Abstract
The effects of ventilation and sucrose concentration on proliferation and organogenesis of pistachio cutting and photosynthetic performance of two in vitro cultures of pistachio rootstocks have been assessed. The apical leaf buds (Qazvini and UCB1 cultivars) were cultured in filter vessels containing Murashige and Skoog medium supplemented with 0, 10, 15, and 30 g L −1 of sucrose. The plants treated with 10, 15, and 30 g L −1 sucrose showed no significant differences regarding the measured traits; therefore, this treatment was set aside from the final statistical analyses. Use of different ventilation systems showed to be suitable for increasing the growth of pistachio. Referring to root production difficulties under in vitro cultivation of pistachio, ventilation increased the root production and length. However, the full ventilation system was more effective in improving the growth properties. Regression between fluorescence feature vs root length showed that F v/F m had a significant positive relationship with root length. Stomata of cell parameters under ventilation systems improved compared to no ventilation, which was highly similar to the trend in the greenhouse. The overall results indicated that low concentrations of sucrose (e.g., 10 g L −1) and full ventilation are recommended for producing high quality and vigorous pistachio plantlets under in vitro conditions.
1 Introduction
Perennial trees, such as pistachio (Pistacia vera), require a long time to go from cutting to a plant with root and shoot. In pistachio, the average time from the cutting to a tree that can produce the nuts is about 2–3 years. In addition, the diseases and some other problems such as insect attach and fungi infections would threaten the maternal plants under in vivo conditions. Therefore, using tissue culture for producing such plants can shorten the production period from the cutting to tree in comparison with in vivo conditions; in pistachio, from 3 years to 1 years, it can also provide new plants without any infection [1]. The maximum time required for transforming the produced plants from acclimatization to the point when the transported plant starts to continue its normal growth is 1 year, whereas its final nut yield is not significantly different from in vivo produced trees in the following years. Furthermore, the breeding of perennial trees has always been hard to improve, whereas using tissue culture would provide much more opportunity to improve the quality and quantity of these plants. In tissue culture, a researcher can assess the impacts of different substances with mutation effects and other ways to test and improve the quality of perennial trees. However, cultivating pistachio has had some essential improvement since its first achievement in growing under the in vitro condition as rooting and root genesis of the tissues in any environment and medium have faced some difficulties [2]. Successful rooting in pistachio trees under in vitro conditions, or any other provided condition to grow vigorous root, would improve its growth and the economic production of pistachio. Aside from improving the organogenesis of pistachio under in vitro condition, producing healthy and high-quality plants in high quantities is also among the main goals of in vitro production of pistachio. By providing and producing healthy and vigorous plants under in vitro conditions, the adaptability of such plants would successfully increase and allow them to have a higher chance to stand along under outdoor conditions [1]. One of the most important features for improving the quality and quantity of plants is to increase the rate of photosynthesis in plants in any possible way. Under in vitro conditions, the air usually is not substitutable. The content of oxygen and carbon dioxide is limited; accordingly, inventing some safe ways to circulate the air in the culture containers would probably improve plant production efficiency and produce high-quality plants. In addition, increasing the photosynthesis efficiency and the ability to use air and light efficiently have not gained enough interest in pistachio, and it practically has not shown any progress; therefore, pistachio requires extra consideration regarding the photosynthesis and improving its mechanism. Moreover, the chlorophyll (Chl) fluorescence emission assessment has been a helpful method for monitoring the plants’ photosynthesis ability and for determining the relative effects of different stress conditions on the plants [3].
In outdoor conditions, there is no limitation related to carbon dioxide and oxygen sources for being provided by the environments to the pistachio plants; whereas, under in vitro conditions, there are limitations for the amount of these sources for the plants. The amount of these sources is strictly dependent on and limited to their container volume. Meanwhile, ventilation can create better conditions for plantlets’ growth by increasing both the waxy leaf layer and stomatal functions [4]. Previous references have indicated that the thin leaf waxy layer and disordered stomatal cell function, especially open stomata, could lead to weak transpiration adjustment on the in vitro-grown plants [5]. Similarly, stomatal density seems to be a major adaptive trait in tissue-cultured plants [6], so that low stomatal density has been considered a critical determinant for high-efficient water use [7]. A similar report showed that stomatal density can tightly control plant water loss through the plant leaf surface and their closing mechanism in environmentally abnormal conditions, especially in tissue-cultured plants [8]. It has been also demonstrated that reducing stomatal density could increase the water-use efficiency [6]. Other stomatal traits, such as stomatal density, the stomatal index, epidermal cell density, and stomatal width, are required to be considered in tissue-cultured plants under different provided conditions and media to set up the best combinations for improving the quality of such traits in pistachio plants produced under in vitro conditions [9].
To the best of our knowledge, no studies on the effect of ventilation on the pistachio plants and their photosynthetic performance under these conditions were performed. Therefore, the main objectives of this study were to focus on the effect of ventilation mechanism on quality and quantity of pistachio plantlets growing under in vitro condition and to study the stomata anatomy and fluorescence emission under different ventilations interacting with sucrose treatment as the most important source of energy in in vitro plants.
2 Materials and methods
2.1 Plant material
Pistachio apical leaf buds from two rootstocks (Qazvini and UCB1) were cultured in vessels containing modified MS media optimized for both rootstocks and supplemented with 6-benzyladenine (BA; 1.5 mg L−1) and indole-3-butyric acid (0.1 mg L−1), which solidified with agar (7 g L−1). The sucrose source was mixed with media before its solidification by the rate of 0, 10, 15, and 30 g L −1 as the sucrose treatments. The media pH was adjusted to 5.7 before mixing the applied substances and autoclaving (20 min at 121°C) the liquid from media. UCB1 rootstock as a vigorous hybrid rootstock and Qazvini as a native stock were selected for this experiment because they are both highly tolerant of harsh conditions, especially salty and dry soil.
