Abstract
C21H11F4NO4, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Orange block |
| Size: | 0.21 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.14 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θ max, completeness: | 29.3°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 15,149, 8030, 0.020 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 5734 |
| N(param)refined: | 543 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3,4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| F1 | 0.69627 (15) | 1.12057 (9) | −0.00684 (9) | 0.0559 (3) |
| F2 | 0.82500 (14) | 1.00955 (10) | −0.09927 (8) | 0.0531 (3) |
| F3 | 0.90785 (15) | 0.84375 (10) | −0.02169 (9) | 0.0576 (4) |
| F4 | 0.87001 (13) | 0.79328 (8) | 0.14903 (9) | 0.0487 (3) |
| O1 | 0.71524 (15) | 1.16530 (10) | 0.19803 (10) | 0.0413 (3) |
| H1B | 0.6631 | 1.2044 | 0.2057 | 0.062* |
| O2 | 0.50202 (15) | 1.05756 (11) | 0.16415 (11) | 0.0484 (4) |
| O3 | 0.72255 (16) | 0.96539 (10) | 0.31652 (9) | 0.0410 (3) |
| O4 | 0.70463 (19) | 0.81013 (10) | 0.27344 (10) | 0.0533 (4) |
| C14 | 0.74873 (19) | 0.92731 (13) | 0.16122 (12) | 0.0296 (4) |
| C15 | 0.70823 (19) | 1.01367 (13) | 0.12094 (12) | 0.0295 (4) |
| C16 | 0.7354 (2) | 1.03931 (14) | 0.03400 (13) | 0.0361 (4) |
| C17 | 0.8004 (2) | 0.98272 (16) | −0.01483 (13) | 0.0383 (5) |
| C18 | 0.8416 (2) | 0.89931 (15) | 0.02424 (14) | 0.0380 (5) |
| C19 | 0.8176 (2) | 0.87294 (14) | 0.11191 (13) | 0.0339 (4) |
| C20 | 0.7232 (2) | 0.89721 (14) | 0.25773 (13) | 0.0313 (4) |
| C21 | 0.6295 (2) | 1.07989 (14) | 0.16496 (12) | 0.0327 (4) |
| F5 | 0.14775 (18) | 0.39433 (10) | 0.95474 (12) | 0.0776 (5) |
| F6 | 0.17855 (19) | 0.53081 (11) | 1.09996 (11) | 0.0798 (5) |
| F7 | 0.34957 (17) | 0.70678 (10) | 1.10945 (8) | 0.0659 (4) |
| F8 | 0.48495 (14) | 0.74706 (9) | 0.97145 (8) | 0.0517 (3) |
| O5 | 0.34896 (16) | 0.35423 (10) | 0.83519 (10) | 0.0457 (4) |
| H5A | 0.3167 | 0.3054 | 0.7963 | 0.069* |
| O6 | 0.18248 (19) | 0.42105 (13) | 0.71838 (11) | 0.0651 (5) |
| O7 | 0.50219 (17) | 0.55649 (11) | 0.77185 (11) | 0.0501 (4) |
| O8 | 0.42477 (17) | 0.69855 (10) | 0.76425 (10) | 0.0471 (4) |
| N1 | 0.59769 (16) | 0.90183 (11) | 0.44963 (10) | 0.0310 (3) |
| H1 | 0.6355 | 0.9193 | 0.4037 | 0.037* |
| C1 | 0.8498 (2) | 0.90658 (14) | 0.55949 (14) | 0.0382 (5) |
| H1A | 0.8890 | 0.9246 | 0.5088 | 0.046* |
| C2 | 0.9432 (2) | 0.89492 (15) | 0.65227 (16) | 0.0442 (5) |
| H2 | 1.0467 | 0.9046 | 0.6645 | 0.053* |
| C3 | 0.8857 (2) | 0.86837 (16) | 0.73020 (15) | 0.0469 (5) |
| H3 | 0.9518 | 0.8615 | 0.7932 | 0.056* |
| C4 | 0.7362 (2) | 0.85288 (15) | 0.71450 (14) | 0.0413 (5) |
| H4 | 0.7001 | 0.8350 | 0.7665 | 0.050* |
| C5 | 0.2219 (2) | 0.84377 (15) | 0.47590 (16) | 0.0442 (5) |
| H5 | 0.1769 | 0.8273 | 0.5242 | 0.053* |
| C6 | 0.1349 (2) | 0.85265 (17) | 0.38120 (18) | 0.0524 (6) |
| H6 | 0.0305 | 0.8409 | 0.3645 | 0.063* |
| C7 | 0.2016 (2) | 0.87969 (16) | 0.30746 (17) | 0.0510 (6) |
| H7 | 0.1399 | 0.8856 | 0.2428 | 0.061* |
