Abstract
The areca nut is one of the most commonly consumed psychoactive substances worldwide, with an estimated consumption by approximately 10% of the world’s population, especially in some regions of South Asia, East Africa, and the tropical Pacific. Arecoline, the major areca nut alkaloid, has been classified as carcinogenic to humans as it adversely affects various organs, including the brain, heart, lungs, gastrointestinal tract, and reproductive organs. Earlier studies have established a link between areca nut chewing and cardiac arrhythmias, and yet research pertaining to the mechanisms underlying cardiotoxicity caused by arecoline is still preliminary. The main purpose of this study is to test the hypothesis that arecoline causes cardiac fibrosis through transforming growth factor-β (TGF-β)/Smad-mediated signaling pathways. Male Wistar rats were injected intraperitoneally with low (5 mg/kg/day) or high (50 mg/kg/day) doses of arecoline for 3 weeks. Results from Masson’s trichrome staining indicated that arecoline could induce cardiac fibrosis through collagen accumulation. Western blot analysis showed that TGF-β and p-Smad2/3 protein expression levels were markedly higher in the arecoline-injected rat hearts than in those of the control rats. Moreover, arecoline upregulated other fibrotic-related proteins, including SP1-mediated connective tissue growth factor expression. Tissue-type plasminogen activator and its inhibitor, plasminogen activator inhibitor, and matrix metalloproteinase (MMP) 9 were upregulated, and the inhibitor of MMP9 was downregulated. This study provides novel insight into the molecular mechanisms underlying arecoline-induced cardiac fibrosis. Taken together, the areca nut is a harmful substance, and the detrimental effects of arecoline on the heart are similar to that caused by oral submucous fibrosis.
1 Introduction
More than 600 million people are regular consumers of areca nuts, especially people in Asian populations [1]. Following nicotine, ethanol, and caffeine, the areca nut is considered the fourth most commonly abused substances by people [2]. Numerous psychiatric studies have shown that chronic areca nut chewers are prone to developing drug dependence, and their presenting symptoms meet the criteria for a substance use disorder of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [3]. In some regions of the tropical Pacific, East Africa, and South Asia, the areca nut is served as a food substance in social gatherings and religious festivities for people with lower socioeconomic status [1]. Chewing the areca nut usually slightly elevates the body temperature and causes people to feel energetic, euphoric, and vigilant. Several epidemiological studies have demonstrated that chronic areca nut chewers can develop systematic diseases in various organs, including the brain, heart, lungs, gastrointestinal tract, reproductive organs, and immune system [4]. Consuming areca nuts has been demonstrated to cause hyperlipidemia, vasospasm, and cardiac arrhythmias, which can subsequently lead to an increased risk of myocardial ischemia [5]. Arecoline, N-methyl-1,2,5,6-tetrahydropyridine-3-carboxylic acid methyl ester (Figure 1), the principle alkaloid in the areca nut, has been classified as carcinogenic to humans [6]. Arecoline-induced collagen deposition through multisignaling pathways has been postulated as the primary causative event for increased collagen production in oral submucous fibrosis [7]. However, information regarding the cardiac dysfunction caused by arecoline is relatively limited.
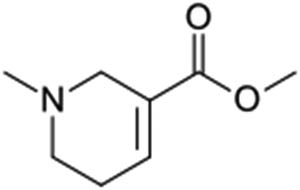
Chemical structure of arecoline, the principal alkaloid in the areca nut.
Fibrosis is characterized by scarring and tissue hardening and is typically caused by excessive extracellular matrix (ECM) protein deposition by myofibroblasts while responding to chronic inflammation [8,9]. A variety of noxious stimuli, including toxins, infectious pathogens, autoimmune reactions, and mechanical stress, are capable of causing fibrotic cellular responses [10]. Fibrosis can occur in almost all organs, including the heart, lungs, liver, and kidneys [11]. Upon tissue damage, myofibroblasts, which are derived from various sources such as resident fibroblasts, circulating fibrocytes, and mesenchymal cells, undergo a wound healing process to restore tissue integrity and promote the replacement of parenchymal cells through the remodeling of the extracellular environment. This pro-fibrotic program is then typically terminated once the tissue healing process has been completed. However, persistent insults or tissue damage can cause dysregulation of the process, resulting in excessive deposition of ECM proteins and reinforced myofibroblast activities [12].
Transforming growth factor-β (TGF-β), the best-known fibrogenic growth factor, is the main regulator of tissue growth, regeneration, remodeling, and fibrosis [13]. The TGF-β family is composed of three subtypes, namely TGF-β1, TGF-β2, and TGF-β3, where TGF-β1 is considered as the major driver of human fibrotic pathologies [14]. Activation of TGF-β1 signaling has been postulated as the main causative event for increased collagen production in oral submucous fibrosis [15]. Recently, it has been shown that the expression of TGF-β is increased during fibrosis, and its downstream mediator Smad2/3 is involved in cardiac fibrosis [16]. Activation of the Smad3 signaling pathway has been reported to play an important role in modulating TGF-β-induced ECM protein synthesis [17]. TGF-β exerts matrix-preserving actions via modulating the activity of matrix metalloproteinases (MMPs) and their inhibitor, tissue inhibitor of metalloproteinases (TIMPs) [18], as well as via regulating the expression of plasminogen activator (PA) and its inhibitors, including PA inhibitor (PAI) [19].
Connective tissue growth factor (CTGF), a cysteine-rich 36–38 kDa secreted protein, is a matricellular protein involved in regulating cell survival, proliferation, adhesion, migration, and ECM production [20]. In addition, TGF-β can induce the Ctgf gene expression in various cell types, especially in fibrotic lesions, and the promoter of the Ctgf gene includes a TGF-β1 response element [21]. Elevated CTGF expression was found in infarcted hearts [22], as well as in cardiac samples obtained from patients who suffer from heart failure [23]. Moreover, the CTGF-stained areas corresponded to the myocardial fibrosis area [24]. Conversely, some of the pro-fibrotic effects of TGF-β have been reported to be mediated via upregulation of its downstream effector CTGF. TGF-β induces the expression of CTGF via a functional Smad3 binding site in the Ctgf gene promoter, which subsequently promotes the synthesis of collagen and the differentiation of myofibroblasts [25]. Furthermore, other studies of in vivo models have concluded that CTGF potentiates fibrogenic actions via regulating TGF-β. However, inhibition of CTGF enhances cardiac repair and limits fibrosis after myocardial infarction [26].
The behavior of chewing areca nuts habitually has been found to cause cardiotoxicity, including coronary artery disease [27], heart failure, and premature ventricular contractions [28]. However, there is a lack of research investigating the effect of arecoline on cardiac fibrosis. As such, we aimed to determine whether arecoline can cause cardiac fibrosis in an animal model and, if so, whether the underlying mechanisms involve the TGF-β/Smad signaling pathway.