To assess the effect of ventilation, changeable vessel filters made out of 50 µm microporous polypropylene membrane (Pardis Co, Iran®) were applied. Accordingly, after mixing with agarose, the liquid medium was immediately poured into the bottom of the vessels, where the cuttings were planted. The neck of the vessels was connected with three different ventilation conditions, including full ventilation (FV), half ventilation (HV), and without ventilation (NV). In the vessels’ neck, two filters were placed at 5 cm distance from each other. The ventilation systems obtained the air from a container that had two fans for inserting the air. The air in the air container was properly disinfected by using UV light. The used vessels had one input path connected with the air container and contained two extra filters to insert the bottom of the vessels directly on the cutting and output path for exiting the air. At the end of the vessels’ output path, some mechanisms were used to ensure that air could not get in. Eight suction engines were used for inserting the air from the air container, and each output part of these engines was divided between ten ventilator systems. In FV systems, the period of 8 h out of 16 light hours (16/8 light–dark condition in the growth chamber) was used for inserting the air, but for HV, the period of 4 h was used. After each inserting period, the output and input parts of the vessels were sealed until the nest ventilation period. To be able to compare the ventilation treatments with in vivo control conditions, some pistachio cuttings were grown under greenhouse conditions (greenhouse plants [GP]). For each treatment combination, three ventilation vessels were used as repetitions, and the three separate cuttings were placed in each vessel’s bottom. Accordingly, the overall experimental units were equal to 72 (four sucrose levels × three ventilations × two rootstock × three repeats). The experimental units, alongside the air container, were placed in a growth chamber having 25 ± 2°C temperature and 16/8 h light–dark cycles. In addition, the control plants in the greenhouse condition were repeated three times and cultivated in pots with 500 mL volume, where each pot consisted of two cultivate cuttings.
2.2 Plantlet growth parameters
Forty-five days after starting the experiment, one of the three planting plantlets in each experimental unit was carefully taken out for measuring plantlet height and root length by using digital collis (caliper) according to the millimeter unit. Other growth parameters contained the number of shoots (proliferation) and leaves that were measured from all three plantlets in each experimental unit and used their average as the final result. Similar measurements were performed on GP.
2.3 Fluorescence emission assessment
For analyzing fluorescence emission, fully developed leaves were used for surveying the maximum quantum efficiency of Photosystem II (F v/F m). First, vessels were inserted in dark condition for at least 20 min to adapt to the dark. Next, plantlets were used to measure Chl fluorescence of in vitro plants and GP using a fluorometer system (Handy FluorCam FC 1000-H Photon Systems Instruments, PSI, the Czech Republic®) immediately. Images taken by FluorCam were recorded during short measuring flashes in dark conditions. Then, based on the FluorCam protocol, the leaf samples were then exposed to a pulse of saturating light (3,900 μmol m−2 s−1), resulting in a temporary photochemistry saturation and primary Quinone acceptor reduction of PSII [3,4]. After reaching a steady state of fluorescence, two main fluorescence data involving F 0 and F m were digitalized, during short measuring flashes in the dark (F 0), and during the saturating exposition (F m) along with F m′ were obtained. Later, according to the ratio between F m and F 0, the variable fluorescence (F v) was calculated. Finally, the F v/F m ratio and nonphotochemical chlorophyll fluorescence quenching (NPQ) were estimated according to the F m/F m ratio [10] (Figure 1).

Fluorescence emission from two pistachio rootstocks UCB1 (a) and Qazvini (b) leaves. Vertical values are F v/F m amounts for each rootstock, yellow-to-red-colored sections parts, maximum F v/F m amounts.
2.4 Stomatal studding
To analyze stomatal of the tissue-cultured plants, leaf samples were taken from samples supplied with sucrose 15 (g L−1) for all ventilation treatments. Next, based on a procedure described in a study [9], four-leaf samples from greenhouse pistachio plants and four vessels with two explants per vessel (as in vitro pistachio plantlets) were used for comparison. Furthermore, we used the nail varnish method for imaging the stomata cell, as explained in a previous study [10]. It should also be noted that plants were randomly selected from each replicate. Afterward, a thin layer of nail polish was applied to the abaxial surface of the second leaf of each sample. After 5 min, the dried varnish was gently peeled off, and the lower surface of the leaf epidermis was removed and placed on a lam. Finally, images of epidermal strips were taken (Figure 2) with a stereo microscope (SZM-3 model, Italy®). These images were used for calculating density (no mm−2), dimension (width and length [µm]), width-to-length ratio of stomata, as well as the stomatal index using Image Tools (University of Texas, TX), and the following equation was reported according to an earlier study [11]:
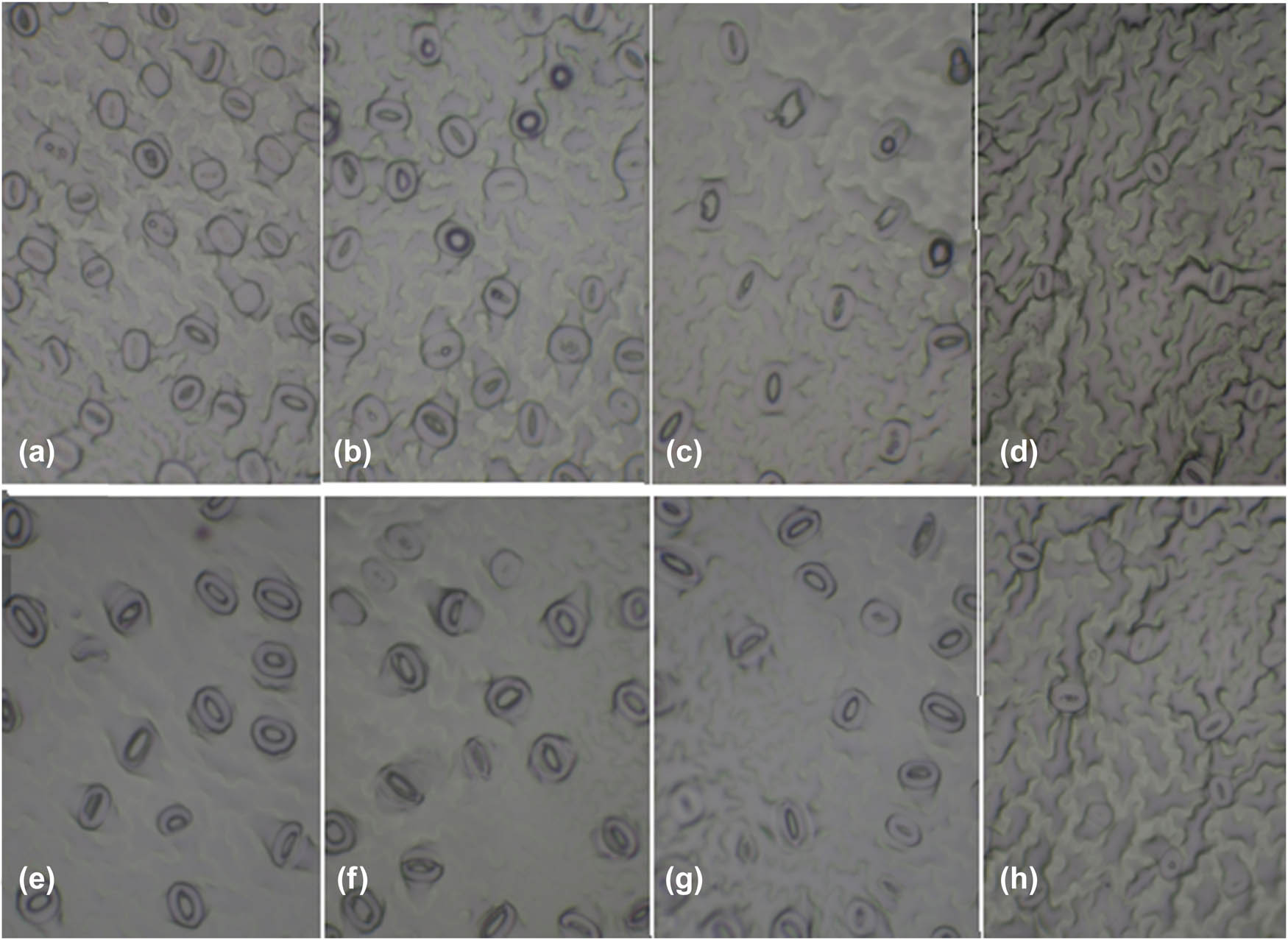
The shape of stomatal and epidermal cells of pistachio rootstocks Qazvini and UCB1, (a) UCB1 no ventilation, (b) UCB1 HV, (c) UCB1 FV, (d) UCB1 greenhouse plant, (e) Qazvini no ventilation, (f) Qazvini HV, (g) Qazvini FV, and (h) Qazvini greenhouse plant.
