Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
-
Agnieszka Tomkowiak
, Roksana Skowrońska
, Michał Kwiatek
, Julia Spychała
, Danuta Kurasiak-Popowska
, Janetta Niemann
, Sylwia Mikołajczyk
, Jerzy Nawracała
, Przemysław Łukasz Kowalczewski
and Kinza Khan
Abstract
Leaf rust caused by the fungus Puccinia recondita f. sp. tritici is one of the most dangerous diseases of common wheat. Infections caused by fungal pathogens reduce the quantity and quality of yields of many cereal species. The most effective method to limit plant infection is to use cultivars that show rust resistance. Genetically conditioned horizontal-type resistance (racial-nonspecific) is a desirable trait because it is characterized by more stable expression compared to major (R) genes that induce racially specific resistance, often overcome by pathogens. Horizontal resistance is conditioned by the presence of slow rust genes, which include genes Lr34 and Lr46. This study aimed to identify markers linked to both genes in 64 common wheat lines and to develop multiplex PCR reaction conditions that were applied to identify both genes simultaneously. The degree of infestation of the analyzed lines was also assessed in field conditions during the growing season of 2017 and 2018. Simple sequence repeat anchored-polymerase chain reaction (SSR-PCR) marker csLV was identified during analysis in line PHR 4947. The presence of a specific sequence has also been confirmed in multiplex PCR analyses. In addition to gene Lr34, gene Lr46 was identified in this genotype. Lines PHR 4947 and PHR 4819 were characterized by the highest leaf rust resistance in field conditions. During STS-PCR analyses, the marker wmc44 of gene Lr46 was identified in most of the analyzed lines. This marker was not present in the following genotypes: PHR 4670, PHR 4800, PHR 4859, PHR 4907, PHR 4922, PHR 4949, PHR 4957, PHR 4995, and PHR 4997. The presence of a specific sequence has also been confirmed in multiplex PCR analyses. Genotypes carrying the markers of the analyzed gene showed good resistance to leaf rust in field conditions in both 2017 and 2018. Research has demonstrated that marker assisted selection (MAS) and multiplex PCR techniques are excellent tools for selecting genotypes resistant to leaf rust.
1 Introduction
Common wheat (Triticum aestivum L. ssp. aestivum) is a crop that occupies a leading place in global food production [1]. The presence of many cultivars allows us to grow wheat in most climate zones, which results in its largest proportion among cultivated cereals in the world. Because of the dynamic development of wheat cultivation and production, diseases of seed plantations are increasingly being observed. Fungal diseases pose a particular threat, as they limit the leaf assimilation area and cause a significant yield loss [2,3]. Wheat leaf rust caused by Puccinia recondita f. sp. tritici is one of the most dangerous and common fungal diseases. Currently, the most beneficial method of plant protection is conducting resistance breeding, consisting in the identification and selection of resistant cultivars and transferring resistance genes to other cultivars for their pyramidization.
In hexaploid wheat, leaf rust resistance genes are widespread in the genome. Different genes determine resistance to emerging types of infection [4]. An essential direction in resistance breeding is the search for new and effective sources of resistance to wheat diseases [5]. The number of genes that determine avirulence and their expression vary between Puccinia recondita f. sp. tritici populations [4]. Systematic appearance of new pathotypes is a significant problem in resistance breeding. Fungi of the genus Puccinia are characterized by high genetic variability and show adaptability to climatic conditions. Therefore, it is necessary to constantly search and select new sources of resistance to create new, resistant wheat cultivars [6].
The most numerous group of genes responsible for leaf rust resistance are the Lr (leaf rust) genes. A small group of leaf rust resistance genes is referred to as slow rust genes, and it includes Lr34 [7] and Lr46 [8]. They are characterized by persistent and nonspecific adult plant resistance (APR), while their expression effect is more limited compared to that of racially specific genes. Inheritance of these genes is quantitative, and literature data very often indicate specific quantitative trait locus (QTL) regions associated with them, which are often dispersed throughout the whole wheat genome. Horizontal resistance is a very desirable trait because it provides a longer and more stable response than the major genes, whose racial-specific resistance is often overcome by the pathogen. Literature data shows that for slow rust resistance there are three gene complexes of major importance: Lr34/Yr18/Pm38, Lr46/Yr29/Pm38, and Sr2/Yr30 [9,10].