| C8 | 0.3527 (2) | 0.89718 (14) | 0.32853 (14) | 0.0409 (5) |
| H8 | 0.3946 | 0.9159 | 0.2793 | 0.049* |
| C9 | 0.4779 (2) | 0.84906 (14) | 0.59706 (13) | 0.0358 (4) |
| H9 | 0.4373 | 0.8319 | 0.6473 | 0.043* |
| C10 | 0.6334 (2) | 0.86367 (13) | 0.61889 (13) | 0.0323 (4) |
| C11 | 0.6931 (2) | 0.89095 (13) | 0.54115 (13) | 0.0298 (4) |
| C12 | 0.4461 (2) | 0.88672 (13) | 0.42639 (13) | 0.0306 (4) |
| C13 | 0.3820 (2) | 0.85944 (13) | 0.50230 (14) | 0.0324 (4) |
| N2 | 0.57485 (17) | 0.60620 (11) | 0.61271 (11) | 0.0324 (3) |
| H2A | 0.5481 | 0.5902 | 0.6636 | 0.039* |
| C22 | 0.3122 (2) | 0.59166 (15) | 0.51758 (16) | 0.0425 (5) |
| H22 | 0.2843 | 0.5728 | 0.5726 | 0.051* |
| C23 | 0.2055 (3) | 0.59900 (16) | 0.42848 (18) | 0.0541 (6) |
| H23 | 0.1042 | 0.5849 | 0.4231 | 0.065* |
| C24 | 0.2453 (3) | 0.62744 (17) | 0.34429 (18) | 0.0597 (7) |
| H24 | 0.1702 | 0.6323 | 0.2844 | 0.072* |
| C25 | 0.3909 (3) | 0.64765 (16) | 0.34940 (15) | 0.0521 (6) |
| H25 | 0.4154 | 0.6662 | 0.2931 | 0.063* |
| C26 | 0.9263 (3) | 0.66317 (17) | 0.5534 (2) | 0.0562 (6) |
| H26 | 0.9594 | 0.6793 | 0.4997 | 0.067* |
| C27 | 1.0271 (3) | 0.65335 (19) | 0.6427 (2) | 0.0654 (7) |
| H27 | 1.1291 | 0.6623 | 0.6499 | 0.079* |
| C28 | 0.9799 (3) | 0.62975 (18) | 0.7250 (2) | 0.0612 (7) |
| H28 | 1.0515 | 0.6241 | 0.7863 | 0.073* |
| C29 | 0.8314 (2) | 0.61495 (15) | 0.71700 (16) | 0.0462 (5) |
| H29 | 0.8018 | 0.5995 | 0.7722 | 0.055* |
| C30 | 0.6602 (2) | 0.65850 (14) | 0.45079 (15) | 0.0430 (5) |
| H30 | 0.6893 | 0.6770 | 0.3961 | 0.052* |
| C31 | 0.5079 (2) | 0.64085 (14) | 0.44057 (13) | 0.0371 (4) |
| C32 | 0.4660 (2) | 0.61307 (13) | 0.52530 (13) | 0.0327 (4) |
| C33 | 0.7231 (2) | 0.62325 (13) | 0.62397 (14) | 0.0333 (4) |
| C34 | 0.7697 (2) | 0.64919 (14) | 0.54046 (15) | 0.0383 (5) |
| C35 | 0.3776 (2) | 0.59317 (14) | 0.88412 (12) | 0.0315 (4) |
| C36 | 0.2951 (2) | 0.50102 (14) | 0.88244 (13) | 0.0353 (4) |
| C37 | 0.2308 (3) | 0.48186 (16) | 0.95586 (16) | 0.0471 (5) |
| C38 | 0.2459 (3) | 0.55112 (18) | 1.03069 (15) | 0.0507 (6) |
| C39 | 0.3301 (3) | 0.64016 (16) | 1.03478 (14) | 0.0458 (5) |
| C40 | 0.3965 (2) | 0.66066 (14) | 0.96212 (13) | 0.0366 (4) |
| C41 | 0.4416 (2) | 0.61909 (14) | 0.80056 (13) | 0.0333 (4) |
| C42 | 0.2695 (2) | 0.42142 (15) | 0.80113 (15) | 0.0379 (4) |
Source of material
A 3 mL EtOH solution of tetrafluorophthalic acid (TFPA, 23.8 mg, 0.1 mmol) was slowly added into a 3 mL EtOH solution of acridine (AD, 17.9 mg, 0.1 mmol) in a 50 mL beaker. The mixture was stirred for 5 min at room temperature. Orange block crystals of the title compound were obtained after about 10 h (CCDC number 2151627).
Experimental details
Empirical absorption correction was performed using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm.
Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program and refined with the ShelXL [4] refinement package.
The carbon bound hydrogen atoms were placed in calculated positions and refined using a riding model.