2.5 Stomatal response to desiccation
Leaf relative water content (RWC) was calculated as a percentage during the desiccation period according to an earlier study [9]. To do so, first, the petioles were removed, and the fresh weight of pistachio leaf samples from greenhouse plants as well as in vitro plants was calculated in an environment with 50% relative humidity (RH) and temperature of 21°C resulted in 1.24 kPa vapor pressure deficit (VPD) and 50 μmol m−2 s−1 irradiance. Next, the leaves were dipped in distilled water for 24 h, and their turgid weight (TW) was measured. The procedure was continued with the gravimetrical weighing of TW every 5 min within 60 min. After desiccation, leaves were dried for 48 h at 70°C to calculate their dry weight (DW). RWC during the desiccation period was also calculated based on the following equation according to an earlier study [9].
2.6 Statistical analysis
The factorial experiment with two factors (ventilation and sucrose treatment) based on a completely randomized design (CRD) was used in this study. The mean comparison for sucrose treatment at all three levels of 10, 15, and 30 mL −1 showed no significant difference from one another. Since the effect of different rates of sucrose (10, 15, and 30 g L −1) was not significant for any of the measured traits, these treatment levels were considered as extra repetitions for each of the ventilation treatments. The sucrose control level (no sucrose application) was set aside from the final analyses. Finally, the obtained data for all measured traits were subjected to one-way analysis of variance based on CRD and multiple mean comparisons using Duncan’s test (honestly significant difference [HSD]; P ≤ 0.05). The data analysis was performed in SAS 9.4 by means of Proc GLM, a general linear model. Linear regression [14] between F v/F m as an independent variable and root length as the response variable in addition to the linear relationship between leaf desiccation (independent) and measured RWC (dependent) were carried out by proc reg in SAS and depicted in Excel 2019 software.
3 Results
3.1 Vegetative growth features
Ventilation and sucrose concentration variably affected the in vitro plant vegetative growth parameters in both Qazvini and UCB1 rootstocks. Based on the results, vegetative growth parameters were increased by ventilation (FV treatment). Pistachio plants grown in a culture media containing different concentrations of sugar (0, 10, 15, and 30 g L −1) did not show significant differences in proliferation (in both species), shoot height (in Qazvini), and leaf number (in UCB1; Table 1). Moreover, the interaction effects of ventilation with sucrose concentration were significant for both species in all growth parameters. The maximum rootstock proliferation was seen in FV treatment and midrange concentration of sugar (10 and 15 g L −1), whereas the minimum of this parameter was observed in NV treatment and sucrose-free treatment. The maximum mean values of shoot height and root length were obtained in FV treatment with either low concentration or no application of sucrose. Also, leaf number was higher in ventilated vessels with sucrose (15 g L −1) for Qazvini rootstock, which was significantly different from all other treatments. For UCB1 rootstock, however, the maximum leaf number was obtained in full ventilated sugar-free media (Table 1).
Multiple mean comparison for some growth-related features in pistachio cv. Qazvini and UCB1 under in vitro cultivation
| Ventilation | Sucrose (g L −1) | Proliferation | Shoot height (mm) | Root length (mm) | Leaf number | ||||
|---|---|---|---|---|---|---|---|---|---|
| Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | ||
| FV | 0 | 10.75ab ± 0.54 | 13.15ab ± 0.66 | 39.12a ± 1.96 | 33.14a ± 1.66 | 4.57a ± 0.23 | 3.76a ± 0.19 | 17.2abc ± 0.86 | 18.6ab ± 0.93 |
| 10 | 11.14ab ± 0.56 | 13.55a ± 0.68 | 38abc ± 1.9 | 33.09a ± 1.65 | 4.5a ± 0.23 | 3.77a ± 0.19 | 17.4ab ± 0.87 | 18.89a ± 0.94 | |
| 15 | 11.88a ± 0.59 | 12.54ab ± 0.63 | 38.61ab ± 1.93 | 33.02a ± 1.65 | 4.18b ± 0.21 | 3.7ab ± 0.19 | 17.68a ± 0.88 | 18.88a ± 0.94 | |
| 30 | 10.75ab ± 0.54 | 12.57ab ± 0.63 | 37.91bc ± 1.9 | 32.93a ± 1.65 | 4.07bc ± 0.2 | 3.44cd ± 0.17 | 17.1abc ± 0.86 | 17.9abc ± 0.9 | |
| HV | 0 | 9.9bc ± 0.5 | 12.2abc ± 0.61 | 36.69d ± 1.83 | 33.03a ± 1.65 | 4.45a ± 0.22 | 3.27d,e ± 0.16 | 16.1d ± 0.81 | 16.9cd ± 0.85 |
| 10 | 10.36b ± 0.52 | 12.54ab ± 0.63 | 37.43bc ± 1.87 | 32.92a ± 1.65 | 4bcd ± 0.2 | 3.61abc ± 0.18 | 16.6abc ± 0.83 | 16.9cd ± 0.85 | |
| 15 | 11.14ab ± 0.56 | 12.2abc ± 0.61 | 36.79d ± 1.84 | 32.90a ± 1.65 | 3.8cde ± 0.19 | 3.5bcd ± 0.18 | 17.4ab ± 0.87 | 17.43c ± 0.87 | |
| 30 | 9.9bc ± 0.5 | 11.4bcd ± 0.57 | 36.49d ± 1.82 | 31.83b ± 1.59 | 3.8def ± 0.19 | 2.98fg ± 0.15 | 16.4bc ± 0.82 | 17.6bc ± 0.88 | |