One of the well-characterized racially nonspecific resistance genes is Lr34 [11], found on wheat chromosome 7DS. This gene determines partial resistance in adult plants to all known of P. recondita f. sp. tritici. Analyzing the effect of this gene, it was found that genotypes carrying Lr34 also had increased resistance to stripe rust [12], stem rust [10,13], and powdery mildew [14]. These genes (also located at the locus of chromosome 7DS) have been designated as Yr18, Sr57, and Pm38, respectively [11]. Gene Lr34, previously referred to as LrT2 [13], is present in older South American (Frontana) cultivars and derivatives of this variety. It is also found in cultivars of Chinese origin [10]. An important feature of this gene is that the resistance conditioned by Lr34 has not been overcome in many regions, which has contributed to the stability of wheat cultivars that carry the above gene complex [11].
The leaf rust resistance gene Lr46 is also a slow rust-type gene. Like Lr34, it does not provide the host plant with complete resistance to all races of Puccinia recondita f. sp. tritici, while the effect of its expression can also delay the infection process in adult plants or slow down the development of disease caused by the pathogen (https://maswheat.ucdavis.edu). As with Lr34, the Lr46/Yr29/Sr58/Pm39 gene complex is located at a locus on chromosome 1BL. It has been characterized in wheat derived from CIMMYT as well as in lines originating from South American wheat cultivars [11]. Research on the effect of the Lr46/Yr29/Sr58/Pm39 gene complex on multifactorial resistance to leaf rust, stripe rust, stem rust, and powdery mildew was described by William et al. [15]. The presence of both genes significantly extends the latency period of the disease and reduces its severity relative to the susceptible media.
2 Materials and methods
2.1 Plant materials
The plant materials used in the study were 64 lines of common wheat (Triticum aestivum L. ssp. aestivum) of various origins from different countries of Europe. The plant material comes from the common wheat collection at the experimental station of Poznańska Hodowla Roślin sp. z o.o. in Poland. According to the literature sources, some of these materials have good leaf rust resistance and may contain Lr34 and Lr46 resistance genes. Frontana was the control variety used in the experiment that carried both analyzed genes. The control variety Myna was also used in multiplex PCR analyses. Both cultivars were derived from the National Small Grain Collection in Agriculture Research Station Aberdeen, USA.
2.2 Field experiment
Field experiment with the tested wheat genotypes was conducted on experimental plots belonging to Poznańska Hodowla Roślin Sp. z o.o. (N: 51°30′44″, E: 17°50′51″) in the years 2017–2018. In the spring, along with the beginning of the vegetation period, close observations were carried out on wheat plots to find clusters of leaf rust urediniospores. Depending on the size of the leaves, 100–150 wheat leaves were harvested randomly per plot. Field evaluation of wheat was carried out in accordance with the recommendations of the European and Mediterranean Plant Protection Organization using a 9° scale. Observations were carried out on the basis of the total number of leaves in the sample, number of infected leaves (i.e., number of leaves with urediniospore clusters), percentage of infected leaves, total number of urediniospore clusters, and average number of urediniospore per wheat leaf. Wheat leaf analyses for the presence of urediniospore clusters were repeated regularly every few days until the end of the vegetation period.
Favorable conditions for plant growth and development were recorded during the 2017 growing season in the area where the field experiment was established: N: 51°30′44″, E: 17°50′51″. In March, the average temperature was 4°C. In April, despite spring frosts, the temperature exceeding 7°C also did not damage the crops. The second half of May brought a higher temperature, as a result of which vegetation accelerated significantly and low rainfall delayed the development of fungal diseases, which began to develop in the second half of June. The growing season of 2018 in this area was extremely warm compared to the entire five decades of 1966–2015. The average temperature of the growing season (from April to September) was higher than the norm (1966–2015) by about +3°C. A small amount of rainfall in March and April significantly weakened the plants, and fungal diseases appeared on them in May.
2.3 DNA isolation
DNA isolation of all tested common wheat samples was performed using the Plant & Fungi DNA Purification Kit (EURx), according to the recommendations attached to the protocol. Concentration and purity of the resulting preparation were measured using a spectrophotometer (DeNovix). The samples were subsequently diluted with the supplied elution buffer to obtain a uniform concentration of 70 ng/µL.
2.4 Genes identification
PCR-SSR amplification was carried out using a B3 Tet thermocycler (Biometra) under appropriate conditions for the analyzed genes Lr34 and Lr46. The PCR reaction was performed in a 20 µL volume of the mixture per sample, where 1 µL of the mixture constituted previously isolated DNA. Mixtures differed in the volume of individual components depending on the gene tested [16].
