.051* |
| C17 | 0.5058 (3) | 0.6151 (7) | 0.6315 (2) | 0.0416 (12) |
| H17 | 0.4723 | 0.5549 | 0.6508 | 0.050* |
| C18 | 0.7262 (3) | 0.7990 (6) | 0.68939 (19) | 0.0240 (9) |
| C19 | 0.7676 (3) | 0.9366 (6) | 0.6696 (2) | 0.0310 (10) |
| H19 | 0.7410 | 0.9944 | 0.6280 | 0.037* |
| C20 | 0.8475 (3) | 0.9901 (6) | 0.7102 (2) | 0.0305 (10) |
| H20 | 0.8736 | 1.0844 | 0.6963 | 0.037* |
| C21 | 0.8883 (3) | 0.9030 (5) | 0.77151 (19) | 0.0226 (9) |
| C22 | 0.8487 (3) | 0.7664 (6) | 0.7927 (2) | 0.0280 (9) |
| H22 | 0.8758 | 0.7083 | 0.8341 | 0.034* |
| C23 | 0.7686 (3) | 0.7158 (6) | 0.7521 (2) | 0.0295 (10) |
| H23 | 0.7422 | 0.6240 | 0.7670 | 0.035* |
| C24 | 1.0065 (3) | 0.9623 (5) | 0.87996 (19) | 0.0236 (9) |
| H24 | 0.9795 | 0.9277 | 0.9090 | 0.028* |
| C25 | 1.0311 (3) | 1.0175 (7) | 0.7869 (2) | 0.0333 (10) |
| H25 | 1.0252 | 1.0276 | 0.7414 | 0.040* |
| C26 | 1.0991 (3) | 1.0595 (6) | 0.8418 (2) | 0.0321 (10) |
| H26 | 1.1488 | 1.1055 | 0.8404 | 0.039* |
| Cu1 | 0.15767 (3) | 0.40843 (6) | 0.49484 (2) | 0.02090 (17) |
| N1 | 0.2577 (2) | 0.5376 (4) | 0.49077 (16) | 0.0241 (7) |
| N2 | 0.3883 (2) | 0.6299 (4) | 0.52229 (17) | 0.0244 (8) |
| N3 | 0.9721 (2) | 0.9567 (5) | 0.81159 (16) | 0.0233 (7) |
| N4 | 1.0841 (2) | 1.0240 (4) | 0.90034 (15) | 0.0225 (7) |
| N5 | 0.4148 (2) | 0.0848 (5) | 0.63521 (19) | 0.0356 (9) |
| O1 | 0.23246 (17) | 0.2575 (4) | 0.57209 (13) | 0.0242 (6) |
| O1W | 0.06542 (17) | 0.2820 (4) | 0.51083 (14) | 0.0273 (7) |
| H1WA | 0.0153 | 0.3134 | 0.5032 | 0.041* |
| H1WB | 0.0764 | 0.1692 | 0.5171 | 0.041* |
| O2 | 0.2087 (2) | 0.0575 (4) | 0.48755 (14) | 0.0365 (8) |
| O2W | −0.0406 (2) | 0.0301 (6) | 0.61584 (17) | 0.0583 (11) |
| H2WA | −0.0057 | 0.0243 | 0.5960 | 0.088* |
| H2WB | −0.0865 | 0.0106 | 0.5831 | 0.088* |
| O3 | 0.09033 (19) | −0.0537 (4) | 0.56467 (16) | 0.0357 (8) |
| O4 | 0.10246 (18) | −0.3459 (4) | 0.54288 (15) | 0.0319 (7) |
| O5 | 0.4051 (2) | 0.1294 (5) | 0.57752 (17) | 0.0484 (9) |
| O6 | 0.4727 (3) | 0.1381 (7) | 0.6843 (2) | 0.0919 (18) |
Source of material
All chemicals were of reagent grade and used as received without further purification. The 4,4′-bis(imidazolyl)biphenyl was obtained from Jinan Henghua Technology Co., Ltd.. The 3-nitrobenzene-1,2-dicarboxylic acid was purchased from Beijing Bailingwei Technology Co., Ltd.. Other chemical reagents were obtained from the Tianjin Deen Chemical Reagent Co., Ltd.. The mixture of 3-nitrobenzene-1,2-dicarboxylic acid (H23-Nbdc 21.3 mg, 0.1 mmol), 4,4′-bis(imidazolyl)biphenyl (bibp, 28.6 mg, 0.1 mmol), Cu(OAc)2·4H2O (20.3 mg, 0.1 mmol), EtOH (2 mL) and H2O (4 mL) was placed in a 23 ml Teflon-lined autoclave at 393 K for four days, then cooled to room temperature. Blue block crystals were obtained in ca. 58% yield. Elemental analysis calcd. (%) for C26H21N5O8Cu: C, 52.48; H, 3.56; N, 11.77 Found: C, 52.42; H, 3.74; N, 11.62.