| No ventilation | 0 | 7.53d ± 0.38 | 9.9d ± 0.5 | 33.16e ± 1.66 | 30.44c ± 1.52 | 3.8cde ± 0.19 | 3.27de ± 0.16 | 13.5d ± 0.68 | 14.47e ± 0.72 |
| 10 | 7.53d ± 0.38 | 11.4bcd ± 0.57 | 33.42e ± 1.67 | 30.52c ± 1.53 | 3.71de ± 0.19 | 2.99fg ± 0.15 | 14.7d ± 0.74 | 15.03e ± 0.75 | |
| 15 | 8.59cd ± 0.43 | 10.75cd ± 0.54 | 33.33e ± 1.67 | 30.49c ± 1.52 | 3.69ef ± 0.18 | 3.2ef ± 0.16 | 16.1d ± 0.81 | 16.14d ± 0.81 | |
| 30 | 8.59cd ± 0.43 | 10.75cd ± 0.54 | 33.63e ± 1.68 | 29.75d ± 1.49 | 3.67f ± 0.18 | 2.7g ± 0.14 | 16.3bc ± 0.82 | 16.14d ± 0.81 | |
| Ventilation | ** | ** | ** | ** | ** | ** | ** | ** | |
| Sucrose | n.s. | n.s. | n.s. | ** | ** | ** | ** | n.s. | |
| Interaction | ** | ** | ** | ** | ** | ** | ** | ** | |
Means with the same superscript letters are not significantly different (Duncan 5%).
3.2 The effects of ventilation and sucrose concentration on fluorescence emission
Dark-adapted leaves of control and ventilated in vitro plants exposed to different sucrose concentrations were used to measure the induction of Chl-a fluorescence. Aside from NPQ, which showed no significant difference regarding the application of either sucrose or ventilation factors, all other measured parameters in relation to Chl fluorescence showed a significant difference in response to both factors and their interaction effects (Table 2). The results showed that F 0, F m, and F m′ responded positively to ventilation and sucrose application. However, their highest mean values were recorded mostly under HV treatment and middle concentration of sucrose (10 and 15 g L −1) in both cv. Qazvini and UCB1. In addition, Qazvini rootstock (Figure 1a and Table 2) showed maximum F v/F m in FV treatment with 15 g L −1 of sucrose. The minimum F v/F m was detected in NV treatment with 30 g L −1 of sucrose. This ratio was increased by 36% through FV treatment and decreasing sucrose concentration to 10 g L −1. F v/F m value was also increased by ventilation and decrease in sucrose concentration; however, the effect of ventilation was more significant than sucrose concentration. In Table 2, it can be seen that there is no significant difference between FV-treated plants exposed to 15 and 30 g L −1 sucrose. For UCB1 rootstock, the results were slightly different so that FV and HV treatments with sucrose-free medium showed the highest F v/F m values. There was also no significant difference between HV treatment in sucrose-free medium and FV treatment exposed to 10, 15, and 30 g L −1 sucrose. Therefore, it can be suggested that sucrose concentration, notably sucrose elimination in the medium, had the most significant effect on F v/F m value. For UCB1 rootstock, F v/F m was increased by 40% through FV treatment with sucrose-free medium compared to NV plants, which had been exposed to 30 g L −1 of sucrose (Table 2). Since the effect of neither ventilation and sucrose was significant on NPQ, multiple mean comparisons for this feature are not presented.
Multiple mean comparison for fluorescents-related parameters in pistachio cv. Qazvini and UCB1 under in vitro cultivation
| Air ventilation | Sucrose (g L −1) | F 0 | F m | F m' | F v | F v/F m | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | ||
| FV | 0 | 359.4ef ± 17.95 | 371.1ef ± 18.55 | 1536.06d ± 76.8 | 1552.5b ± 77.6 | 2087.4b ± 104.35 | 2108.05a,b ± 105.4 | 1176.6bc ± 58.8 | 1181.3ab ± 59.05 | 0.77a ± 0.04 | 0.76a ± 0.04 |
| 10 | 468.1de ± 23.4 | 390def ± 19.5 | 1636cd ± 81.8 | 1287.2bc ± 64.35 | 2051.1b ± 102.55 | 1593.9bcd ± 79.65 | 1168.1bc ± 58.4 | 896.7cd ± 44.8 | 0.71bc ± 0.04 | 0.69b ± 0.03 | |
| 15 | 511cd ± 25.55 | 329.34f ± 16.45 | 1714bcd ± 85.7 | 1343.9bc ± 67.15 | 2227.92b ± 111.35 | 1775.13bc ± 88.75 | 1203.1bc ± 60.15 | 1014.5bc ± 50.7 | 0.7bcd ± 0.04 | 0.75a ± 0.04 | |
| 30 | 308.56f ± 15.4 | 597.93a ± 29.85 | 1004.8e ± 50.2 | 1857.2a ± 92.85 | 1358.1c ± 67.9 | 2515.09a ± 125.75 | 696.28d ± 34.8 | 1259.28a ± 62.95 | 0.69bcd ± 0.03 | 0.68bc ± 0.03 | |
| HV | 0 | 446.4de ± 22.3 | 432.8cde ± 21.6 | 1689bcd ± 84.45 | 1366.4bc ± 68.3 | 2122.4b ± 106.1 | 1723.96bc ± 86.15 | 1243ab ± 62.15 | 933.59cd ± 46.65 | 0.73ab ± 0.04 | 0.68bc ± 0.03 |
| 10 | 765.85a ± 38.25 | 395.5def ± 19.75 | 2199.87a ± 109.95 | 1236.18c ± 61.8 | 2766.2a ± 13.8 | 1604.1bcd ± 80.2 | 1434.02a ± 71.7 | 840.62cd ± 42 | 0.65de ± 0.03 | 0.68bc ± 0.03 | |
| 15 | 371.8ef ± 18.55 | 502.03bc ± 25.1 | 1114.7e ± 55.7 | 1378.3bc ± 68.9 | 1501.8c ± 75.05 | 1857.63bc ± 92.85 | 742.91d ± 37.1 | 876.3cd ± 43.8 | 0.67cde ± 0.03 | 0.63cd ± 0.03 | |
| 30 | 805.53a ± 40.25 | 560.1ab ± 28 | 1879bc ± 93.95 | 1361.6bc ± 68.05 | 2346.9ab ± 117.3 | 1707.54bc ± 85.35 | 1073.4bc ± 53.65 | 801.54cd ± 40.05 | 0.57f ± 0.03 | 0.59d ± 0.03 | |