2 Materials and methods
2.1 Antibodies and reagents
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Primary antibodies used in the study include p-Smad2/3 (Cell Signaling Technology, Danvers, MA), TGF-β, MMP9, TIMP-2, CTGF, tissue-type PA (tPA), and β-actin (Santa Cruz Biotechnology, Dallas, TX). All secondary antibodies (anti-rabbit, anti-mouse, and anti-goat horseradish peroxidase-conjugated antibodies) were purchased from Santa Cruz Biotechnology (Dallas, TX).
2.2 Animal procedure
Sprague-Dawley (SD) rats were purchased from BioLASCO Co., Ltd. (Taipei, Taiwan). The rats were provided with standard laboratory chow and water ad libitum. Eight-week-old male SD rats were individually housed in a temperature-controlled room at 25 ± 2°C with a 12 h dark–light cycle. After a 4-week acclimation period, the rats were divided into three groups, with eight animals in each group: control group, arecoline low-dosage group (5 mg/kg/day), and arecoline high-dosage group (50 mg/kg/day). Phosphate-buffered saline was used as the medium in all groups. Rats were given arecoline with low dosage or high dosage via intraperitoneal (i.p.) injection every day for 3 weeks (Figure 2). After the treatment, the animals were weighed, anesthetized via isoflurane exposure, and sacrificed via cervical decapitation, and the heart tissue was collected and stored at −80°C until further Western blot analysis. For histological analysis, the collected whole heart tissues from each group were immersed in 10% formalin with gently shaking for 48 h and then dehydrated by consecutive immersion in alcohols and fixed with paraffin wax.

Administration of arecoline to animals.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and was approved by the Animal Research Committee of China Medical University, Taichung, Taiwan and the Animal Care and Use Committee at China Medical University (approval number 100-4-B).
2.3 Masson’s trichrome (MT) staining
Two-micron thick paraffin sections from the hearts of rats in each group were cut from paraffin-embedded tissue blocks. Whole-heart cross sections, derived from serial sectioning, were deparaffinized by immersion in xylene and then rehydrated. The samples were then exposed to MT stain (ScyTek Laboratories, Inc., Logan, UT) and histologically evaluated for fibrotic changes in the heart sections. Images of the samples were obtained microscopically (Zeiss Axiophot, Oberkochen, Deutschland, Germany) under 400× magnification. Semiquantitative scoring (0 to 4) of trichrome sections was assigned in a blinded manner independently by two researchers (Dr Ku and Dr Ou).
2.4 Tissue protein extraction
The left ventricle tissues were collected and homogenized using lysis buffer (20 mM Tris, 2 mM ethylenediaminetetraacetic acid, 50 mM β-mercaptoethanol, 10% glycerol, protease inhibitor, and phosphatase inhibitor, pH 7.4). The homogenates were centrifuged at 12,000×g for 40 min, and the supernatant was collected and stored for further analysis [29].
2.5 Western blotting
Western blot analysis for protein expression was similar to previously described, with slight modifications [30]. The protein concentration of the tissue was analyzed by Lowry’s protein assay [31]. Proteins (40 μg/lane) were separated by 10–15% gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis with a mini gel apparatus at 75 V for 3 h and then transferred to a polyvinylidene fluoride membrane and blocked with 5% nonfat dry milk in tris-buffered saline buffer. Primary antibodies (TGF-β1, p-Smad2/3, SP1, CTGF, tPA, PAI-1, MMP9, TIMP, and β-actin) were diluted to 1:500 and added to hybridize to the membrane overnight at 4°C. Then, the membranes were washed with tris buffered saline buffer with Tween 20 buffer for 30 min, and the secondary antibody solution (1:5,000 dilution) was added and allowed to incubate for 1 h. The proteins were visualized using a enhanced chemiluminescence Western blotting reagent (Millipore, Burlington, MA) in Fujifilm LAS-3000 (GE Healthcare Life Sciences, Marlborough, MA). The intensity of the protein bands was quantified using ImageJ software https://imagej.nih.gov/ij/download.html, and the densitometric data were normalized using β-actin as an internal control.
2.6 Statistical analysis
Statistical analysis was performed with GraphPad Prism software, version 5.0 (Graph-Pad Software, San Diego, CA, USA). All data are expressed as mean ± standard error of the mean. Comparative analysis between groups was conducted using one-way analysis of variance. Significance between the individual means was determined by Tukey’s test. A P value of less than 0.05 was considered significant.
3 Results
3.1 Arecoline induces cardiac fibrosis
To investigate the role of arecoline-affected signaling molecules on cardiac fibrosis, MT staining was implemented to analyze the collagen accumulation in ventricular tissue. The collagen fibers are stained blue, the nuclei are stained black, and the background is stained red. Compared to control rats (Figure 3a, left panel), heart damage due to slight collagen accumulation in rats subjected to low-dosage arecoline treatment was observed (Figure 3a, middle panel); however, the content of accumulated collagen was significantly higher in high-dosage arecoline treatment (Figure 3a, right panel). The accumulation of cardiac collagen fibers clearly indicates the fibrotic injury in the arecoline treatment group.
