Abstract
C20H22F3N3OS, triclinic, P1 (no. 1), a = 6.9678(8) Å, b = 10.7614(14) Å, c = 13.0503(14) Å, α = 76.870(3)°, β = 88.004(4)°, γ = 87.275(4)°, V = 951.60(19) Å3, Z = 2, R gt (F) = 0.0629, wR ref (F2) = 0.1626, T = 100 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.54 × 0.23 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.22 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II D8 venture, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 21,069, 6660, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 6248 |
| N(param)refined: | 511 |
| Programs: | Bruker [1], SHELX [2, 3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1A | 0.3734 (2) | −0.06030 (14) | 0.44740 (12) | 0.0166 (4) |
| O1A | 0.1263 (6) | 0.0610 (4) | 0.3004 (3) | 0.0131 (9) |
| N1A | 0.0897 (7) | 0.2555 (5) | 0.3300 (4) | 0.0136 (11) |
| N2A | 0.2170 (7) | 0.1807 (5) | 0.4005 (4) | 0.0133 (10) |
| N3A | 0.4741 (7) | 0.2654 (5) | 0.4833 (4) | 0.0153 (11) |
| H3AB | 0.572 (7) | 0.215 (6) | 0.510 (5) | 0.018* |
| F1A | 0.8885 (10) | 0.7712 (6) | 0.2557 (4) | 0.0644 (19) |
| F2A | 0.5965 (10) | 0.8309 (5) | 0.2232 (5) | 0.0644 (18) |
| F3A | 0.7488 (7) | 0.7206 (4) | 0.1308 (3) | 0.0379 (11) |
| C1A | −0.1006 (9) | 0.2087 (6) | 0.1860 (4) | 0.0131 (13) |
| C2A | −0.2851 (9) | 0.1361 (7) | 0.2270 (5) | 0.0188 (14) |
| H2AA | −0.254193 | 0.043201 | 0.247696 | 0.023* |
| H2AB | −0.338981 | 0.164466 | 0.289477 | 0.023* |
| C3A | −0.4324 (9) | 0.1639 (7) | 0.1390 (5) | 0.0211 (14) |
| H3AA | −0.551853 | 0.117547 | 0.164934 | 0.025* |
| C4A | −0.4816 (9) | 0.3078 (7) | 0.1086 (5) | 0.0235 (15) |
| H4AA | −0.578978 | 0.325877 | 0.053066 | 0.028* |
| H4AB | −0.535929 | 0.337093 | 0.170541 | 0.028* |
| C5A | −0.2999 (10) | 0.3787 (6) | 0.0684 (5) | 0.0193 (14) |
| H5AA | −0.332776 | 0.472425 | 0.048326 | 0.023* |
| C6A | −0.1501 (9) | 0.3533 (6) | 0.1556 (5) | 0.0157 (13) |
| H6AA | −0.202663 | 0.382915 | 0.217727 | 0.019* |
| H6AB | −0.032805 | 0.400366 | 0.129951 | 0.019* |
| C7A | −0.3492 (10) | 0.1177 (6) | 0.0425 (5) | 0.0182 (14) |
| H7AA | −0.316444 | 0.024952 | 0.062525 | 0.022* |
| H7AB | −0.445849 | 0.132579 | −0.013265 | 0.022* |
| C8A | −0.1686 (10) | 0.1909 (6) | 0.0014 (5) | 0.0179 (13) |
| H8AA | −0.114654 | 0.161871 | −0.061632 | 0.022* |
| C9A | −0.2162 (9) | 0.3350 (6) | −0.0287 (5) | 0.0170 (13) |
| H9AA | −0.310615 | 0.354184 | −0.085687 | 0.020* |
| H9AB | −0.098380 | 0.381364 | −0.054519 | 0.020* |
| C10A | −0.0181 (9) | 0.1631 (6) | 0.0893 (5) | 0.0153 (13) |