| No ventilation | 0 | 752.65a ± 37.6 | 363.6ef ± 18.15 | 1966.2ab ± 98.3 | 1179.22c ± 58.95 | 2496.7ab ± 124.8 | 1595.6bcd ± 79.75 | 1213.6bc ± 60.65 | 815.6cd ± 40.75 | 0.62ef ± 0.03 | 0.68bc ± 0.03 |
| 10 | 687.3ab ± 34.35 | 472bcd ± 23.6 | 1699bcd ± 84.95 | 1296.3bc ± 64.8 | 2292.2ab ± 114.6 | 1755.32bc ± 87.75 | 1012.47c ± 50.6 | 823.4cd ± 41.15 | 0.6f ± 0.03 | 0.63cd ± 0.03 | |
| 15 | 497.52d ± 24.85 | 468bcd ± 23.4 | 991.18e ± 49.55 | 1190.05c ± 59.5 | 1236.49c ± 61.8 | 1491.91cd ± 74.55 | 493.66e ± 24.65 | 721.4d ± 36.05 | 0.5g ± 0.03 | 0.61d ± 0.03 | |
| 30 | 625.4bc ± 31.25 | 417c–f ± 20.85 | 1224.41e ± 61.2 | 813.21d ± 40.65 | 1558.65c ± 77.9 | 1095.55d ± 54.75 | 598.99de ± 29.9 | 395.73e ± 19.75 | 0.48g ± 0.02 | 0.49e ± 0.02 | |
| Ventilation | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Sucrose | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
| Interaction | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | |
Means with the same letters are not significantly different (Duncan 5%).
The scatter plots of all fluorescence feature vs root length, as response variables, were prepared, and the results showed that only F v/F m has a significant relationship with root length (Figure 3; r 2 >0.74). The root length of both cv. Qazvini and UCB1 responded positively to the increase in the amount of F v/F m with a linear model (Figure 3b). Although cv. UCB1 showed a higher slope than cv. Qazvini, the difference between the slopes of these two cultivars was not significant according to t-student test (P > 0.05).

Scatter plot of root vs F v (a) and F v/F m (b) in pistachio cv. Qazvini and UCB1 under in vitro cultivation.
3.3 Stomatal cell density
Morphologically, stomatal traits were completely different between the leaves of in vitro pistachio plants in different ventilation treatments as well as those plants in greenhouse conditions. Totally, the stomatal cell density in tissue-cultured plants was higher than that in GP, but in Qazvini rootstocks, there was a significant difference between plants treated in a greenhouse and tissue-cultured FV conditions (Table 3). Moreover, our results indicated a significant difference between different ventilation treatments so that the stomatal density of Qazvini rootstock decreased by half in FV treatment compared with NV ones. Also, the stomatal density of full ventilated UCB1 rootstocks was 54% less than NV ones. For HV treatment, stomatal density was intermediate for both UCB1 and Qazvin. Furthermore, the stomatal density of Qazvini rootstock grown in greenhouse conditions was 60% less than NV treatment. However, for UCB1, this percentage was equal to 66%. Moreover, the stomatal density of UCB1 rootstocks was more than that of Qazvini rootstocks, both in a greenhouse and in vitro conditions (Table 3).
Stomatal and epidermal cell properties in leaves of two in vitro pistachio rootstocks Qazvini and UCB1
| Ventilation level | Stomatal density (no mm−2) | Stomatal index | Epidermal cell density (no mm−2) | Stomatal Length (µm) | Stomatal width (µm) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | Qazvini | UCB1 | |
| FV | 115bc ± 5.75 | 145c ± 7.25 | 8.25bc ± 0.41 | 8.25c ± 0.41 | 1244.8bc ± 62.2 | 2516.9ab ± 125.8 | 13.88a ± 0.69 | 12.02b ± 0.6 | 10.89a ± 0.54 | 9.93ab ± 0.5 |
| HV | 162.5b ± 8.1 | 277.5b ± 13.85 | 9.47ab ± 0.47 | 8.77b ± 0.44 | 1529.3b ± 76.45 | 2697.1ab ± 134.85 | 12.76a ± 0.64 | 11.72b ± 0.59 | 10.72a ± 0.54 | 9.12bc ± 0.46 |
| No ventilation | 230a ± 11.5 | 320a ± 16 | 10.75a ± 0.54 | 10.62a ± 0.53 | 1885.8a ± 94.25 | 2881.8a ± 144.05 | 13.84a ± 0.69 | 14.16a ± 0.71 | 11.13a ± 0.56 | 10.61a ± 0.53 |
| Greenhouse plant | 90.75c ± 4.54 | 107.5d ± 5.35 | 7.2c ± 0.36 | 4.55c ± 0.23 | 1172.3c ± 58.6 | 2247.2b ± 112.35 | 14.27a ± 0.71 | 11.53b ± 0.58 | 10.44a ± 0.52 | 8.36c ± 0.42 |
| Significance level | ** | ** | ** | ** | ** | * | n.s. | * | n.s. | ** |
Means with the same letters are not significantly different (Duncan 5%).
3.4 Stomatal index
The present study also showed that ventilation treatments had significant effects on the stomatal index in pistachio in vitro plants. For Qazvini rootstocks, the stomatal index in NV treatment was almost 23% higher than FV treatment, whereas there was no significant difference between FV and HV treatments (Table 3). In UCB1, however, the stomatal index in NV treatment was almost twofold higher than FV treatment. Similar to the stomatal density, the stomatal index was significantly more in tissue-cultured plants than those grown in greenhouse conditions; however, there was no significant difference between greenhouse and tissue-cultured FV plants in both Qazvini and UCB1 species. Totally, stomatal index in Qazvini was higher than UCB1 (Table 3).