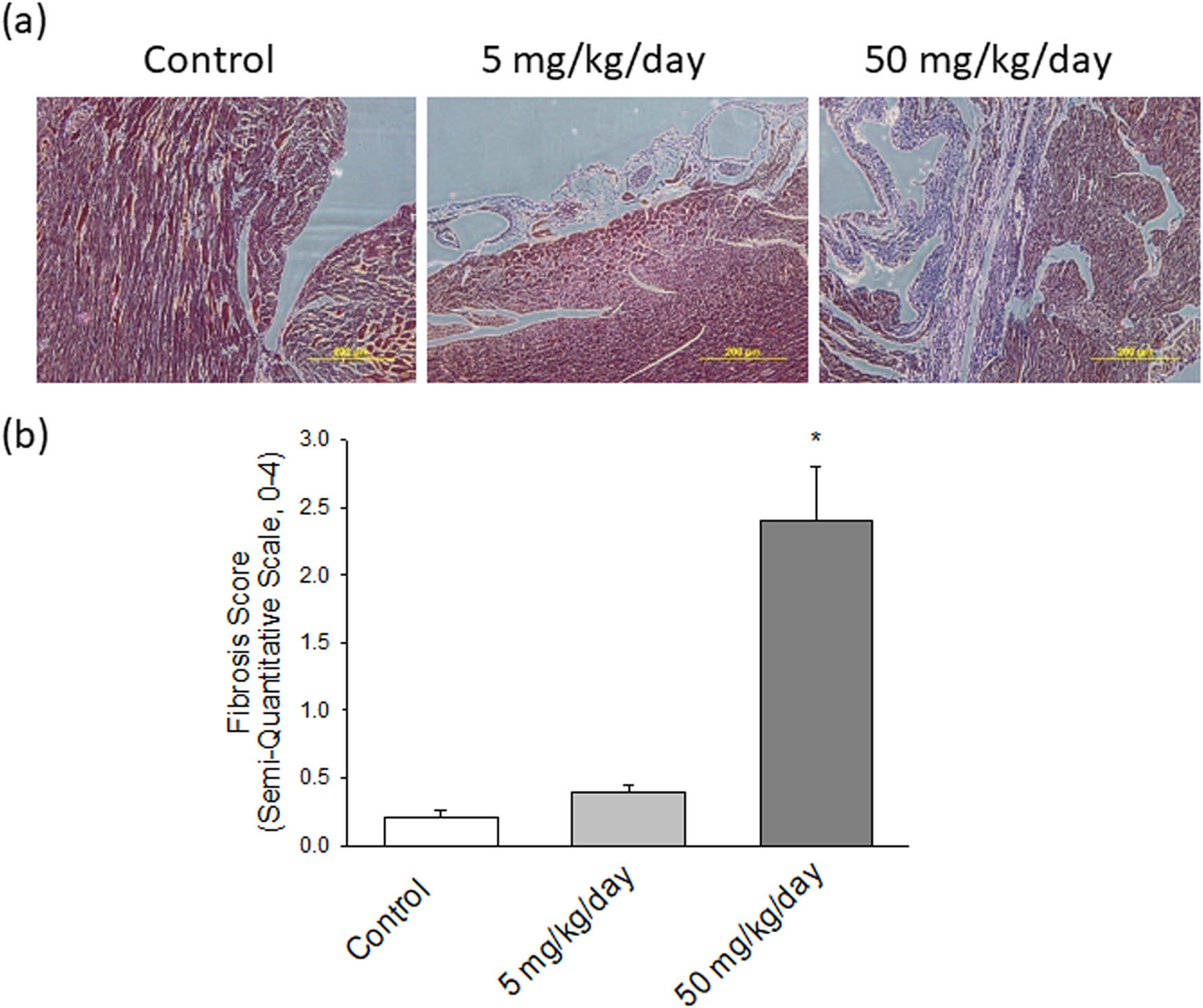
(a) Pathological changes in the left ventricles of experimental rats. MT staining of cardiac tissues from control, low-dosage arecoline, and high-dosage arecoline rats. Collagen accumulation is shown in blue. The myocardial architecture images were magnified at ×400. The scale bar is 200 µm. (b) Semiquantitative grade morphormetric fibrosis scoring for trichrome slides of left ventricular cardiac section. * Significant difference (P < 0.05).
3.2 Arecoline induces TGF-β1/Smad2/3 signaling
To investigate the pro-fibrotic protein expression signaling pathways affected by arecoline, we first assessed the levels of molecules involved in the progress of cardiac remodeling, namely TGF-β1 and p-Smad2/3, in control, low-dose arecoline, and high-dose arecoline rats. The TGF-β1 expression was slightly lower in the low-dose arecoline rats compared to the control rats, and it exhibited an approximately 1.7-fold increase in high-dose arecoline rats compared to the control rats (Figure 4). In terms of p-Smad2/3, the downstream molecule of TGF-β1, the expression levels were increased by 2- and 4.8-fold in low-dose and high-dose arecoline rats compared to control rats, respectively. The results suggest that TGF-β1 signaling is involved in the cardiac fibrosis caused by arecoline, especially in the high-dose group. Notably, the expression levels of TGF-β1 in low-dose group rats are slightly decreased compared to that of the control group.
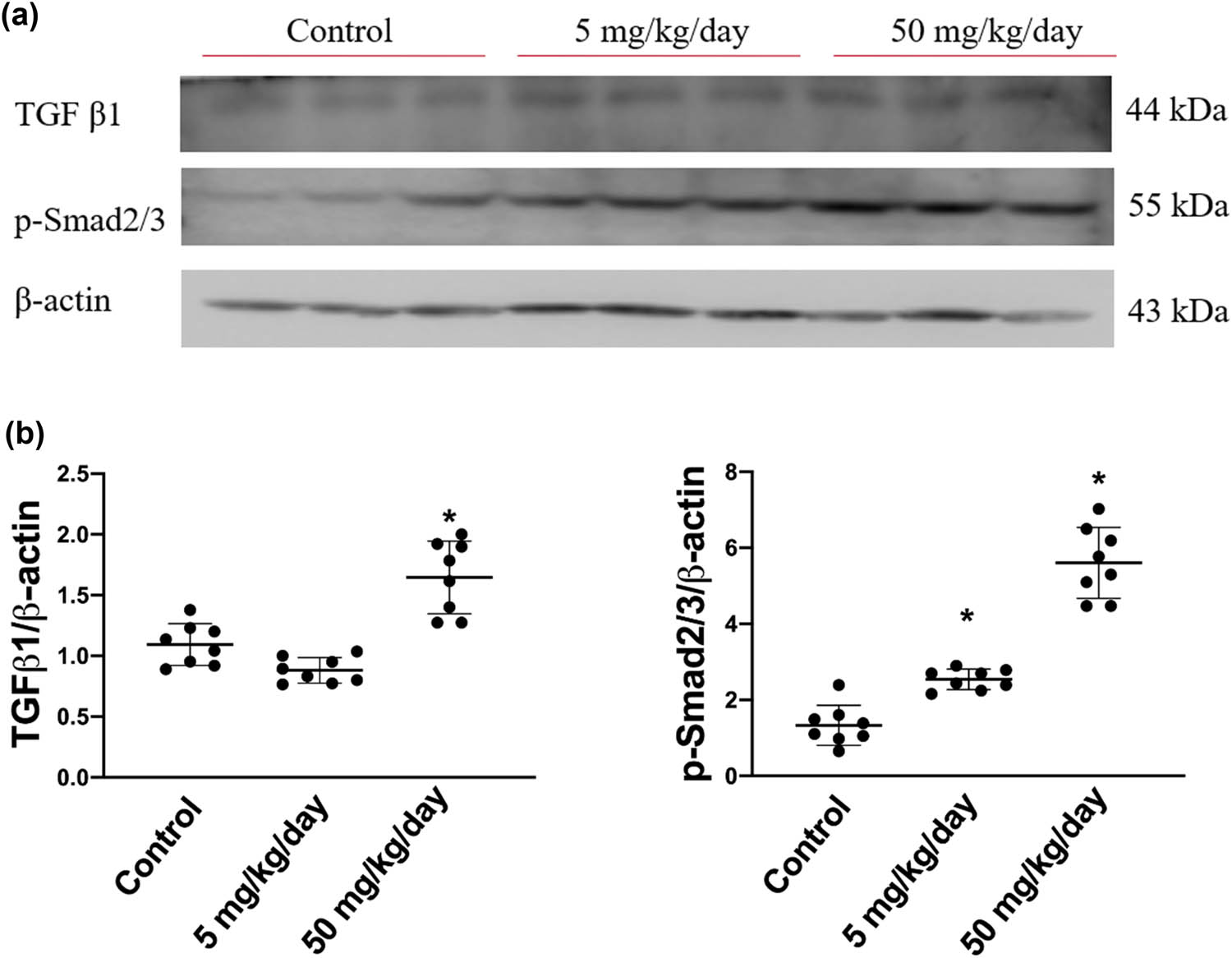
TGF-β1 and phosphorylated Smad2/3 protein expression levels in arecoline-treated rats. (a) TGF-β1 and p-Smad2/3 protein expression levels were examined by Western blot analysis of left ventricular samples from control, low-dosage, and high-dosage arecoline-treated rats. (b) Data were quantified densitometrically and expressed as mean ± SEM. Protein expression was normalized to β-actin expression. *Significant difference (P < 0.05).