Identification of individual genes was performed using primers with sequences derived from the MASWheat database (https://maswheat.ucdavis.edu), as listed in Table 1. The 20 µL reaction mixture for gene Lr34 contained the following: 5× Green GoTaq® Flexi Buffer – 4 µL, MgCl2 (2 mM) – 1.6 µL, dNTP Mix (0.2 mM each) – 0.45 µL, GoTaq® DNA Polymerase (1.25 µ) – 0.2 µL, water – 11.75 µL, primers (0.25 µM) – 2 × 0.5 µL, and DNA (50 ng/µ) – 1 µL. The 20 µL reaction mixture for gene Lr46 consisted of the following: 5× Green GoTaq® Flexi Buffer – 4 µL, MgCl2 (2 mM) – 1.8 µL dNTP Mix (0.2 mM each) – 0.5 µL, GoTaq® DNA Polymerase (1.25 µ) – 0.25 µL, water – 11.05 µL, primers (0.25 µM) – 2 × 0.7 µL, and DNA template (50 ng/µ) – 1 µL. The PCR reaction profile for both genes differed in primer annealing temperature, determined on the basis of their melting temperature, and was as follows: initial denaturation – 5 min at 94°C, then 45 cycles (denaturation – 45 s at 94°C, annealing – 30 s at 55°C (Lr34) or 59°C (Lr46), synthesis – 1 min at 72°C), final synthesis – 7 min at 72°C, and storage at 4°C for a maximum of 24 h [16].
Primer sequences and their annealing temperatures for the identification of Lr34 and Lr46 genes
| No. | Gene – primer | Primer sequence | Annealing temperature |
|---|---|---|---|
| 1 | Lr34 – csLV34F | 5′-GTTGGTTAAGACTGGTGATGG-3′ | |
| 2 | Lr34 – csLV34R | 5′-TGCTTGCTATTGCTGAATAGT-3′ | 55°C |
| 3 | Lr46 – wmc44F | 5′-GGTCTTCTGGGCTTTGATCCT-3′ | |
| 4 | Lr46 – wmc44R | 5′-GTTGCTAGGGACCCGTAGTGG-3′ | 59°C |
The resulting PCR products were subjected to electrophoretic separation on a 2.5% agarose gel, using 2 µL of Midori Green Stain dye. O’RangeRuler™ 100 bp DNA Ladder was used as the molecular mass size standard. Electrophoretic separation was carried out in TBE 1× buffer for 2.5 h at 70 V. Then, to visualize PCR products, a Gel Doc™ XR UV Molecular Imager transilluminator was used together with ImageLab™ Software (Bio-Rad, USA).
In the next stage, multiplex PCR conditions were developed for Lr34 and Lr46 gene markers. The 20 µL PCR multiplex reaction mixture consisted of the following: 5× Green GoTaq® Flexi Buffer – 4 µL, MgCl2 – 1.8 µL, dNTP Mix – 0.5 µL, GoTaq® DNA Polymerase – 0.3 µL, water – 10.4 µL, primers csLV34F i csLV34R – 2 × 0.6 µL, primers WMC44-F i WMC44-R – 2 × 0.4 µL, and DNA template – 1 µL. Amplification of the multiplex PCR reaction products was carried out in the following temperature profile: initial denaturation – 5 min at 94°C, then 45 cycles (denaturation – 45 s at 94°C, primer annealing – 30 s at 57°C, and the synthesis – 1 min at 72°C), final synthesis – 7 min at 72°C, and storage at 4°C for a maximum of 24 h.
3 Results
The Lr34 allele yields a 150 bp product, and a 229 bp band is amplified in non-Lr34 germplasm. For gene Lr46, a 242 bp product was identified in lines carrying this gene.
3.1 Identification of the gene Lr34 marker csLV34
Of the 64 lines tested, the Lr34 marker was only present in genotype PHR 4947 (Figure A3) where a 150 bp product appeared, and in the control variety Frontana. For other lines, a 229 bp product was identified as evidence of gene absence (Figures A1–A4).
3.2 Identification of the gene Lr46 marker wmc44
Of the 64 lines tested, the gene Lr46 marker was present in most of the genotypes, as evidenced by the presence of a 242 bp product (Figures A5–A8). The marker wmc44 was not observed in the following lines: PHR 4670, PHR 4800, PHR 4859, PHR 4907, PHR 4922, PHR 4949, PHR 4957, PHR 4995, and PHR 4997. Nonspecific products were also observed in most lines.
3.3 Simultaneous identification of the gene Lr46 marker wmc44 and gene Lr34 marker csLV34 (multiplex PCR)
Comparing the results of multiplex PCR analyses with single PCR, the result was reproducible for gene Lr34, i.e., the csLV marker of gene Lr34 was only present in line PHR 4947 (Figure A3) where a 150 bp product appeared, and in the control variety Frontana. For gene Lr46, single gene and multiplex PCR analysis results were also reproducible. A 242 bp product characteristic of gene Lr46 was not observed in the genotypes: PHR 4670, PHR 4800, PHR 4859, PHR 4907, PHR 4922, PHR 4949, PHR 4957, PHR 4995, and PHR 4997 (Figures A9–A12).