| H10A | 0.014370 | 0.070330 | 0.109004 | 0.018* |
| H10B | 0.100871 | 0.208062 | 0.063410 | 0.018* |
| C11A | 0.0406 (8) | 0.1820 (6) | 0.2727 (4) | 0.0116 (12) |
| C12A | 0.2407 (8) | 0.0624 (6) | 0.3843 (4) | 0.0103 (12) |
| C13A | 0.2859 (9) | 0.2254 (6) | 0.4916 (5) | 0.0158 (13) |
| H13A | 0.199478 | 0.297127 | 0.502702 | 0.019* |
| H13B | 0.274240 | 0.155203 | 0.555075 | 0.019* |
| C14A | 0.5328 (9) | 0.3822 (6) | 0.4224 (5) | 0.0143 (13) |
| C15A | 0.4044 (10) | 0.4654 (6) | 0.3557 (5) | 0.0194 (14) |
| H15A | 0.275917 | 0.442040 | 0.350387 | 0.023* |
| C16A | 0.4670 (11) | 0.5816 (6) | 0.2979 (5) | 0.0220 (15) |
| H16A | 0.379578 | 0.639995 | 0.255293 | 0.026* |
| C17A | 0.6570 (11) | 0.6128 (7) | 0.3021 (5) | 0.0223 (15) |
| C18A | 0.7850 (10) | 0.5317 (7) | 0.3681 (5) | 0.0216 (15) |
| H18A | 0.914460 | 0.553968 | 0.371951 | 0.026* |
| C19A | 0.7184 (9) | 0.4165 (6) | 0.4287 (5) | 0.0172 (13) |
| H19A | 0.803501 | 0.360982 | 0.475187 | 0.021* |
| C20A | 0.7246 (12) | 0.7329 (7) | 0.2305 (6) | 0.0302 (18) |
| S1B | 0.8594 (2) | 1.06071 (14) | 0.55707 (12) | 0.0181 (4) |
| O1B | 0.6111 (6) | 0.9361 (4) | 0.7018 (3) | 0.0121 (9) |
| N1B | 0.6062 (7) | 0.7430 (5) | 0.6693 (4) | 0.0138 (11) |
| N2B | 0.7321 (7) | 0.8202 (5) | 0.6002 (4) | 0.0144 (11) |
| N3B | 1.0073 (8) | 0.7352 (5) | 0.5130 (4) | 0.0166 (11) |
| H3BB | 1.098 (8) | 0.787 (6) | 0.484 (5) | 0.020* |
| F1B | 1.1988 (13) | 0.1684 (5) | 0.7620 (5) | 0.081 (2) |
| F2B | 1.4793 (10) | 0.2431 (6) | 0.7529 (4) | 0.072 (2) |
| F3B | 1.2775 (7) | 0.2737 (5) | 0.8712 (3) | 0.0413 (12) |
| C1B | 0.3907 (9) | 0.7883 (6) | 0.8138 (5) | 0.0116 (12) |
| C2B | 0.1965 (9) | 0.8522 (7) | 0.7710 (5) | 0.0171 (13) |
| H2BA | 0.209521 | 0.945584 | 0.746741 | 0.021* |
| H2BB | 0.159649 | 0.818357 | 0.710316 | 0.021* |
| C3B | 0.0406 (9) | 0.8245 (7) | 0.8585 (5) | 0.0197 (14) |
| H3BA | −0.084207 | 0.866512 | 0.831068 | 0.024* |
| C4B | 0.0191 (9) | 0.6804 (7) | 0.8921 (5) | 0.0227 (15) |
| H4BA | −0.084525 | 0.661078 | 0.946554 | 0.027* |
| H4BB | −0.016148 | 0.646910 | 0.830956 | 0.027* |
| C5B | 0.2072 (10) | 0.6167 (6) | 0.9358 (5) | 0.0182 (14) |
| H5BA | 0.191746 | 0.522441 | 0.958370 | 0.022* |
| C6B | 0.3673 (10) | 0.6434 (6) | 0.8500 (5) | 0.0187 (14) |
| H6BA | 0.489875 | 0.601295 | 0.878336 | 0.022* |
| H6BB | 0.333932 | 0.608230 | 0.789419 | 0.022* |
| C7B | 0.0991 (10) | 0.8778 (6) | 0.9527 (5) | 0.0199 (14) |
| H7BA | 0.114163 | 0.971073 | 0.929898 | 0.024* |