3.3 Arecoline induces SP1-mediated CTGF expression
Based on the abovementioned findings, we further determined the protein expression levels of CTGF and its transcription factor SP1, which are both regulated by the TGF-β1-activated p-Smad2/3 pathway. As expected, the expression levels of SP1 were dose dependently increased in arecoline-treated rats compared to the control rats (all P < 0.05). Similarly, the protein levels of CTGF are significantly elevated in both low- and high-dose arecoline-treated groups (Figure 5). The results confirm our hypothesis that the signaling pathway of TGF-β1/Smad2/3/SP1/CTGF is involved in, at least in part, the cardiac fibrosis caused by arecoline.
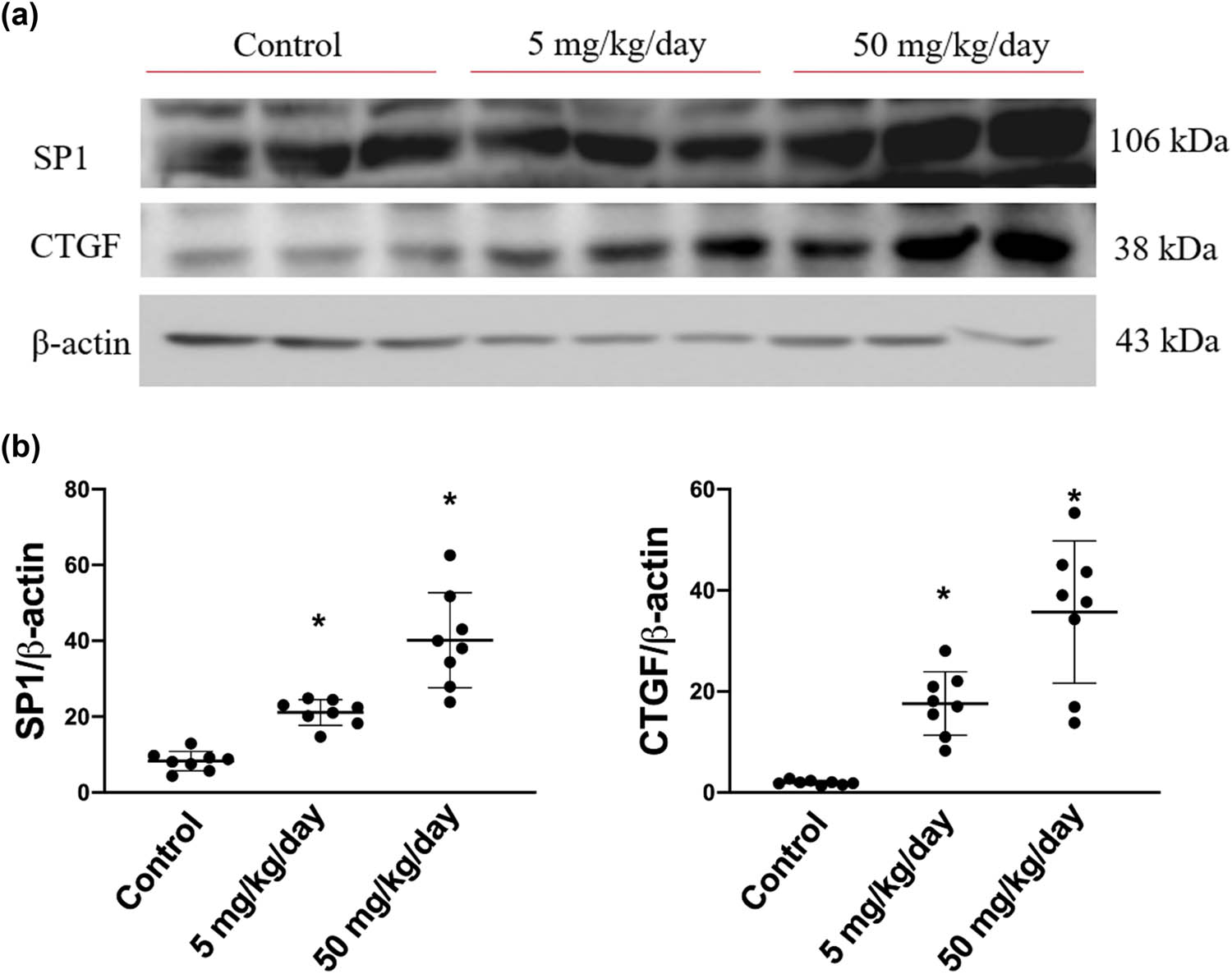
SP1 and CTGF protein expression levels in arecoline-treated rats. (a) SP1 and CTGF protein expression levels were examined by Western blot analysis of left ventricular samples from control, low-dosage, and high-dosage arecoline-treated rats. (b) Data were quantified densitometrically and expressed as mean ± SEM. Protein expression was normalized to β-actin expression. *Significant difference (P < 0.05).
3.4 Arecoline induces PAI/tPA
Subsequently, we sought to determine whether the expression levels of PAI/tPA are involved by arecoline-induced cardiac fibrosis. As shown in Figure 6, the expression levels of PAI were significantly increased in rats in the high-dosage group compared to rats in the low-dosage and the control groups. Similarly, the expression levels of tPA were markedly elevated only in the high-dose group (Figure 6). Notably, the expression levels of PAI-1 in low-dosage group rats are slightly reduced compared to those in the control group. Based on the results, we suggest that arecoline-induced cardiac fibrosis through tPA/PAI-1 regulated the signaling pathway, especially in high-dosage group rats.