3.4 Observations of infection degree of the analyzed wheat genotypes in 2017–2018
In the field experiment, the highest degree of resistance to infection caused by Puccinia recondita sp. tritici was observed in PHR 4819 and PHR 4947 lines, which in 2018 and 2019 were characterized by resistance of 9 on a 9° scale (Table 2). In the case of line PHR 4819, marker wmc44 of gene Lr46 was identified during molecular analyses, while markers of both analyzed genes (Lr34 and Lr46) were identified in line PHR 4947. Genotypes in which none of the markers of the analyzed genes were found were characterized by an average field resistance from 3.5 in 2018 (PHR 4859) to 8 in 2017 (PHR 4670, PHR 4922, PHR 4995, and PHR 4997).
Field and laboratory evaluation of 64 wheat cultivars
| Degree | Genotype name | Field infection score | Laboratory evaluation | ||
|---|---|---|---|---|---|
| 9° scale | Lr34 | Lr46 | |||
| 2017 | 2018 | csLV34 | Xwmc44 | ||
| 1 | PHR 4666 | 6 | 4 | − | + |
| 2 | PHR 4670 | 8 | 7 | − | − |
| 3 | PHR 4671 | 7 | 5.3 | − | + |
| 4 | PHR 4672 | 9 | 8.5 | − | + |
| 5 | PHR 4777 | 8 | 7 | − | + |
| 6 | PHR 4792 | 5 | 6 | − | + |
| 7 | PHR 4794 | 5 | 6 | − | + |
| 8 | PHR 4795 | 8 | 7.5 | − | + |
| 9 | PHR 4796 | 6 | 6 | − | + |
| 10 | PHR 4800 | 6 | 8.3 | − | − |
| 11 | PHR 4813 | 7 | 5.5 | − | + |
| 12 | PHR 4817 | 6 | 6 | − | + |
| 13 | PHR 4818 | 8 | 8 | − | + |
| 14 | PHR 4819 | 9 | 9 | − | + |
| 15 | PHR 4820 | 9 | 8.8 | − | + |
| 16 | PHR 4856 | 8 | 8 | − | + |
| 17 | PHR 4859 | 7 | 3.5 | − | − |
| 18 | PGR 4860 | 7 | 3.8 | − | + |
| 19 | PHR 4861 | 9 | 7.8 | − | + |
| 20 | PHR 4862 | 8 | 6.5 | − | + |
| 21 | PHR 4867 | 8 | 5 | − | + |
| 22 | PHR 4868 | 9 | 6 | − | + |
| 23 | PHR 4870 | 9 | 7.5 | − | + |
| 24 | PHR 4872 | 9 | 8.5 | − | + |
| 25 | PHR 4875 | 8 | 8.8 | − | + |
| 26 | PHR 4876 | 6 | 5.5 | − | + |
| 27 | PHR 4877 | 9 | 5.8 | − | + |
| 28 | PHR 4879 | 8 | 7 | − | + |
| 29 | PHR 4888 | 8 | 7 | − | + |
| 30 | PHR 4895 | 7 | 8 | − | + |
| 31 | PHR 4896 | 8 | 8.3 | − | + |
| 32 | PHR 4897 | 5 | 4.8 | − | + |
| 33 | PHR 4903 | 8 | 7 | − | + |
| 34 | PHR 4905 | 9 | 8.3 | − | + |
| 35 | PHR 4906 | 8 | 8 | − | + |
| 36 | PHR 4907 | 6 | 5.2 | − | − |
| 37 | PHR 4922 | 8 | 7.2 | − | − |
| 38 | PHR 4923 | 6 | 6.25 | − | + |
| 39 | PHR 4932 | 8 | 6 | − | + |
| 40 | PHR 4933 | 6 | 6.3 | − | + |
| 41 | PHR 4934 | 8 | 7 | − | + |
| 42 | PHR 4936 | 9 | 8.75 | − | + |
| 43 | PHR 4937 | 8 | 6 | − | + |
| 44 | PHR 4947 | 9 | 9 | + | + |
| 45 | PHR 4948 | 7 | 4.5 | − | + |
| 46 | PHR 4949 | 7 | 6.3 | − | − |
| 47 | PHR 4953 | 9 | 6 | − | + |
| 48 | PHR 4955 | 9 | 8.3 | − | + |
| 49 | PHR 4956 | 7 | 5 | − | + |
| 50 | PHR 4957 | 5 | 7.3 | − | − |
| 51 | PHR 4962 | 9 | 7.8 | − | + |
| 52 | PHR 4963 | 7 | 6.3 | − | + |
| 53 | PHR 4973 | 7 | 5.5 | − | + |
| 54 | PHR 4977 | 9 | 8.7 | − | + |
| 55 | PHR 4979 | 7 | 6 | − | + |
| 56 | PHR 4995 | 8 | 7.3 | − | − |
| 57 | PHR 4997 | 8 | 6 | − | − |
| 58 | PHR 5000 | 8 | 7.5 | − | + |
| 59 | PHR 5003 | 6 | 6.3 | − | + |
| 60 | PHR 5004 | 7 | 5.8 | − | + |
| 61 | PHR 5005 | 9 | 8.8 | − | + |
| 62 | PHR 5007 | 9 | 7.3 | − | + |
| 63 | PHR 5013 | 9 | 8.5 | − | + |
| 64 | PHR 5017 | 9 | 6.8 | − | + |
+ variety of the analyzed gene.