Comment
Over the past few decades, the rational design and synthesis of luminescent materials have attracted great attention due to their excellent performance in several fields. Among them, two-component crystal materials, assembled by two or more different molecules assembled through hydrogen bond, π–π or charge transfer interactions, have been favored by scientific researchers because of its simple synthetic route, and versatile molecular arrangement [5], [6], [7], [8], [9]. It is well known that acrinidine (AD) is an excellent chromophore and fluorinated organic ligand can enhance the luminescence intensity of complex [10], [11], [12]. In this work, AD was used as the electron acceptor to react with TFPA, and a complex salt was constructed by forming intermolecular hydrogen bonds.
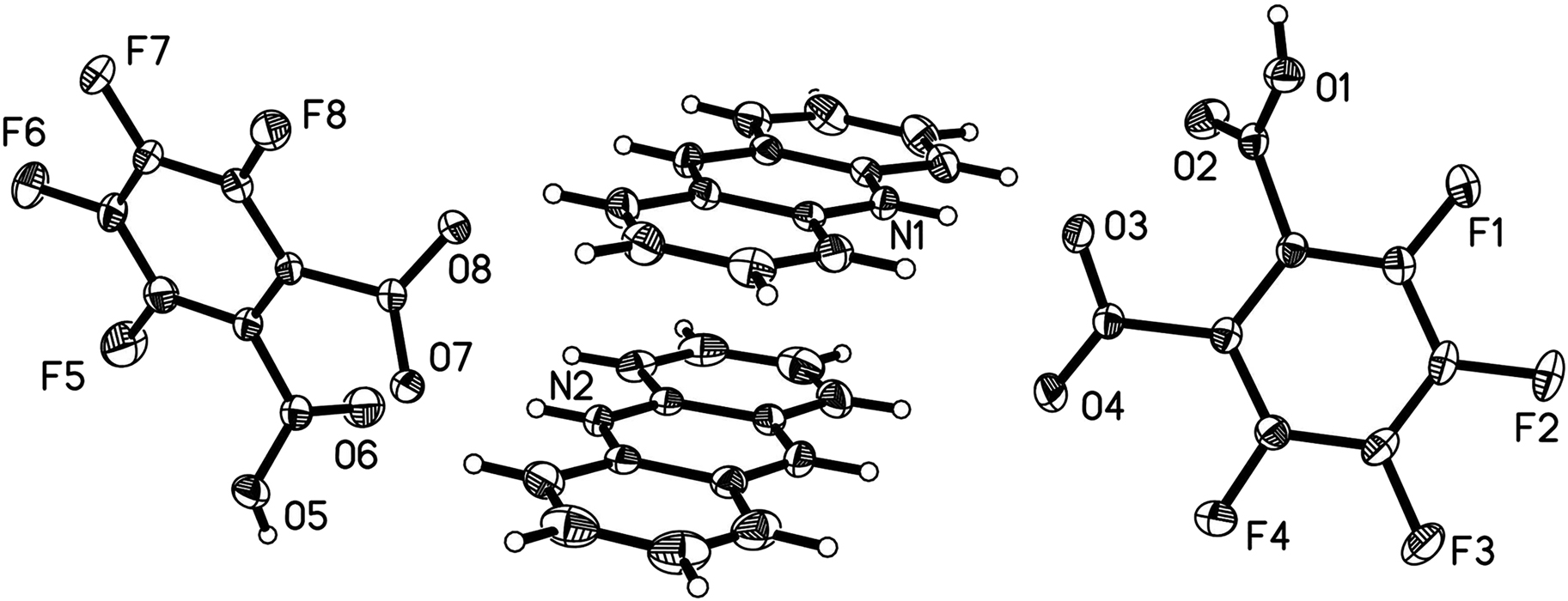
The asymmetric unit of the title compound consists of two molecules of TFPA anions and two molecules of AD cations. As shown in the figure, one of the carboxyl groups of TFPA is deprotonated and the proton is transfered to the nitrogen atom of AD, and the proton transfer is proved by the comparable C–O bond lengths (C20–O3, 1.253(2) Å; C20–O4, 1.240(2) Å) in the carboxyl group [13,14]. Bond lengths and angles in all ions are in the expected ranges [15,16]. Each AD cation is hydrogen bonded to a TFPA anion by N–H⃛O hydrogen bond to form a TFPA-AD ion pair, which is further linked by the O–H⃛O hydrogen bonds and π–π interactions into a 3D supramolecular structure. From another perspective, the TFPA anions located adjacent to each other are connected through O–H⃛O hydrogen bonds, forming an infinite 1D chain along the b direction, and the AD cations are fixed together by multiple TFPA chains in an antiparallel packing manner through N–H⃛O hydrogen bonds and π–π interactions, forming a 3D supramolecular structure.