Experimental details
Using Olex2 [2]. The structure was solved with the SheLXT [3] structure solution program and refined with the SheLXL [4] refinement package. Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. The Uiso of the H-atoms were constrained to 1.2 times Ueq of their bonding carbon atoms with C–H = 0.93 Å (aromatic) and 1.5 times Ueq for the hydrogen atoms at water with O–H = 0.85 Å.
Comment
The aromatic 1,2-benzenedicarboxylic acids are widely used constructing coordination polymers (CPs) with interesting structures and properties not only because of their diversities coordination modes (monodentate, bridging, chelating), but also because of their strong thermal and chemical stabilities. The nitro-1,2-benzenedicarboxylates are widely used as O-donor ligands to enrich the structural and functional diversities of coordination polymers because the N and O atoms in the electron-withdrawing group (–NO2) can be not only used as coordination sites, but also as donors or acceptors of hydrogen-bond interactions. We and other authors have synthesized a large number of CPs with interesting structures and excellent properties based on 3-nitrobenzene-1,2-dicarboxylic acid (H23-Nbdc) [5–15]. However, the literature on the synthesis of CPs based on the mixed H23-Nbdc and 4,4′-bis(imidazolyl)biphenyl(bibp) ligands is relative rare [8].
X-ray crystallographic analysis reveals that the title complex crystallizes in monoclinic crystal system, space group P21/c and features a two-dimensional grid layer. The asymmetric unit contains one Cu(II) ion, one 3-Nbdc dianion, one bibp molecule, one coordinated water and one guest water molecule, as shown in Figure (A: x, 1 + y, z; B: −1 + x, 1.5 − y, −0.5 + z). The Cu(II) ion is five-coordinated in a slightly distorted tetragonal-pyramidal geometry[CuO3N2] with the one carboxylate O(1) atom belonging to 3-Nbdc anion, one O(1W) atom from coordinated water molecule, and N(1) and N(4B) atoms from two symmetry-related bibp molecules, while another carboxylate O(4A) atom from symmetry-related 3-Nbdc dianion occupies the apical site. The Cu–O bond lengths are 1.974(3), 2.002(3) and 2.429(3) Å, and the Cu–N bond lengths are 2.013(5) and 2.037(5) Å, respectively.
Since the fivefold coordination geometry in the complex does not correspond to a perfect tetragonal pyramid, we used a convenient distortion parameter τ5 for the quantitative characterisation of the copper coordination polyhedron [16]. The extreme values of distortion parameter (0 and 1) are associated with an ideal tetragonal pyramid and a regular trigonal bipyramid. The distortion parameter of the title compound is 0.23, the result reveals that the copper coordination polyhedron is the predominantly tetragonal pyramidal geometry (77%), additionally including a 23% contribution of the trigonal-bipyramid state.
The adjacent copper ions are linked by the carboxylate groups of 3-Nbdc dianions adopting monodentate coordination mode to form a metal-carboxylate chain with the Cu···Cu distance of 7.3474 Å. The metal-carboxylate chains are further connected by bibp molecules adopting exo-bidentate coordinated mode to produce a two-dimensional grid layer with the through-ligand Cu···Cu separation of 17.3226 Å. The discussed layers, stacking along the c direction adopting a -ABAB- mode, are cohered by interlayer hydrogen bonds forming a bilayer structure. There is a hydrogen bond between the coordinated water O(1W) and the carboxylate O(4) atom from 3-Nbdc anion (O(1W)–H(1WA)···O(4): d = 2.732(4) Å). Secondly, it there is a hydrogen bond between free water O(2W) and the carboxylate O(3) and O(2) atoms from 3-Nbdc anions (O(2W)–H(2WA)···O(3): d = 2.901(5) Å; O(2W)–H(2WB)···O(2): d = 2.989(5) Å). Bilayers further stack together to accomplish its entire three-dimensional structure by offset-stacking π–π interactions between imidazole ring and benzene ring of bibp molecules with the centroid distance of 4.1312 Å and the planar angle of 18.2°. It is obvious that H-bond interactions and π–π interactions play important roles in the self-assembly and enhanced the stability of resultant structure.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 21571093