| H7BB | −0.002375 | 0.862112 | 1.008562 | 0.024* |
| C8B | 0.2873 (10) | 0.8127 (7) | 0.9953 (5) | 0.0242 (15) |
| H8BA | 0.323632 | 0.846582 | 1.056910 | 0.029* |
| C9B | 0.2664 (10) | 0.6671 (6) | 1.0307 (5) | 0.0198 (14) |
| H9BA | 0.389877 | 0.625215 | 1.057680 | 0.024* |
| H9BB | 0.167595 | 0.647761 | 1.087787 | 0.024* |
| C10B | 0.4459 (9) | 0.8404 (6) | 0.9099 (5) | 0.0168 (13) |
| H10C | 0.569004 | 0.799209 | 0.938070 | 0.020* |
| H10D | 0.462561 | 0.933660 | 0.887926 | 0.020* |
| C11B | 0.5385 (8) | 0.8157 (6) | 0.7282 (4) | 0.0116 (12) |
| C12B | 0.7359 (8) | 0.9358 (6) | 0.6192 (5) | 0.0136 (13) |
| C13B | 0.8156 (9) | 0.7771 (6) | 0.5067 (5) | 0.0168 (13) |
| H13C | 0.738634 | 0.706928 | 0.494871 | 0.020* |
| H13D | 0.801939 | 0.848788 | 0.444338 | 0.020* |
| C14B | 1.0726 (9) | 0.6173 (6) | 0.5754 (5) | 0.0157 (13) |
| C15B | 0.9477 (10) | 0.5316 (6) | 0.6376 (5) | 0.0183 (13) |
| H15B | 0.813251 | 0.550759 | 0.638783 | 0.022* |
| C16B | 1.0248 (10) | 0.4166 (7) | 0.6984 (5) | 0.0216 (15) |
| H16B | 0.940911 | 0.356650 | 0.739494 | 0.026* |
| C17B | 1.2184 (11) | 0.3892 (6) | 0.6996 (5) | 0.0214 (15) |
| C18B | 1.3421 (9) | 0.4726 (6) | 0.6362 (5) | 0.0183 (14) |
| H18B | 1.476321 | 0.452406 | 0.635334 | 0.022* |
| C19B | 1.2684 (10) | 0.5859 (6) | 0.5737 (5) | 0.0188 (14) |
| H19B | 1.352845 | 0.642595 | 0.529361 | 0.023* |
| C20B | 1.2923 (12) | 0.2697 (7) | 0.7700 (5) | 0.0284 (17) |
Source of material
Formaldehyde solution (37%, 1.5 mL) and 4-trifluoromethylaniline (1.61 g, 0.01 mol) were added to a solution of 5-(adamantan-1-yl)-1,3,4-oxadiazole-2(3H)-thione (2.36 g, 0.01 mol) in ethanol (15 mL), and the mixture was stirred at 293 K for 2 h and allowed to stand overnight. The precipitated crude product was filtered, washed with water, dried and crystallised from aqueous ethanol to yield 3.36 g (82%) of the title compound as colourless prisms. M.pt: 447–449 K (uncorrected). Anal. Calc. for C20H22F3N3OS: C, 58.67; H, 5.42; N, 10.26; S, 7.83%. Found: C, 58.63; H, 5.45; N, 10.24; S, 7.82%. 1 H-NMR (CDCl3, 500.13 MHz): δ 1.74–1.80 (m, 6H, Adamantane-H), 1.97–1.99 (m, 6H, Adamantane-H), 2.12 (s, 3H, Adamantane-H), 5.46 (s, 1H, NH), 5.78 (d, 2H, CH2, J = 6.8 Hz), 6.96 (d, 2H, Ar–H, J = 8.5 Hz), 7.43 (d, 2H, Ar–H, J = 8.5 Hz). 13C-NMR (CDCl3, 125.76 MHz): δ 27.41, 34.33, 36.04, 39.04 (adamantane–C), 59.0 (CH2), 123.85 (CF3), 114.50, 119.88, 128.09, 147.12 (Ar–C), 165.45 (oxadiazole–C), 177.43 (C=S). ESI-MS m/z: 410.3 [M + H]+.
Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95–1.00 Å) and refined as riding with U iso (H) = 1.2U eq (C). The N-bound H atoms were located from a difference Fourier map and refined with N–H = 0.88 + −0.01 Å, and with Uiso(H) set to 1.2Ueq(N).
Comment
The highly lipophilic adamantane cage is frequently found in biologically-active compounds possessing various pharmacological activities [5], [6], [7]. Amantadine was discovered early as an efficient drug for the control of Influenza A infection [8, 9] and further approved for the treatment of Parkinson’s disease [10]. Further studies based on adamantane derivatives resulted in the evolution of more active anti-viral drugs such as tromantadine [11] and rimantadine [12]. Adamantane-based analogues were also reported to display significant inhibitory effects against human immunodeficiency virus (HIV) [13], [14], [15]. The adamantane ethylenediamine analogue, SQ109, and the related dipiperidine derivative, SQ609, were further approved as efficient therapies against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains [16, 17]. The adamantane carboxamide derivative Opaganib (ABC294640) is a newly approved anti-tumour drug for treating patients suffering from advanced solid tumours [18], [19], [20]. Furthermore, various adamantane derivatives were recognised as efficient anti-bacterial and anti-fungal candidates [21], [22], [23], [24]. On the other hand, the 1,3,4-oxadiazole nucleus was reported to constitute the core of several chemotherapeutic agents possessing anti-bacterial [25, 26], anti-fungal [27], anti-cancer [28, 29] and anti-viral [30] activities. Besides, 1,3,4-oxadiazole derivatives are presently utilised as safe herbicides for crop protection [31]. In an earlier study, we described the synthesis, anti-HIV, and anti-bacterial activities of adamantane-1,3,4-oxadiazole hybrid N-Mannich bases [15]. The previously reported compounds showed strong inhibitory activity against Gram-positive bacteria and limited activity against Gram-negative bacteria. The anti-bacterial structure-activity relationship of these compounds proved that the spectrum of the anti-bacterial efficacy is fundamentally contingent on the nature of the substituents of the aryl moiety. Further optimisation studies based on the previously prepared compounds resulted in development of the title compound (I), which exhibited strong broad-spectrum anti-bacterial activity.