Funding source: Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province
Award Identifier / Grant number: 2018GGJS128
Funding source: Science and Technology Development Project in Henan Province
Award Identifier / Grant number: 172101410037
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province (No. 2018GGJS128) and Science and Technology Development Project in Henan Province (No. 172101410037).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPRO (version 1.171.39.6a); Rigaku Oxford Diffraction: England, 2018.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Li, S. Z., Yan, D. P. Two-component aggregation-induced emission: two-component aggregation-induced emission materials: tunable one/two-photon luminescence and stimuli-responsive switches by co-crystal formation. Adv. Opt. Mater. 2018, 6, 1870076; https://doi.org/10.1002/adom.201870076.Search in Google Scholar
6. Yang, X. G., Zhai, Z. M., Lu, X. M., Ma, L. F., Yan, D. Fast crystallization-deposition of orderly molecule level heterojunction thin films showing tunable up-conversion and ultrahigh photoelectric response. ACS Cent. Sci. 2020, 6, 1169–1178; https://doi.org/10.1021/acscentsci.0c00447.Search in Google Scholar PubMed PubMed Central
7. Khan, A., Wang, M., Usman, R., Sun, H., Du, M., Xu, C. Molecular marriage via charge transfer interaction in organic charge transfer co-crystals toward solid-state fluorescence modulation. Cryst. Growth Des. 2017, 17, 1251–1257; https://doi.org/10.1021/acs.cgd.6b01636.Search in Google Scholar
8. Mandal, A., Kim, Y., Kim, S. J., Park, J. H. Unravelling the fluorescence and semiconductor properties of a new coronene: TCNB charge transfer cocrystal polymorph. CrystEngComm 2021, 23, 7132–7140; https://doi.org/10.1039/d1ce00741f.Search in Google Scholar
9. Wang, Y., Shang, H., Li, B., Jiang, S. Reversible luminescence “off–on” regulation based on tunable photodimerization via crystal-to-cocrystal transformation. J. Mater. Chem. C 2022, 10, 734–741; https://doi.org/10.1039/d1tc04401j.Search in Google Scholar
10. Varaksina, E. A., Kiskin, M. A., Lyssenko, K. A., Puntus, L. N., Korshunov, V. M., Silva, G. S., Freiref, R. O., Taydakov, I. V. Tuning the luminescence efficiency by perfluorination of side chains in Eu3+ complexes with β-diketones of the thiophene series. Phys. Chem. Chem. Phys. 2021, 23, 25748–25760; https://doi.org/10.1039/d1cp02951g.Search in Google Scholar PubMed
11. Yao, X., Wang, X., Han, Y., Yan, P., Li, Y., Li, G. Structure, color-tunable luminescence, and uv-vis/nir benzaldehyde detection of lanthanide coordination polymers based on two fluorinated ligands. CrystEngComm 2018, 20, 3335–3343; https://doi.org/10.1039/c8ce00516h.Search in Google Scholar
12. Devi, R., Bala, M., Khatkar, S. P., Taxak, V. B., Boora, P. Investigations of luminescent behavior and intramolecular energy transfer mechanism of Europium(III) complexes with fluorinated β-ketoester ligand. J. Fluor. Chem. 2006, 181, 36–44.10.1016/j.jfluchem.2015.11.004Search in Google Scholar
13. Sedghiniya, S., Soleimannejad, J., Janczak, J. The salt-cocrystal spectrum in salicylic acid-adenine: the influence of crystal structure on proton-transfer balance. Acta Crystallogr. 2019, C75, 412–421; https://doi.org/10.1107/s2053229619003127.Search in Google Scholar PubMed
14. D’Vries, R. F., Moreno-Fuquen, R., Camps, I., Ayala, A. P., Kennedy, A. R., Ellena, J. Order-disorder phase transition induced by proton transfer in a co-crystal of 2,4-dichlorobenzoic acid and trimethylamine n-oxide. CrystEngComm 2017, 19, 3753–3759; https://doi.org/10.1039/c7ce00835j.Search in Google Scholar
15. Chang, X. Crystal structure of bis(acridin-10-ium) 2,5-dihydroxy terephthalate-2,5-dihydroxyterephthalic acid (1/1), C21H15NO6. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1253–1254; https://doi.org/10.1515/ncrs-2019-0377.Search in Google Scholar
16. Häcker, H.-G., Schnakenburg, G., Hoffbauer, W., Daniels, J., Pietsch, M., Gütschow, M. Isopropylammonium tetrafluorohydrogenphthalate: structural characterization and comparison to two related salts with different stoichiometric ratios. J. Mol. Struct. 2009, 934, 23–27; https://doi.org/10.1016/j.molstruc.2009.06.013.Search in Google Scholar
© 2022 Xin-Fang Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