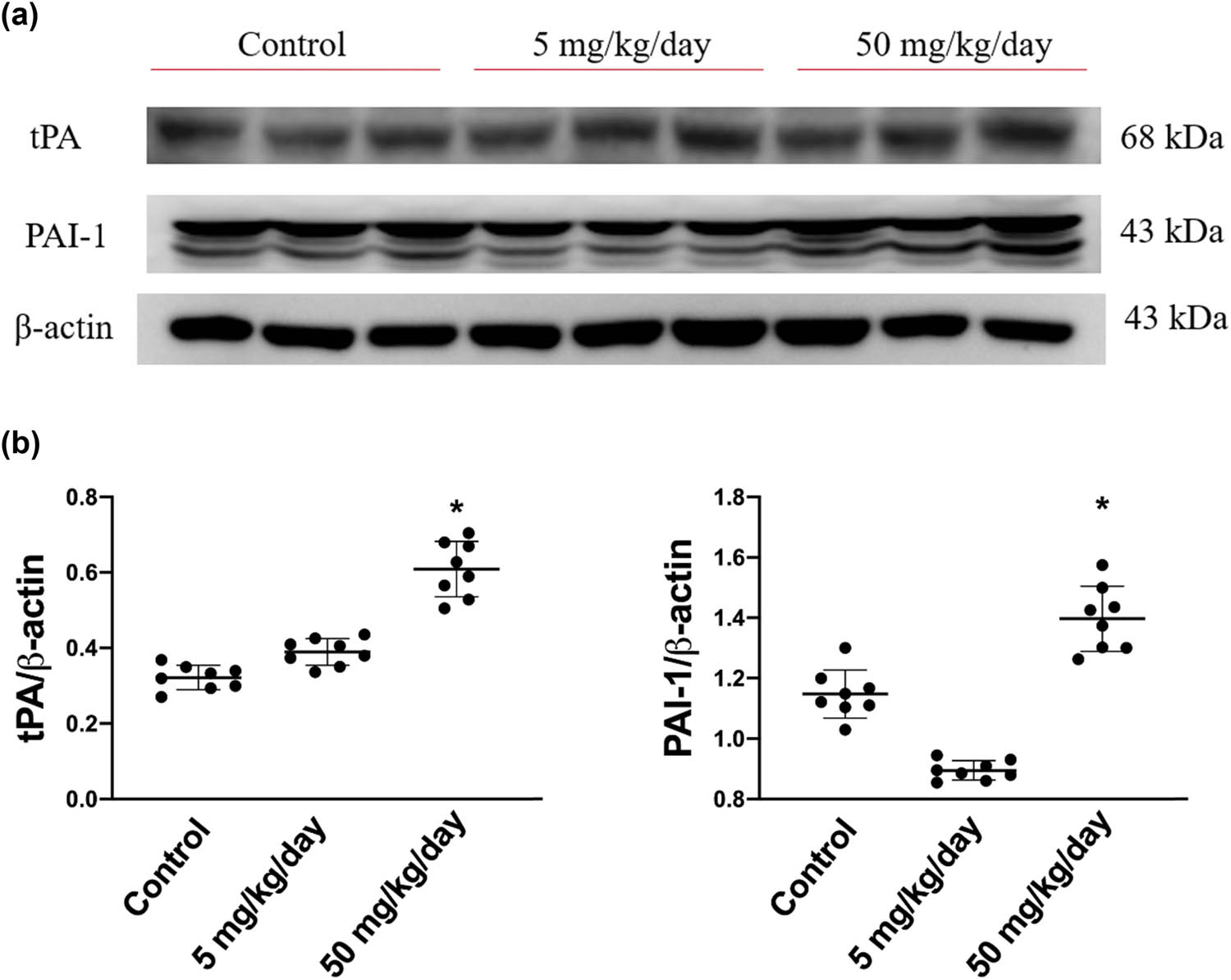
tPA and its inhibitor, PAI protein expression levels in arecoline-treated rats. (a) PAI and tPA protein expression levels were examined by Western blot analysis of left ventricular samples from control, low-dosage, and high-dosage arecoline-treated rats. (b) Data were quantified densitometrically and expressed as mean ± SEM. Protein expression was normalized to β-actin expression. *Significant difference (P < 0.05).
3.5 Arecoline induces compensatory inhibition of TIMP2/MMP9 signaling
We subsequently measured the expression levels of MMP9 and its inhibitor, TIMP2. We found that the expression levels of MMP9 were markedly elevated in high-dosage group rats, whereas the expression level of TIMP2 was significantly reduced in both low-dosage and high-dosage arecoline-treated groups (Figure 7). The results are consistent with earlier studies that MMP-9 influences the metabolism of collagen and promotes the fibrosis of the myocardium. Accordingly, we suggested that MMP-9 participates in arecoline-induced cardiac fibrosis, especially in high-dosage group rats.

MMP9 and its inhibitor, TIMP2, protein expression levels in arecoline-treated rats. (a) MMP9 and TIMP2 protein expression levels were examined by Western blot analysis of left ventricular samples from control, low-dosage, and high-dosage arecoline-treated rats. (b) Data were quantified densitometrically and expressed as mean ± SEM. Protein expression was normalized to β-actin expression. *Significant difference (P < 0.05).
4 Discussion
To the best of our knowledge, this is the first study to detail the molecular mechanisms through which arecoline induces cardiac fibrosis. In accordance with earlier research that found that arecoline induces oral submucous fibrosis through activation of the TGF-β1/Smad signaling pathway, our data provided show that the i.p. administration of arecoline significantly increased the protein expression levels of fibrotic modulators, including TGF-β1, p-Smad2/3, SP1, and CTGF. Furthermore, ECM remodeling proteins, including tPA and MMP9, were upregulated, and TIMP2 was downregulated during arecoline-induced cardiac fibrosis (Figure 8).
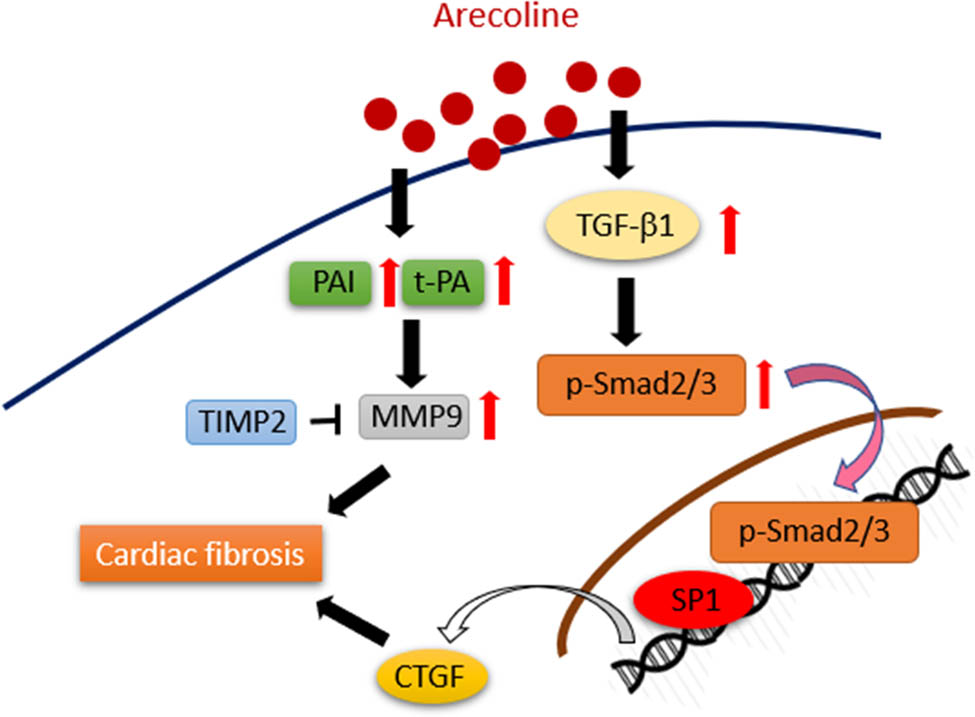
Schematic showing how arecoline induces cardiac fibrosis through upregulation of the TGF-β1/Smad2/3/CTGF as well as tPA/MMP9 signaling pathways.