3.5 Epidermal cell density
The epidermal cell density of in vitro pistachio leaves was considerably higher than GP, but there was no significant difference between greenhouse and tissue-cultured FV treatments in both Qazvini and UCB1 plants (Table 3). Additionally, the epidermal cell density of Qazvini plants under NV treatment was 33% more than FV treatment and 37% more than GP. These values were 12 and 22% in UCB1. Moreover, the epidermal cell density of UCB1 rootstock was more than Qazvini, both in the greenhouse and in vitro conditions (Table 3).
3.6 Stomatal width and length
Based on the results, there were no significant differences in stomatal width and length of different ventilation treatments as well as greenhouse Qazvini plants (Table 3). In the case of UCB1, stomatal width and length in a tissue-cultured NV treatment were higher than other treatments, so that stomatal length in NV treatment was 15% higher than FV treatment and 18% higher than GP (Table 3). Similarly, stomatal width was 6% higher in NV treatment than FV treatment and 21% higher than greenhouse ones. Besides, the stomatal length-to-width ratio in GP was the same as that of in vitro plants treated with different ventilation conditions.
3.7 Stomatal responses to desiccation
By desiccation, RWC was decreased in all in vitro plants with different ventilation levels for both species. Regarding in vitro pistachio plants, RWC was sharply decreased as a result of leaf desiccation, especially in NV plantlets in the first 60 min, whereas this decrease was less strong for GP (Figure 4). For Qazvini plants, the slope of the RWC curve in NV treatment was equal to 22.58, which was 28, 31, and 49% steeper than HV, FV, and GP, respectively. As shown in Figure 4a, the slope of the RWC curve in FV treatment is closer to GP. For UCB1, on the other side, the slope of the RWC curve in NV treatment was equal to 29.52, which was 0.11, 7.2, and 69% steeper than the slope of the RWC curve HV treatment, FV treatment, and GP (Figure 4b). Although the slope of RWC curve for GP was less steep than that of in vitro plants, the slope of RWC curve in FV treatment was closer to the GP.
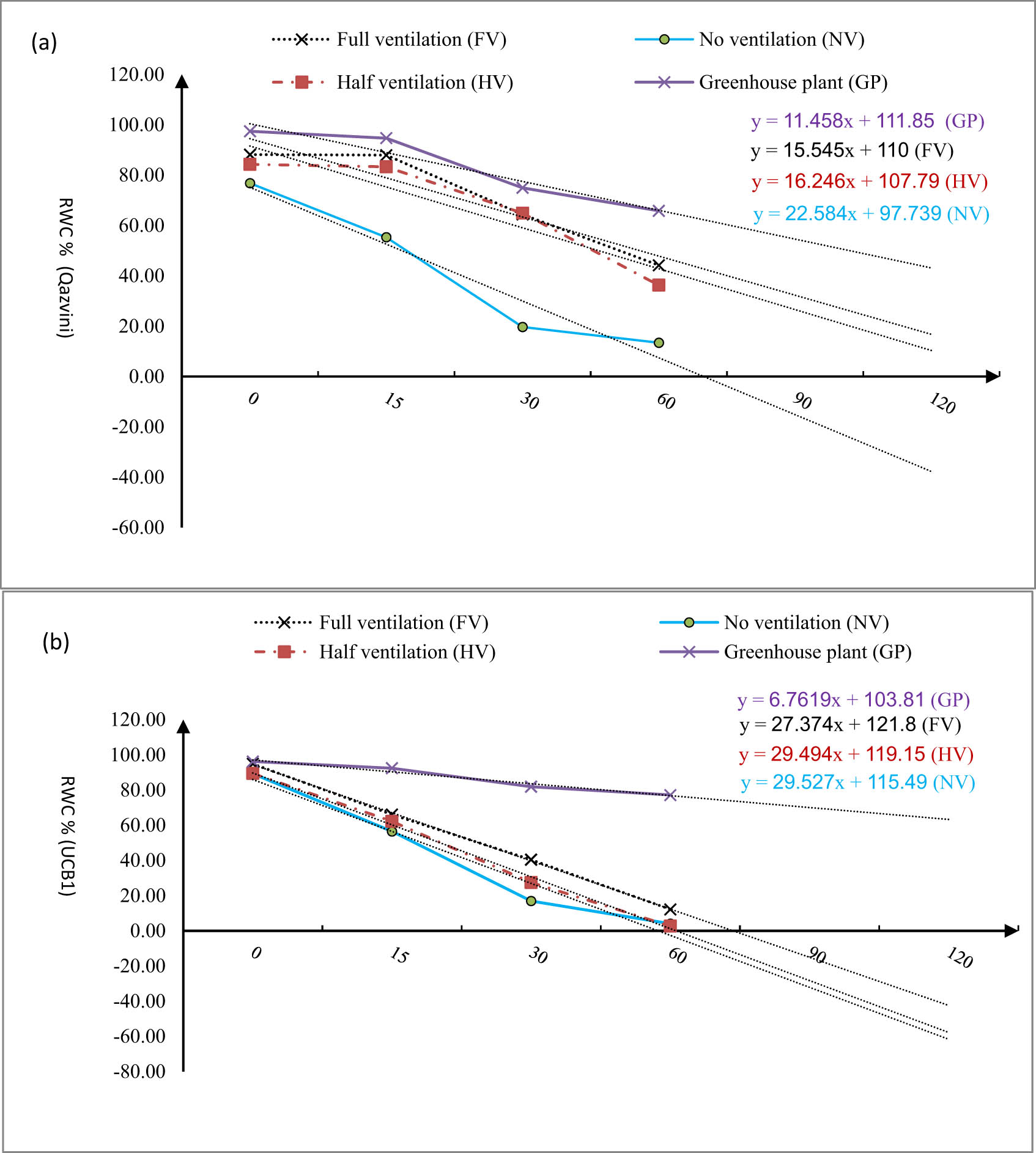
RWC% changing, as result of leaf desiccation in the Qazvini pistachio rootstock, affected by different ventilation levels in the in vitro conditions vs greenhouse plant in the first 60 min for cv. Qzvinig (a) and UCB1 (b), horizontal axis consists of different experimental times, vertical: RWC% changing.
4 Discussion
Although the effect of ventilation and controlling the amount of photosynthetic and respirational gasses and RH of the cultured environments for the plants under in vitro conditions are among significant and effective factors, the effect of ventilation on the quality and quantity production of pistachio plantlets has never been assessed, to the best of our knowledge. The results of this study clearly showed that using ventilation using microfilters that can absorb microsubstances is a proper method to increase the growth of pistachio plantlets, especially the root growth, under in vitro culture. The shoot and root length of the pistachio plantlets have been achieved under the FV treatment and application of low to no sucrose in the media.