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (no. 21571093).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO Software System; Rigaku Corporation: Oxford, UK, 2015.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
5. Wang, X. L., Xiong, Y., Sha, X. T., Liu, G. C., Lin, H. Y. Various polycarboxylate-directed Cd(II) coordination polymers based on a semirigid bis-pyridyl-bis-amide ligand: construction and fluorescent and photocatalytic properties. Cryst. Growth Des. 2017, 17, 483–496; https://doi.org/10.1021/acs.cgd.6b01299.Search in Google Scholar
6. Liu, G., Li, Y., Lu, Z., Li, X., Chen, X. Carboxylates directed versatile structures of ten 1D-3D Ni(II) coordination polymers: fluorescent behaviors and electrochemical activities. CrystEngComm 2019, 21, 5344–5355; https://doi.org/10.1039/c9ce01060b.Search in Google Scholar
7. Xu, W., Hu, K. K., Jin, S., Zhang, Y., Wang, D. Constructions of seven noncovalent-bonded supramolecules from reactions of Cu(II)/Cd(II)/Zn(II) with isonicotinamide and carboxylates. Inorg. Nano-Metal Chem. 2021, 51, 1842–1859; https://doi.org/10.1080/24701556.2020.1862207.Search in Google Scholar
8. Xiao, Q. Q., Song, Z. W., Li, Y. H., Cui, G. H. Two difunctional Co(II) coordination polymers for natural sunlight photocatalysis of methylene blue and selective fluorescence sensing of Cr(VI) ion in water media. J. Solid State Chem. 2019, 276, 331–338; https://doi.org/10.1016/j.jssc.2019.05.025.Search in Google Scholar
9. Liu, S., Li, L. L., Wang, W. Z., Jia, X. G., Lin, C. L. A carboxylate-bridged Co(II) layer complex: synthesis, structure and magnetic property. Chin. J. Struct. Chem. 2018, 37, 973–980.Search in Google Scholar
10. Huan, D. H., Liu, Y. G., Dong, G. Y., Wang, S. C. Three cobalt(II) coordination polymers constructed from flexible bis(thiabendazole) and dicarboxylate linkers: crystal structures, fluorescence, and photocatalytic properties. Transition Met. Chem. 2016, 41, 447–457; https://doi.org/10.1007/s11243-016-0040-9.Search in Google Scholar
11. Li, G. L., Liu, G. Z., Xin, L. Y., Li, X. L., Ma, L. F., Wang, L. Y. Syntheses, structures and fluorescence properties of four Zn/Cd(II) coordination polymers with 3-nitrobenzene-1,2-dicarboxylate and dipyridyl-typed coligands. J. Inorg. Organomet. Polym. 2015, 25, 694–701; https://doi.org/10.1007/s10904-014-0147-4.Search in Google Scholar
12. Li, G. L., Yin, W. D., Liu, G. Z., Ma, L. F., Huang, L. L., Li, L., Wang, L. Y. Single-crystal to single-crystal photochemical structure transformation of a ladder-like coordination polymer with dinuclear Zn(II) platform. Inorg. Chem. Commun. 2014, 43, 165–168; https://doi.org/10.1016/j.inoche.2014.02.037.Search in Google Scholar
13. Yin, W. D., Li, G. L., Liu, G. Z., Xin, L. Y., Li, X. L., Ma, L. F. Syntheses, structures and properties of two coordination polymers constructed by 3-nitrobenzene-1,2-dicarboxylate acid and Zn/Co. Chin. J. Inorg. Chem. 2015, 31, 1439–1446.Search in Google Scholar
14. Yin, W. D., Liu, Q. L., Li, G. L. Crystal structure of catena-poly[triaqua-(1,3-di(1H-imidazol-1-yl)benzene- κ2N:N′)- (3-nitrophthalato-κ1O)cobalt(II)]-water (2/3), C20H22N5O10.5Co. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 125–128.10.1515/ncrs-2019-0531Search in Google Scholar
15. Yin, W. D., Liu, Q. L., Zhao, Y. J., Gong, X. R., Li, G. L. Crystal structure of catena-poly[bis(μ2-3,5-bis(1-imidazolyl) pyridine-κ2N:N′)-(μ2-3-nitrophthalato-κ3O,O′:Oʺ)cadmium(II)] dihydrate, C30H25N11O8Cd. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 899–902; https://doi.org/10.1515/ncrs-2021-0133.Search in Google Scholar
16. Rodinaa, T. A., Losevaa, O. V., Smolentsevb, A. I., Antzutkind, O. N., Ivanova, A. V. Crystal structure, solid-state 13C and 15N NMR characterisation, chemisorption activity and thermal behaviour of new mercury(II) dipropyldithiocarbamate: binuclear, pseudo- binuclear and heteronuclear complexes of [Hg2(PrDtc)4], [Hg(PrDtc)2]2 and [Au(PrDtc)2]2[Hg2Cl6]. Inorg. Chim. Acta 2020, 508, 119630; https://doi.org/10.1016/j.ica.2020.119630.Search in Google Scholar
© 2022 Gui-Lian Li et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