The molecular pathogenesis of arecoline on the oral cavity, which is the primary exposure site of areca nut chewing, has long been explored. Several arecoline-induced pro-fibrotic factors are accompanied by a reduced intracellular thiol content, and this impairment can be reversed in the presence of antioxidants [32]. Indeed, reactive oxygen species (ROS) generation by arecoline is considered an upstream cause of the various arecoline-driven effects that initiate oral submucous fibrosis [33]. Interaction in a reciprocal manner can be observed between ROS and TGF-β1. For instance, arecoline-induced ROS activates diverse downstream signaling molecules, including TGF-β1, which is followed by ECM deposition, and TGF-β1 reversely increases ROS production coupled with suppression of the antioxidative enzymes [33].
Cardiac fibroblasts play a significant role in the homeostasis of the ECM in cardiac tissue, and they have recently been identified as inflammatory supporter cells. TGF-β1 is a critical regulator of tissue growth, regeneration, remodeling, and fibrosis. TGF-β1 in the injured heart is expressed by resident macrophages and cardiac fibroblasts [34]. Through the activation of the downstream Smad pathway, TGF-β1 induces cardiac fibroblast to myofibroblast differentiation and collagen deposition [35]. Although the TGF-β superfamily is considered the main activator of Smad signaling, accumulating evidence suggests that Smad activation may also be attributed to TGF-β-independent mechanisms in certain cell types [14]. In this study, i.p. administration of arecoline at 5 mg/kg/day did not cause an increase of TGF-β1, and yet it did lead to an increase in p-Smad2/3, suggesting that Smad activation in arecoline-induced fibrotic cardiac conditions is due to both TGF-β-dependent and -independent pathways. In addition, a recent study published by our research group demonstrated that phosphor-Akt, a survival signaling molecule, was tended to increase in low-dosage arecoline-treated rats compared to the control group and high-dosage group rats. The results inferred that the myocardial tissue might still be protected by the survival mechanism under the lower dosage of arecoline but not at high dosage [36]. Consistently, results from this study also showed that the expression levels of TGF-β1 were slightly lower than those in control group rats.
CTGF induced the proliferation of fibroblasts, promoted the transformation from cardiac fibroblast to myofibroblasts, and increased ECM production. Earlier research has indicated that in addition to TGF-β stimulated Smads, several signaling molecules, such tumour necrosis factor α, epidermal growth factor, and wingless/integrated, are involved in the induction of CTGF in normal fibroblasts [37]. This study demonstrated that while arecoline did not change the expression levels of TGF-β1 at a low dose, there was still an increase in CTGF protein expression. This suggests that arecoline may have induced CTGF expression through TGF-β-dependent and -independent pathways.
PAs and their inhibitors, PAIs, play crucial roles in the balance of proteolytic and antiproteolytic enzymatic cascades that regulate ECM turnover. Fibrotic lesions occur when normal control of the balance becomes compromised, which leads to excessive deposition of ECM in the tissues. Consistent with a prior study that showed that both tPA and PAI are increased in areca nut chewer fibroblasts compared to control individual fibroblasts [38], our results indicated that the protein expression levels of tPA and PAI were significantly higher in rats that received a high dose of arecoline than in rats from the low-dose and the control groups. Furthermore, it has been reported that increased tPA has detrimental consequences by inducing MMP9 expression [39]. We found that MMP9 was upregulated, and its inhibitor TIMP2 was downregulated in a dose-dependent manner during arecoline-induced cardiac fibrosis.
In addition to the well-known pathogenic mechanisms underlying oral submucous fibrosis caused by arecoline, the adverse effects of areca nut in other organs have been discovered. For instance, habitual betel quid chewing caused hepatocellular carcinoma complicating cirrhosis [40], and arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes [41]. Betel nut chewing causes bronchoconstriction [42]. Arecoline causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system [43]. Several studies have been reported that betel nut chewing induced cardiac dysrhythmias [28,44]. However, the molecular mechanisms in which arecoline-induced cardiac dysfunction is needed to be further elucidated.
Areca nuts have been classified as a Class I carcinogen by IARC (IARC working Group on the Evaluation of Carcinogenic Risks to Human, 2004) [45]. Arecoline is an important alkaloid that can be detected in the blood plasma, hair samples, and breast milk of areca nut consumers [46]. The lower dose, 5 mg/kg/day, used in our study has been reported in an earlier pharmacokinetic study that the peak concentrations of 1142 ± 554 and 923 ± 368 ng/mL were measured in plasma after 1 min in 3- and 24-month-old rats, respectively. Thereafter, arecoline demonstrated a monophasic disappearance with t l/2 values of 5.8 and 3.5 min [47]. As such, the concentrations of arecoline in the plasma after i.p. administration at the dose of 5 mg/kg/day were likely depleted within an hour. Accordingly, we assumed that the expression levels of TGF-β1 in rats that received the low-dosage arecoline remained unchanged or slightly lower than the control is attributed to the short half-life of arecoline in the plasma. Another human pharmacokinetics study reported that the concentration of arecoline of areca nut consumed 1 day before the blood was drawn remained at about 7 ng/mL [46]. Indeed, the pathophysiology of cardiac fibrosis is highly complicated, as elements of cardiac fibrosis are involved in a wide range of multifactorial disorders. This study has certain limitations. First, the expression trends of some proteins were not consistent in rats that received low and high doses; thus, we need more doses to validate the effects of arecoline in cardiac fibrosis. Second, although our results showed that cardiac fibrosis is attributed to the activation of the TGF-β/Smad pathway, whether or not other signaling molecules are involved in arecoline-induced cardiac fibrosis remains to be elucidated. Third, there is still a small number of repetitions and that additional research is necessary for confirmation.
5 Conclusion
Unlike the quick decline of arecoline in our animal model, the plasma concentration of arecoline in habitual areca nut chewers remains substantial, which may lead to more profoundly harmful effects. Therefore, the use of areca nuts must be tightly regulated for the welfare of society.