Several researches have provided evidence that micropropagated shoots grown in jars with high RH have shown many abnormalities, such as stomata malfunctioning with wide pore aperture [12,13]. It has also been demonstrated that exposing the vessels to high RH conditions can induce stomatal defects [14]. Based on our results, the highest stomatal density, stomatal index, and epidermal cell density were obtained in tissue-cultured unventilated plants. Furthermore, the current study results revealed that with ventilation stomata cell parameters showed more similarity to GP, and there was no significant difference between FV plants and greenhouse ones. The same results have also been reported [15]. In the present study, the stomatal parameters were less affected by in vitro conditions in FV vessels compared to GP. Additionally, it has been shown that in vitro plants’ stomatal function and their responses to closing have decreased [16]. Under the ventilation condition, the fresh air can come into the vessels and provide required sources, humidity, oxygen, and carbon dioxide to the pistachio plants. By increasing the availability of such sources, the plants can produce more vigorous roots to provide nutrients for themselves and improve their growth. In addition, such conditions would require higher photosynthesis. Therefore, the plants are inclined to produce more Chl and increase their photosynthesis rate and yield. However, different hormones affect the ability of plants to respond to the conditions and change their growth quantity. In close vessel condition, control condition with no ventilation, low evapo-transpiration rate would result in less plant ability to produce abscisic acid (ABA) phytohormone, leading to less growth and photosynthetic ability of control plants in comparison with ventilated plants. Moreover, because of high RH in closed in vitro vessels (more than 95%), the in vitro plants have never been exposed to evapo-transpiration condition to induce synthesis of ABA [17]. The ABA impact on pistachio plants is probably related to the role of ABA in retention and water content handling by the plants. Previous studies have shown that ABA can effectively alter the water condition and the plants’ growth quantity and quality; for example, a positive relationship has also been observed between leaf ABA level and the ability to conserve its water content during desiccation [4]. In agreement with our findings, in an earlier study [7] study, RWC was sharply decreased due to leaf desiccation in pistachio plants, whereas this decrease was smaller in GP. We also observed that the slope of the RWC curve was less steep for FV treated and GP compared with NV treatment. For UCB1, the slope of the RWC curve for GP was less steep than the slope of in vitro plants. However, the slope of RWC curve in FV treatment was closer to GP. Masle et al. [7] also noticed that low stomatal density has a critical determinant for high water-use efficiency. Consistent with our results, another study [8] stated that stomatal density and their closing mechanism in response to environmental abnormalities could tightly control plant water loss, especially in tissue-cultured plants. This can be related to more ABA biosynthesis in FV plants than NV ones and the ability of FV plants to conserve their water content during desiccation. Moreover, exposing pistachio plantlets to ventilation can induce accumulation of more ABA in the leaves and thereby causing a better stomatal closure [18]. However, reduction of photosynthetic capacity for plants can be caused by stomatal defects induced by high RH, or disorder in photosystem II. The first scenario (stomatal defects) has already been discussed in detail. To confirm the second scenario (disorder in photosystem II), we found that maximum amount of (F v/F m) was achieved in FV and in sugar-free medium and (15 g L −1) sucrose treatments for UCB1 and Qazvini pistachio rootstocks, respectively. It also seemed that ventilation could effectively decrease the high ethylene amount in the vessels and decrease damages to photosystem II functioning. In agreement with the previously mentioned results related to higher circulation of air and CO2 and therefore the higher ability of the plant to trap the photon from the light source, under ventilation treatments, the photosynthesis yield was significantly increased. In addition, these results regarding the influence of ventilation on pistachio’s growth parameters, especially the root organogenesis and growth, were confirmed by the significant relationship, the linear regression model [19], between root length of both cultivars and quantum photosynthesis yield of the leaves. This result is once more verifying the positive influence of ventilation on the higher quality and quantity pistachio production under in vitro conditions, and it almost definitely is recommendable for being applied in such systems of pistachio productions. Furthermore, our results about the maximum quantum yield of PSII (F v/F m) are in agreement with [20] as well as [16], who revealed that ventilation treatments could increase photosynthesis. Afreen et al. [21] also supported increasing photosynthetic ability by ventilation. Conversely, decreasing photosynthetic performance at high sucrose levels is consistent with the hypothesis stating that excess sucrose could motive the downregulation of photosynthesis [22]. Moreover, based on our findings, F v/F m was reduced under closed vessels (NV condition) exposed to high sucrose concentration. Hdider and Desjardins [23] reported a higher photosynthetic ability of strawberries with transferring them from a medium containing high sucrose level to a sugar-free media. This was also the case in the present study. Similar results have also been published [24], who verified that Chl synthesis and photosynthetic ability of tobacco tissue-cultured in media with 2% sucrose were more than plants in media containing 8% sucrose. Furthermore, according to obtained results from the current study, sucrose concentration had only a small trace on the photosynthetic performance at each ventilation level, whereas ventilation was noticed to be the main effective factor. Similar results have been stated in a previous study [10] regarding walnut. Our findings are in contrast with that of an earlier study [25], who reported no significant difference between Rehmannia glutinosa plantlets grown in media with different sucrose concentrations in terms of their Chl content and photosynthesis performance. In line with our results, results of an earlier study [21] also indicated that somatic embryos of Coffea arabusta had more Chl content as well as more ability to photosynthesize under photoautotrophic conditions.
5 Conclusion
The results of this study showed that changing the concentration of sucrose in pistachio production under in vitro conditions or in line with the ventilation system had no significant effect on any of the measured traits. In addition, using ventilation showed to be highly effective for increasing the growth of in vitro cultures plantlets of pistachio. Ventilation was able to increase the pistachio plantlet’s root length, which has been one of the difficulties in pistachio in vitro cultivation. Furthermore, the quality related-features of pistachio plantlets increased with the application of ventilation. The results also showed that the photosynthesis yield of the pistachio planets under in vitro conditions is highly improved by applying the ventilation system, leading to improved pistachio production under in vitro conditions. Moreover, stomata cell parameters reposed positively to the ventilation and the trend in the FV system was almost the same as the trend in greenhouse grown plants. The final results of this study indicated that the application of low concentrations of sucrose, such as 10 mL −1 in our study, along with the application of FV systems, is recommended for producing high-quality and vigorous pistachio plantlets under in vitro cultivation systems.
Acknowledgments
The authors thank the Center for Vali-e-Asr University of Rafsanjan, Kerman, for their supports.