− variety without the analyzed gene.
4 Discussion
Genetic disease resistance is a highly desirable trait in plants [17,18]. It is an environmentally friendly and definitely more profitable alternative to cultivations that use pesticides. The genome of common wheat (Triticum aestivum L. ssp. aestivum) is characterized by the presence of a number of genes with high resistance potential [12,19,20]. Unfortunately, most of the long-functioning resistance genes are not effective against current breeds of P. recondita f. sp. tritici, P. striiformis f. sp. tritici, P. graminis, and Blumeria graminis, causing leaf rust, stripe rust, stem rust, and powdery mildew, respectively [21]. Leaf rust is a fungal disease that is one of the biggest threats to present common wheat cultivation [22]. Because of the systematic occurrence and significant reduction in wheat yields, leaf rust is a disease of great economic importance and is associated with yield decreases ranging from 25 to even 45% [23].
The study determined the resistance of 64 common wheat lines originating from the experimental station of Poznańska Hodowla Roślin sp. z o.o. in Poland in the years 2017 and 2018, in field conditions. PHR 4819 and PHR 4947 lines proved to be the most resistant. Both lines were characterized by 9° of resistance in both years. Because of the favorable weather conditions during the growing season in 2017, the vast majority of the lines analyzed had a higher degree of resistance compared to 2018. Resistance in 2017 ranged from 5 to 9°, with as many as 34 lines with resistance at the level of 8–9°.
According to Krattinger et al. [24], Lr genes present in wheat and other cereals can be divided into three groups, regarding their stability and specificity. The first of these includes the major genes, also called R genes, which confer racial-specific resistance to one race of the pathogen. Most of the identified Lr genes belong to this group. Proteins encoded by R genes directly or indirectly receive virulence effects induced by pathogens that are secreted into the cytoplasm of host plant cells. The second group includes genes that are characterized by nonspecific resistance, comprising many races of fungal pathogens. Examples of genes with such resistance are the well-studied genes Lr34 and Lr46. The third group of genes, like the second, includes genes that confer nonspecific resistance; however, resistance is raised against all races within the same pathogen species. A known example of a gene with such resistance is Yr36, which provides resistance to stripe rust in wheat [25].
Most of the known Lr genes have racial-specific resistance that occurs at the seedling stage. However, there are several genes, including Lr12, Lr13, Lr22a, Lr34, Lr46, Lr67, Lr68, and Lr77, also called APR genes, which exhibit resistance at the adult plant stage. The latest literature indicates that rust resistance, which results from the combination of genes conferring racial-specific resistance and genes not specific to a given race of the pathogen, is the more persistent and the most preferred resistance in cultivated wheat cultivars [26]. In common wheat, rust resistance genes from related wild forms of Agropyron, Aegilops, and Scale are currently applied [27].
To date, at least four effective gene complexes have been identified: Lr34/Yr18/Sr57/Pm38, Lr46/Yr29/Pm39, Lr67/Yr46, and Sr2/Yr30, which provide partial resistance but last for a long period. This type of resistance is referred to as slow rust and causes slower disease development [21]. Gene Lr34 codes ATP-binding transporter (ABC transporter – ATP-binding cassette transporter) [24]. The gene Lr34 is present in European cultivars that have Mentana and Ardito cultivars in their pedigree registered in the early twentieth century [28]. Frontana is one of these cultivars.
In the current study, Frontana was used as a reference variety in which the presence of marker csLV of gene Lr34 and marker wmc44 of gene Lr46 was confirmed. The Lr34 gene marker was developed by Lagudah et al. [29]. SSR-PCR marker csLV was identified during analysis in line PHR 4947. The presence of a specific sequence has also been confirmed in multiplex PCR analyses. In addition to gene Lr34, gene Lr46 was identified in this genotype. Lines PHR 4947 and PHR 4819 were characterized by the highest leaf rust resistance in field conditions. For years, the authors have been analyzing the genotypes of various species for the presence of Lr34 gene.