-
Funding information: This research was funded by Hualien Tzu Chi Hospital (grant number: IMAR109-01-04-01), China Medical University, and Asia University (grant number: CMU107-ASIA-10).
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during this study are available from the corresponding author on reasonable request.
References
[1] Nelson BS , Heischober B . Betel nut: a common drug used by naturalized citizens from India, Far East Asia, and the South Pacific Islands. Ann Emerg Med. 1999;34(2):238–43.10.1016/S0196-0644(99)70239-8Search in Google Scholar
[2] Zdrojewicz Z , Kosowski W , Królikowska N , Stebnicki M , Stebnicki MR . [Betel – the fourth most popular substance in the world]. Pol Merkur Lekarski. 2015;39(231):181–5.Search in Google Scholar
[3] Lee CH , Ko AM , Yang FM , Hung CC , Warnakulasuriya S , Ibrahim SO , et al. Association of DSM-5 betel-quid use disorder with oral potentially malignant disorder in 6 betel-quid endemic asian populations. JAMA Psychiatry. 2018;75(3):261–9.10.1001/jamapsychiatry.2017.4307Search in Google Scholar PubMed PubMed Central
[4] Garg A , Chaturvedi P , Gupta PC . A review of the systemic adverse effects of areca nut or betel nut. Indian J Med Paediatr Oncol. 2014;35(1):3–9.10.4103/0971-5851.133702Search in Google Scholar PubMed PubMed Central
[5] Chittivelu S , Chittivelu KS . Betel nut chewing and cardiac arrhythmia. Vet Hum Toxicol. 1998;40(6):368.Search in Google Scholar
[6] Sundqvist K , Liu Y , Nair J , Bartsch H , Arvidson K , Grafström RC . Cytotoxic and genotoxic effects of areca nut-related compounds in cultured human buccal epithelial cells. Cancer Res. 1989;49(19):5294–8.Search in Google Scholar
[7] Rehman A , Ali S , Lone MA , Atif M , Hassona Y , Prime SS , et al. Areca nut alkaloids induce irreparable DNA damage and senescence in fibroblasts and may create a favourable environment for tumour progression. J Oral Pathol Med. 2016;45(5):365–72.10.1111/jop.12370Search in Google Scholar PubMed
[8] Jurisic V , Terzic T , Pavlovic S , Colovic N , Colovic M . Elevated TNF-alpha and LDH without parathormone disturbance is associated with diffuse osteolytic lesions in leukemic transformation of myelofibrosis. Pathol Res Pract. 2008;204(2):129–32.10.1016/j.prp.2007.09.001Search in Google Scholar PubMed
[9] Jurisic V , Terzic T , Colic S , Jurisic M . The concentration of TNF-alpha correlate with number of inflammatory cells and degree of vascularization in radicular cysts. Oral Dis. 2008;14(7):600–5.10.1111/j.1601-0825.2007.01426.xSearch in Google Scholar PubMed
[10] Wynn TA . Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210.10.1002/path.2277Search in Google Scholar PubMed PubMed Central
[11] Wynn TA , Ramalingam TR . Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40.10.1038/nm.2807Search in Google Scholar PubMed PubMed Central
[12] Weiskirchen R , Weiskirchen S , Tacke F . Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Asp Med. 2019;65:2–15.10.1016/j.mam.2018.06.003Search in Google Scholar PubMed
[13] Diegelmann RF , Evans MC . Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9.10.2741/1184Search in Google Scholar PubMed
[14] Biernacka A , Dobaczewski M , Frangogiannis NG . TGF-β signaling in fibrosis. Growth Factors (Chur, Switz). 2011;29(5):196–202.10.3109/08977194.2011.595714Search in Google Scholar PubMed PubMed Central
[15] Rai A , Ahmad T , Parveen S , Parveen S , Faizan MI , Ali S . Expression of transforming growth factor beta in oral submucous fibrosis. J Oral Biol Craniofa Res. 2020;10(2):166–70.10.1016/j.jobcr.2020.03.015Search in Google Scholar PubMed PubMed Central
[16] Mehdipoor M , Damirchi A , Razavi Tousi SMT , Babaei P . Concurrent vitamin D supplementation and exercise training improve cardiac fibrosis via TGF-β/Smad signaling in myocardial infarction model of rats. J Physiol Biochem. 2021;77(1):75–84 10.1007/s13105-020-00778-6Search in Google Scholar PubMed
[17] Iozzo RV , Theocharis AD , Neill T , Karamanos NK . Complexity of matrix phenotypes. Matrix Biol Plus. 2020;6–7:100038.10.1016/j.mbplus.2020.100038Search in Google Scholar PubMed PubMed Central
[18] Su C , Wang Q , Luo H , Jiao W , Tang J , Li L , et al. Si-Miao-Yong-An decoction attenuates cardiac fibrosis via suppressing TGF-β1 pathway and interfering with MMP-TIMPs expression. Biomed Pharmacother. 2020;127:110132.10.1016/j.biopha.2020.110132Search in Google Scholar PubMed
[19] Rabieian R , Boshtam M , Zareei M , Kouhpayeh S , Masoudifar A , Mirzaei H . Plasminogen activator inhibitor Type-1 as a regulator of fibrosis. J Cell Biochem. 2018;119(1):17–27.10.1002/jcb.26146Search in Google Scholar PubMed
[20] Parada C , Li J , Iwata J , Suzuki A , Chai Y . CTGF mediates Smad-dependent transforming growth factor β signaling to regulate mesenchymal cell proliferation during palate development. Mol Cell Biol. 2013;33(17):3482–93.10.1128/MCB.00615-13Search in Google Scholar PubMed PubMed Central
[21] Wong CKS , Falkenham A , Myers T , Légaré JF . Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-β signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis. J Renin-Angiotensin-Aldosterone Syst. 2018;19(1):1470320318759358.10.1177/1470320318759358Search in Google Scholar PubMed PubMed Central
[22] Dean RG , Balding LC , Candido R , Burns WC , Cao Z , Twigg SM , et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53(10):1245–56.10.1369/jhc.4A6560.2005Search in Google Scholar PubMed
[23] Chi H , Feng H , Shang X , Jiao J , Sun L , Jiang W , et al. Circulating connective tissue growth factor is associated with diastolic dysfunction in patients with diastolic heart failure. Cardiology. 2019;143(3–4):77–84.10.1159/000499179Search in Google Scholar PubMed
[24] Wu CK , Wang YC , Lee JK , Chang SN , Su MY , Yeh HM , et al. Connective tissue growth factor and cardiac diastolic dysfunction: human data from the Taiwan diastolic heart failure registry and molecular basis by cellular and animal models. Eur J Heart Fail. 2014;16(2):163–72.10.1002/ejhf.33Search in Google Scholar PubMed