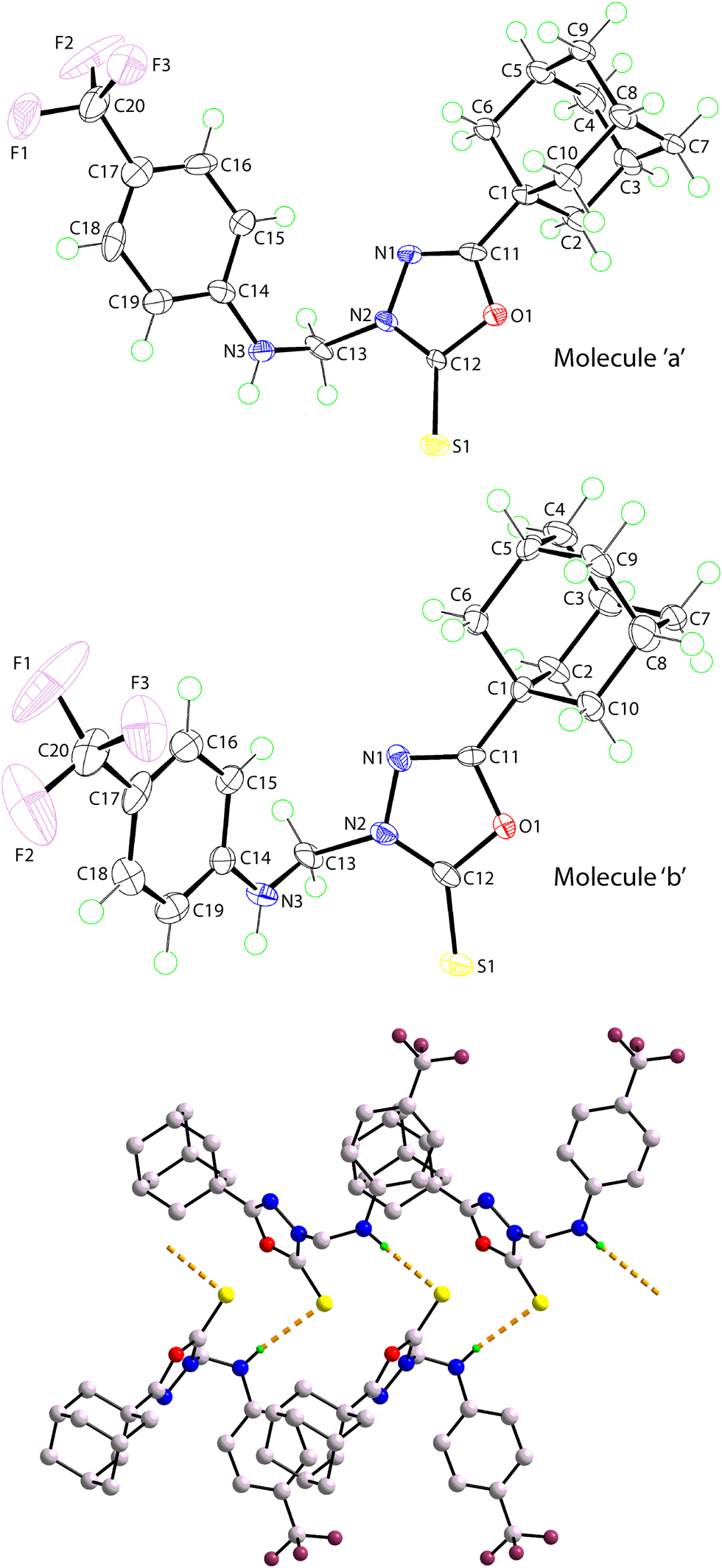
The molecular structures of the independent molecules, i.e. molecule a and molecule b, comprising the crystallographic asymmetric-unit of (I) are shown in the figure (70% probability ellipsoids). The independent molecules resemble each other very closely as seen in the r.m.s. bond and angle fits of 0.0128 Å and 0.745°, respectively [32]. Crucially, there is no pseudo centre of inversion between the molecules, they being directly superimposable.
Each molecule comprises a central, planar 1,3,4-oxadiazole core connected to a thione–S [1.649(6) & 1.665(6) Å for molecules a and b, respectively], an N-bound (4-trifluoromethylanilino)methyl group and the remaining carbon atom carries the adamantan-1-yl group. Within the five-membered ring, the C11–N1 bond of 1.270(8) Å is significantly shorter than the C12–N2 bond of 1.339(8) Å, and the C11–O1 [1.383(7) Å] and C12–O1 [1.379(7) Å] bond lengths are experimentally equivalent, results suggesting limited delocalisation of π-electron density in the ring; the equivalent bond lengths for molecule b are 1.280(8), 1.324(8), 1.379(7) and 1.361(7) Å, respectively. The dihedral angle between the five- and six-membered rings is 47.1(3)°; the equivalent angle for molecule b is 51.3(3)°. Globally, the two central ring-substituents tend to be oriented to the same side of the molecule in a direction away from the thione-S atom.
There are four closely related structures in the literature, namely the phenyl compound, i.e. the parent compound [33], the 4-fluorophenyl [34], the 4-chlorophenyl [35] and the 2-trifluoromethyl [36] derivatives. Each of the previously reported structure resemble that noted above for (I).
The most prominent feature of the molecular packing in the crystal of (I) is a twisted chain aligned along the a-axis. The chains comprise alternating independent molecules and feature amine-N–H⋯S(thione) hydrogen bonds [N3a–H3ab⋯S1bi: H3ab⋯S1bi = 2.54(6) Å, N3a–H3ab⋯S1bi = 3.412(5) Å with angle at H3ab = 171(6)° and N3b–H3bb⋯S1aii: H3bb⋯S1aii = 2.55(6) Å, N3b–H3bb⋯S1aii = 3.407(6) Å with angle at H3bb = 166(5)° for symmetry operations (i): x, −1 + y, z and (ii): 1 + x, 1 + y, z]. A portion of the supramolecular chain is shown in the lower view of the Figure, where the hydrogen bonds are shown as dashed lines and non-participating hydrogen atoms are omitted.