Singh and Huerta-Espino [30] studied a doubled haploid population for the presence of the gene Lr34 marker. Wheat studied by the authors was created from a cross between Japanese Fukuho-komugi wheat and Israeli wheat oligoculm. In another experiment, Fukuho-komugi showed leaf necrosis in field trials in Mexico and was therefore found to be a carrier of the Lr34/Yr18 gene complex. In addition, the latency period of the disease increased, and the size and openness of uredinium decreased. Although the effects of gene Lr34 expression could be observed at every stage of leaf growth, the differences in the latent period and in the number of open uredinia increased significantly, starting from phases 4 to 5. The relative effect of gene Lr34 expression on the latent period was reduced, and with increasing temperature, its sensitivity was also elevated. Temperature and growth stage had the lowest impact on the size of uredinium [30].
Reynolds and Borlaug [23] conducted research on lines with and without the gene Lr34. The authors showed that thecrop loss in cultivars with Lr34 ranged from 11 to 15%, whereas in the absence of this gene, losses ranged from 40 to even 85%, depending on the sowing date.
The gene Lr34 is effective at the adult plant stage and, under favorable conditions, i.e., 4–10°C, at the flag leaf stage and seedling stage [31]. Unfortunately, the resistance conditioned by Lr34 is less effective at high temperatures [32]. Inoculation tests performed in field conditions on inbred wheat lines (F6) with the Lr34res allele showed 50% infestation with a 15% reduction in yield compared to control forms, in which 100% infestation and a 84% decrease in yield were observed [7].
The second of the analyzed genes was Lr46, which is also present as a gene complex and is linked to gene Yr29, which shows the resistance capacity to stripe rust. The gene Lr46 has been identified in the variety “Pavon 76” on chromosome 1B [33]. Its sequence is still unknown, but a number of candidate genes have already been selected.
During our own STS-PCR analyses, the marker wmc44 of gene Lr46 was identified in most of the analyzed lines. This marker was not present in the following genotypes: PHR 4670, PHR 4800, PHR 4859, PHR 4907, PHR 4922, PHR 4949, PHR 4957, PHR 4995, and PHR 4997. The presence of a specific sequence has also been confirmed in multiplex PCR analyses. In addition to the specific 242 bp product characteristic for Lr46, nonspecific products appeared. Genotypes carrying markers of the analyzed genes were characterized by good resistance to leaf rust in the field in both 2018 and 2019. In 2018, the resistance ranged from 5 to 8° and in 2019 from 5.2 to 8.3°, except for line 4859 where 3.5° was recorded.
This gene has been the research object of many scientists. Singh et al. [33] investigated the genetic associations of Lr46 with leaf rust resistance of adult plants. The authors conducted their works on two cultivars “Pavon 76” and “Avocet S,” which were analyzed using the AFLP-PCR technique. Preliminary studies have shown that inbred lines of wheat carrying this gene were characterized by an average of 35% lower infestation (leaf rust) compared to control forms in an experiment where plants were inoculated with fungal spores. These studies also allowed us to develop an STS (sequence-tagged site) marker wmc44, linked to the Lr46 gene locus [11]. In turn, Agarwal and Saini [9] proved that gene Lr46 was ineffective against leaf rust races in India. Lack of expression of this gene in some regions is explained by the temperature that is optimal for the development of the pathogen and unfavorable for the resistance mechanism conditioned by this gene [34]. Other traits of partial resistance of adult plants that exert the slow rust effect have been compared between Lr34 and Lr46 by Martinez et al. [8].
5 Conclusions
In recent years, molecular techniques have become the main tool used in breeding and protecting crop plants. They are used in genotype identification, genetic diversity studies, and genetic map construction. Genetic markers efficiently support the effectiveness of traditional methods based on morphological and phenotypic analyses, which require considerable time and labor. MAS selection using multiplex PCR contributes to the rapid identification of cultivars showing desired functional characteristics, which makes resistance breeding possible. Research shows that the molecular markers csLV34 and Xwmc44 enable the identification of genes Lr34 and Lr46. It is possible to use the multiplex PCR technique for simultaneous identification of both genes, which will shorten the time of selection. PHR line 4947 is a genotype that can be successfully used for further crossbreeding as the source of genes Lr34 and Lr46. This line is also characterized by high resistance in field conditions (9°).