[25] Lang C , Sauter M , Szalay G , Racchi G , Grassi G , Rainaldi G , et al. Connective tissue growth factor: a crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med (Berlin, Ger). 2008;86(1):49–60.10.1007/s00109-007-0249-3Search in Google Scholar PubMed
[26] Vainio LE , Szabó Z , Lin R , Ulvila J , Yrjölä R , Alakoski T , et al. Connective tissue growth factor inhibition enhances cardiac repair and limits fibrosis after myocardial infarction. JACC Basic Transl Sci. 2019;4(1):83–94.10.1016/j.jacbts.2018.10.007Search in Google Scholar PubMed PubMed Central
[27] Khan MS , Bawany FI , Ahmed MU , Hussain M , Khan A , Lashari MN . Betel nut usage is a major risk factor for coronary artery disease. Glob J health Sci. 2013;6(2):189–95.10.5539/gjhs.v6n2p189Search in Google Scholar PubMed PubMed Central
[28] Huang TC , Wu WT , Chen YC , Yang FM , Tsai WC , Lee CH . Betel-quid chewing, heart failure, and premature ventricular contractions in patients with cardiopulmonary symptoms. Int J Environ Res public health. 2020;17:20.10.3390/ijerph17207472Search in Google Scholar PubMed PubMed Central
[29] Lu CH , Ou HC , Day CH , Chen HI , Pai PY , Lee CY , et al. Deep sea minerals ameliorate diabetic-induced inflammation via inhibition of TNFα signaling pathways. Environ Toxicol. 2020;35(4):468–77.10.1002/tox.22882Search in Google Scholar PubMed
[30] Lin CC , Chen KB , Tsai CH , Tsai FJ , Huang CY , Tang CH , et al. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-κB signaling pathways. J Food Biochem. 2019;43(7):e12902.10.1111/jfbc.12902Search in Google Scholar PubMed
[31] Huang TY , Peng SF , Huang YP , Tsai CH , Tsai FJ , Huang CY , et al. Combinational treatment of all-trans retinoic acid (ATRA) and bisdemethoxycurcumin (BDMC)-induced apoptosis in liver cancer Hep3B cells. J Food Biochem. 2020;44(2):e13122.10.1111/jfbc.13122Search in Google Scholar PubMed
[32] Tsai CH , Chou MY , Chang YC . The up-regulation of cyclooxygenase-2 expression in human buccal mucosal fibroblasts by arecoline: a possible role in the pathogenesis of oral submucous fibrosis. J Oral Pathol Med. 2003;32(3):146–53.10.1034/j.1600-0714.2003.00004.xSearch in Google Scholar PubMed
[33] Das A , Giri S . A review on role of arecoline and its metabolites in the molecular pathogenesis of oral lesions with an insight into current status of its metabolomics. Prague Med Rep. 2020;121(4):209–35.10.14712/23362936.2020.19Search in Google Scholar PubMed
[34] Bolivar S , Espitia-Corredor JA , Olivares-Silva F , Valenzuela P , Humeres C , Anfossi R , et al. In cardiac fibroblasts, interferon-beta attenuates differentiation, collagen synthesis, and TGF-β1-induced collagen gel contraction. Cytokine. 2021;138:155359.10.1016/j.cyto.2020.155359Search in Google Scholar
[35] Saadat S , Noureddini M , Mahjoubin-Tehran M , Nazemi S , Shojaie L , Aschner M , et al. Pivotal role of TGF-β/Smad signaling in cardiac fibrosis: non-coding RNAs as effectual players. Front Cardiovasc Med. 2020;7:588347.10.3389/fcvm.2020.588347Search in Google Scholar
[36] Lin WY , Tsai BC , Day CH , Chiu PL , Chen RJ , Chen MY , et al. Arecoline induces heart injure via Fas/Fas ligand apoptotic pathway in heart of Sprague-Dawley rat. Environ Toxicol. 2021;36(8):1567–75.10.1002/tox.23153Search in Google Scholar
[37] Ramazani Y , Knops N , Elmonem MA , Nguyen TQ , Arcolino FO , van den Heuvel L , et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018;68–69:44–66.10.1016/j.matbio.2018.03.007Search in Google Scholar
[38] Yang SF , Hsieh YS , Tsai CH , Chen YJ , Chang YC . Increased plasminogen activator inhibitor-1/tissue type plasminogen activator ratio in oral submucous fibrosis. Oral Dis. 2007;13(2):234–8.10.1111/j.1601-0825.2006.01272.xSearch in Google Scholar
[39] Hu K , Yang J , Tanaka S , Gonias SL , Mars WM , Liu Y . Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J Biol Chem. 2006;281(4):2120–7.10.1074/jbc.M504988200Search in Google Scholar
[40] Tsai JF , Jeng JE , Chuang LY , Ho MS , Ko YC , Lin ZY , et al. Habitual betel quid chewing and risk for hepatocellular carcinoma complicating cirrhosis. Medicine. 2004;83(3):176–87.10.1097/01.md.0000126971.80227.a4Search in Google Scholar
[41] Chou WW , Guh JY , Tsai JF , Hwang CC , Chen HC , Huang JS , et al. Arecoline-induced growth arrest and p21WAF1 expression are dependent on p53 in rat hepatocytes. Toxicology. 2008;243(1–2):1–10.10.1016/j.tox.2007.09.003Search in Google Scholar
[42] Taylor RF , Al-Jarad N , John LM , Conroy DM , Barnes NC . Betel-nut chewing and asthma. Lancet (London, Engl). 1992;339(8802):1134–6.10.1016/0140-6736(92)90732-ISearch in Google Scholar
[43] Shih YT , Chen PS , Wu CH , Tseng YT , Wu YC , Lo YC . Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic Biol Med. 2010;49(10):1471–9.10.1016/j.freeradbiomed.2010.07.017Search in Google Scholar PubMed
[44] Chiang WT , Yang CC , Deng JF , Bullard M . Cardiac arrhythmia and betel nut chewing--is there a causal effect? Vet Hum Toxicol. 1998;40(5):287–9.Search in Google Scholar
[45] IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 85; 2004. p. 1–334.Search in Google Scholar
[46] Wu IC , Chen PH , Wang CJ , Wu DC , Tsai SM , Chao MR , et al. Quantification of blood betel quid alkaloids and urinary 8-hydroxydeoxyguanosine in humans and their association with betel chewing habits. J Anal Toxicol. 2010;34(6):325–31.10.1093/jat/34.6.325Search in Google Scholar
[47] Soncrant TT , Holloway HW , Greig NH , Rapoport SI . Regional brain metabolic responsivity to the muscarinic cholinergic agonist arecoline is similar in young and aged Fischer-344 rats. Brain Res. 1989;487(2):255–66.10.1016/0006-8993(89)90830-5Search in Google Scholar
© 2021 Chang-Wen Ku et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