As indicated above, the molecular structures are very similar as is the primary mode of association between the molecules. This is confirmed by the calculation of the Hirshfeld surfaces and the full and decomposed two-dimensional fingerprint plots. Crystal Explorer 17 [37] was employed for this purpose following established procedures [38]. The differences between the Hirshfeld surfaces for each independent molecule differ by no more than 1%. The most significant contribution are from H⋯H contacts being 39.9 and 39.2% for molecules a and b, respectively. The next most important contributions are of the type F⋯H/H⋯F, i.e. 24.5 and 24.0%, respectively. There are also significant contributions to the Hirshfeld surface by C⋯H/H⋯C [13.6 and 13.9%], S⋯H/H⋯S [9.0 and 9.8%], N⋯H/H⋯N [4.1 and 4.3%] and O⋯H/H⋯O [3.4 and 3.5%]. The only other surface contacts greater than 1.0% are S⋯F/F⋯S [1.5 and 0.9%], S⋯N/N⋯S [1.2 and 1.2%] and S⋯C/C⋯S [1.0 and 1.0%].
Acknowledgements
This research was funded by the Princess Nourah bint Abdulrahman University Researchers Supporting Project No. PNURSP2022R3, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Princess Nourah bint Abdulrahman University Researchers Supporting Project No. PNURSP2022R3, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SADABS, APEX2 and SAINT; Bruker AXS Inc.: Madison, Wisconsin, USA, 2014.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Wanka, L., Iqbal, K., Schreiner, P. R. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604; https://doi.org/10.1021/cr100264t.Search in Google Scholar PubMed PubMed Central
6. Liu, J., Obando, D., Liao, V., Lifa, T., Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963; https://doi.org/10.1016/j.ejmech.2011.01.047.Search in Google Scholar PubMed
7. Lamoureux, G., Artavia, G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978; https://doi.org/10.2174/092986710792065027.Search in Google Scholar PubMed
8. Davies, W. L., Grunert, R. R., Haff, R. F., McGahen, J. W., Neumayer, E. M., Paulshock, M., Watts, J. C., Wood, T. R., Hermann, E. C., Hoffmann, C. E. Antiviral activity of 1-adamantamine (amantadine). Science 1964, 144, 862–863; https://doi.org/10.1126/science.144.3620.862.Search in Google Scholar PubMed
9. Wendel, H. A., Snyder, M. T., Pell, S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Ther. 1966, 7, 38–43; https://doi.org/10.1002/cpt19667138.Search in Google Scholar PubMed
10. Schwab, R. S., England, A. C.Jr., Poskanzer, D. C., Young, R. R. Amantadine in the treatment of Parkinson’s disease. J. Am. Med. Assoc. 1969, 208, 1168–1170; https://doi.org/10.1001/jama.208.7.1168.Search in Google Scholar
11. Rosenthal, K. S., Sokol, M. S., Ingram, R. L., Subramanian, R., Fort, R. C. Tromantadine: Inhibitor of early and late events in herpes simplex virus replication. Antimicrob. Agents Chemother. 1982, 22, 1031–1036; https://doi.org/10.1128/aac.22.6.1031.Search in Google Scholar
12. Wingfield, W. L., Pollack, D., Grunert, R. R. Therapeutic efficacy of amantadine HCl and rimantadine HCl in naturally occurring influenza A2 respiratory Illness in man. N. Engl. J. Med. 1969, 281, 579–584; https://doi.org/10.1056/nejm196909112811102.Search in Google Scholar
13. Balzarini, J., Orzeszko-Krzesińska, B., Maurin, J. K., Orzeszko, A. Synthesis and anti-HIV studies of 2- and 3-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2009, 44, 303–311; https://doi.org/10.1016/j.ejmech.2008.02.039.Search in Google Scholar PubMed PubMed Central