Appendix
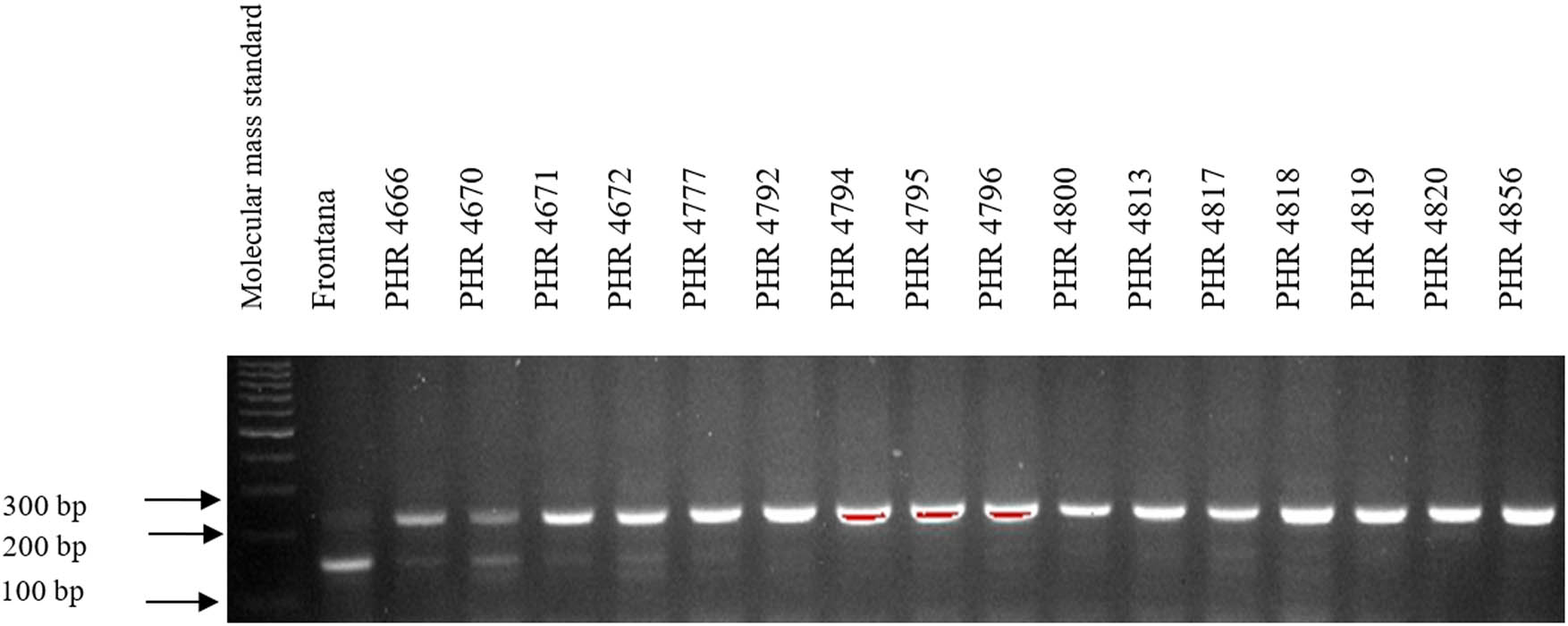
Electropherogram showing the identification of gene Lr34 marker csLV34 in wheat lines of different origin (genotypes 1–16).
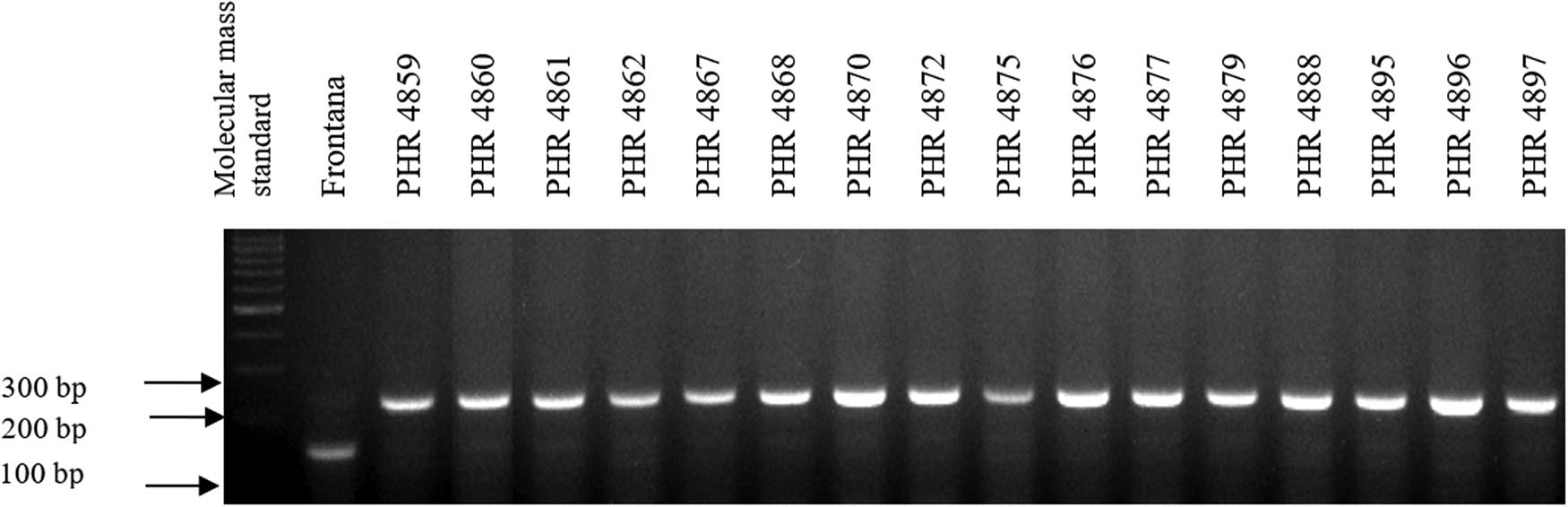
Electropherogram showing the identification of gene Lr34 marker csLV34 in wheat lines of different origin (genotypes 17–32).