-
Funding information: This work was supported by the Vali-e-Asr University of Rafsanjan, Rafsanjan, Iran.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Mozafari A-A, Ghaderi N, Havas F, Dedejani S. Comparative investigation of structural relationships among morpho-physiological and biochemical properties of strawberry (Fragaria × ananassa Duch.) under drought and salinity stresses: a study based on in vitro culture. Sci Hortic. 2019;256:108601.10.1016/j.scienta.2019.108601Search in Google Scholar
[2] Mozafari AA, Havas F, Ghaderi N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. 2018;132(3):511–23.10.1007/s11240-017-1347-8Search in Google Scholar
[3] Aliniaeifard S, van Meeteren U. Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. J Exp Bot. 2014;65(22):6529–42.10.1093/jxb/eru370Search in Google Scholar PubMed PubMed Central
[4] ZoBayed SMA. In vitro propagation of Lagerstroemia spp. from nodal explants and gaseous composition in the culture headspace. Environ Control Biol. 2000;38(1):1–11.10.2525/ecb1963.38.1Search in Google Scholar
[5] Shackel KA, Novello V, Sutter EG. Stomatal function and cuticular conductance in whole tissue-cultured apple shoots. J Am Soc Hortic Sci. 1990;115(3):468–72.10.21273/JASHS.115.3.468Search in Google Scholar
[6] Barbieri G, Vallone S, Orsini F, Paradiso R, De Pascale S, Negre-Zakharov F, et al. Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J Plant Physiol. 2012;169(17):1737–46.10.1016/j.jplph.2012.07.001Search in Google Scholar PubMed
[7] Masle J, Gilmore SR, Farquhar GD. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature. 2005;436(7052):866–70.10.1038/nature03835Search in Google Scholar PubMed
[8] Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV. Regulation of transpiration to improve crop water use. Crit Rev Plant Sci. 2009;28(6):410–31.10.1080/07352680903173175Search in Google Scholar
[9] Vahdati K, Aliniaeifard S. Investigation of physiological components involved in low water conservation capacity of in vitro walnut plants. Sci Hortic. 2017;224:1–7.10.1016/j.scienta.2017.04.023Search in Google Scholar
[10] Hassankhah A, Vahdati K, Lotfi M, Mirmasoumi M, Preece J, Assareh MH. Effects of ventilation and sucrose concentrations on the growth and plantlet anatomy of micropropagated Persian walnut plants. Int J Hortic Sci Technol. 2014;1(2):111–20.Search in Google Scholar
[11] Nejad AR, Van Meeteren U. Stomatal response characteristics of Tradescantia virginiana grown at high relative air humidity. Physiol Plant. 2005;125(3):324.10.1111/j.1399-3054.2005.00567.xSearch in Google Scholar
[12] Ivanova M, Van Staden J. Natural ventilation effectively reduces hyperhydricity in shoot cultures of Aloe polyphylla Schönland ex Pillans. Plant Growth Regul. 2010;60(2):143–50.10.1007/s10725-009-9430-8Search in Google Scholar
[13] Fanourakis D, Heuvelink E, Carvalho SMP. A comprehensive analysis of the physiological and anatomical components involved in higher water loss rates after leaf development at high humidity. J Plant Physiol. 2013;170(10):890–8.10.1016/j.jplph.2013.01.013Search in Google Scholar
[14] Aliniaeifard S, Malcolm Matamoros P, van Meeteren U. Stomatal malfunctioning under low VPD conditions: induced by alterations in stomatal morphology and leaf anatomy or in the ABA signaling? Physiol Plant. 2014;152(4):688–99.10.1111/ppl.12216Search in Google Scholar
[15] Joshi P, Joshi N, Purohit SD. Stomatal characteristics during micropropagation of Wrightia tomentosa. Biol Plant. 2006;50(2):275–8.10.1007/s10535-006-0019-zSearch in Google Scholar
[16] Aliniaeifard S, Van Meeteren U. Stomatal characteristics and desiccation response of leaves of cut chrysanthemum (Chrysanthemum morifolium) flowers grown at high air humidity. Sci Hortic. 2016;205:84–9.10.1016/j.scienta.2016.04.025Search in Google Scholar
[17] Santamaria JM, Davies WJ, Atkinson CJ. Stomata of micropropagated Delphinium plants respond to ABA, CO2, light and water potential, but fail to close fully. J Exp Bot. 1993;44(1):99–107.10.1093/jxb/44.1.99Search in Google Scholar
[18] Patakas A, Nikolaou N, Zioziou E, Radoglou K, Noitsakis B. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci. 2002;163(2):361–7.10.1016/S0168-9452(02)00140-1Search in Google Scholar
[19] Saed-Moucheshi A, Fasihfar E, Hasheminasab H, Rahmani A, Ahmadi A. A review on applied multivariate statistical techniques in agriculture and plant science. Int J Agron Plant Prod. 2013;4(1):127–41.Search in Google Scholar
[20] Lichtenthaler HK, Babani F. Detection of photosynthetic activity and water stressby imaging the red chlorophyll fluorescence. Plant Physiol Biochem. 2000;38(11):889–95.10.1016/S0981-9428(00)01199-2Search in Google Scholar
[21] Afreen F, Zobayed SMA, Kozai T. Photoautotrophic culture of Coffea arabusta somatic embryos: photosynthetic ability and growth of different stage embryos. Ann Bot. 2002;90(1):11–9.10.1093/aob/mcf150Search in Google Scholar PubMed PubMed Central
[22] Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Biol. 1996;47(1):509–40.10.1146/annurev.arplant.47.1.509Search in Google Scholar PubMed
[23] Hdider C, Desjardins Y. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase efficiency by the presence of sucrose during the tissue culture of strawberry plantlets. In Vitro Cell Dev Biol Plant. 1995;31(3):165–70.10.1007/BF02632014Search in Google Scholar
[24] Kaul K, Sabharwal PS. Effects of sucrose and kinetin on growth and chlorophyll synthesis in tobacco tissue cultures. Plant Physiol. 1971;47(5):691–5.10.1104/pp.47.5.691Search in Google Scholar PubMed PubMed Central
[25] Cui Y-Y, Hahn E-J, Kozai T, Paek K-Y. Number of air exchanges, sucrose concentration, photosynthetic photon flux, and differences in photoperiod and dark period temperatures affect growth of Rehmannia glutinosa plantlets in vitro. Plant Cell Tissue Organ Cult. 2000;62(3):219–26.10.1023/A:1006412321864Search in Google Scholar
© 2021 Mohammad Javad Mahmoudi Meimand et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