14. Balzarini, J., Orzeszko, B., Mauri, J. K., Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003; https://doi.org/10.1016/j.ejmech.2007.01.003.Search in Google Scholar PubMed
15. El-Emam, A. A., Al-Deeb, O. A., Al-Omar, M., Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113; https://doi.org/10.1016/j.bmc.2004.07.033.Search in Google Scholar PubMed
16. Protopopova, M., Hanrahan, C., Nikonenko, B., Samala, R., Chen, P., Gearhart, J., Einck, L., Nacy, C. A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005, 56, 968–974; https://doi.org/10.1093/jac/dki319.Search in Google Scholar PubMed
17. Bogatcheva, E., Hanrahan, C., Nikonenko, B., de los Santos, G., Reddy, V., Chen, P., Barbosa, F., Einck, L., Nacy, C., Protopopova, M. Identification of SQ609 as a lead compound from a library of dipiperidines. Bioorg. Med. Chem. Lett. 2011, 21, 5353–5357; https://doi.org/10.1016/j.bmcl.2011.07.015.Search in Google Scholar PubMed PubMed Central
18. McNaughton, M., Pitman, M., Pitson, S. M., Pyne, N. J., Pyne, S. Proteasomal degradation of sphingosine kinase 1 and inhibition of dihydroceramide desaturase by the sphingosine kinase inhibitors, SKi or ABC294640, induces growth arrest in androgen-independent LNCaP-AI prostate cancer cells. Oncotarget 2016, 7, 16663–16675; https://doi.org/10.18632/oncotarget.7693.Search in Google Scholar PubMed PubMed Central
19. Britten, C. D., Garrett-Mayer, E., Chin, S. H., Shirai, K., Ogretmen, B., Bentz, T. A., Brisendine, A., Anderton, K., Cusack, S. L., Maines, L. W., Zhuang, Y., Smith, C. D., Thomas, M. B. A phase I study of ABC294640, a first-in-class sphingosine kinase-2 inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2017, 23, 4642–4650; https://doi.org/10.1158/1078-0432.ccr-16-2363.Search in Google Scholar
20. Zhou, J., Chen, J., Yu, H. Targeting sphingosine kinase 2 by ABC294640 inhibits human skin squamous cell carcinoma cell growth. Biochem. Biophys. Res. Commun. 2018, 497, 535–542; https://doi.org/10.1016/j.bbrc.2018.02.075.Search in Google Scholar PubMed
21. Omar, K., Geronikaki, A., Zoumpoulakis, P., Camoutsis, C., Soković, M., Ćirić, A., Glamočlija, J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010, 18, 426–432; https://doi.org/10.1016/j.bmc.2009.10.041.Search in Google Scholar PubMed
22. Al-Wahaibi, L. H., Hassan, H. M., Abo-Kamar, A. M., Ghabbour, H. A., El-Emam, A. A. Adamantane-isothiourea hybrid derivatives: synthesis, characterization, in vitro antimicrobial, and in vivo hypoglycemic activities. Molecules 2017, 22, 710; https://doi.org/10.3390/molecules22050710.Search in Google Scholar PubMed PubMed Central
23. El-Emam, A. A., Al-Tamimi, A.-M. S., Al-Omar, M. A., Alrashood, K. A., Habib, E. E. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013, 68, 96–102; https://doi.org/10.1016/j.ejmech.2013.07.024.Search in Google Scholar PubMed
24. Al-Abdullah, E. S., Al-Tuwaijri, H. M., Hassan, H. M., Haiba, M. E. Antimicrobial and hypoglycemic activities of novel N-Mannich bases derived from 5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones. Int. J. Mol. Sci. 2014, 15, 22995–23010; https://doi.org/10.3390/ijms151222995.Search in Google Scholar PubMed PubMed Central
25. Ogata, M., Atobe, H., Kushida, H., Yamamoto, K. In vitro sensitivity of mycoplasmas isolated from various animals and sewage to antibiotics and nitrofurans. J. Antibiot. 1971, 24, 443–451; https://doi.org/10.7164/antibiotics.24.443.Search in Google Scholar PubMed
26. Kadi, A. A., El-Brollosy, N. R., Al-Deeb, O. A., Habib, E. E., Ibrahim, T. M., El-Emam, A. A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242; https://doi.org/10.1016/j.ejmech.2006.10.003.Search in Google Scholar PubMed
27. Prakash, O., Kumar, M., Kumar, R., Sharma, C., Aneja, K. R. Hypervalent iodine(III) mediated synthesis of novel unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles as antibacterial and antifungal agents. Eur. J. Med. Chem. 2010, 45, 4252–4257; https://doi.org/10.1016/j.ejmech.2010.06.023.Search in Google Scholar PubMed
28. Gamal El-Din, M. M., El-Gamal, M. I., Abdel-Maksoud, M. S., Yoo, K. H., Oh, C.-H. Synthesis and in vitro antiproliferative activity of new 1,3,4-oxadiazole derivatives possessing sulfonamide moiety. Eur. J. Med. Chem. 2015, 90, 45–52; https://doi.org/10.1016/j.ejmech.2014.11.011.Search in Google Scholar PubMed
29. Zhang, K., Wang, P., Xuan, L.-N., Fu, X.-Y., Jing, F., Li, S., Liu, Y.-M., Chen, B.-Q. Synthesis and antitumor activities of novel hybrid molecules containing 1,3,4-oxadiazole and 1,3,4-thiadiazole bearing Schiff base moiety. Bioorg. Med. Chem. Lett 2014, 24, 5154–5146; https://doi.org/10.1016/j.bmcl.2014.09.086.Search in Google Scholar PubMed
30. Summa, V., Petrocchi, A., Bonelli, F., Crescenzi, B., Donghi, M., Ferrara, M., Fiore, F., Gardelli, C., Gonzalez Paz, O., Hazuda, D. J., Jones, P., Kinzel, O., Laufer, R., Monteagudo, E., Muraglia, E., Nizi, E., Orvieto, F., Pace, P., Pescatore, G., Scarpelli, R., Stillmock, K., Witmer, M. V., Rowley, M. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J. Med. Chem. 2008, 51, 5843–5855; https://doi.org/10.1021/jm800245z.Search in Google Scholar PubMed
31. Shi, W., Qian, X., Zhang, R., Song, G. Synthesis and quantitative structure-activity relationships of new 2,5-disubstituted-1,3,4-oxadiazoles. J. Agric. Food Chem. 2001, 49, 124–130; https://doi.org/10.1021/jf0007941.Search in Google Scholar PubMed
32. Spek, A. L. checkCIF validation ALERTS: what they mean and how to respond. Acta Crystallogr. 2020, E76, 1–11; https://doi.org/10.1107/s2056989019016244.Search in Google Scholar PubMed PubMed Central
33. Al-Tamimi, A.-M. S., Al-Deeb, O. A., El-Emam, A. A., Ng, S. W., Tiekink, E. R. T. 5-(Adamantan-1-yl)-3-anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione. Acta Crystallogr. 2013, E69, o729; https://doi.org/10.1107/s1600536813009835.Search in Google Scholar PubMed PubMed Central
34. Al-Tamimi, A.-M. S., Alafeefy, A. M., El-Emam, A. A., Ng, S. W., Tiekink, E. R. T. 5-(Adamantan-1-yl)-3-[(4-fluoroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione. Acta Crystallogr. 2013, E69, o730; https://doi.org/10.1107/s1600536813009823.Search in Google Scholar
35. Al-Alshaikh, M. A., Ghabbour, H. A., Al-Tamimi, A.-M. S., Abdelbaky, M. S. M., Garcia-Granda, S., El-Emam, A . A. Crystal structure of 5-(adamantan-1-yl)-3-[(4-chloroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C19H22ClN3OS. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 301–303; https://doi.org/10.1515/ncrs-2015-0151.Search in Google Scholar
36. Al-Wabli, R. I., El-Emam, N. A., Ghabbour, H. A., Haress, N. G., El-Emam, A. A. Crystal structure of 5-(adamantan-1-yl)-3-[(2-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS. Z. Kristallogr. N. Cryst. Struct. 2016, 231, 815–817; https://doi.org/10.1515/ncrs-2015-0278.Search in Google Scholar
37. Turner, M. J., Mckinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A. Crystal Explorer v17; The University of Western Australia: Australia, 2017.Search in Google Scholar
38. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Search in Google Scholar PubMed PubMed Central
© 2022 Lamya H. Al-Wahaibi et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