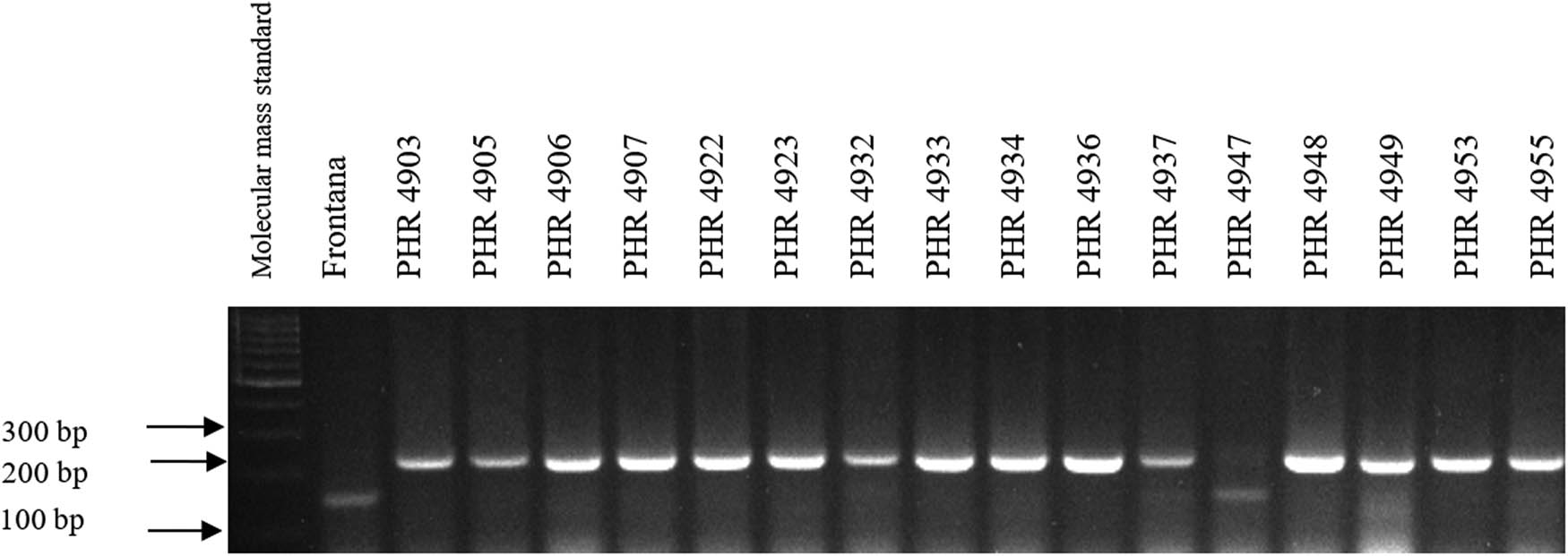
Electropherogram showing the identification of gene Lr34 marker csLV34 in wheat lines of different origin (genotypes 33–48).

Electropherogram showing the identification of gene Lr34 marker csLV34 in wheat lines of different origin (genotypes 49–64).

Electropherogram showing the identification of gene Lr46 marker in wheat lines of different origin (genotypes 1–17).

Electropherogram showing the identification of gene Lr46 marker in wheat lines of different origin (genotypes 18–32).
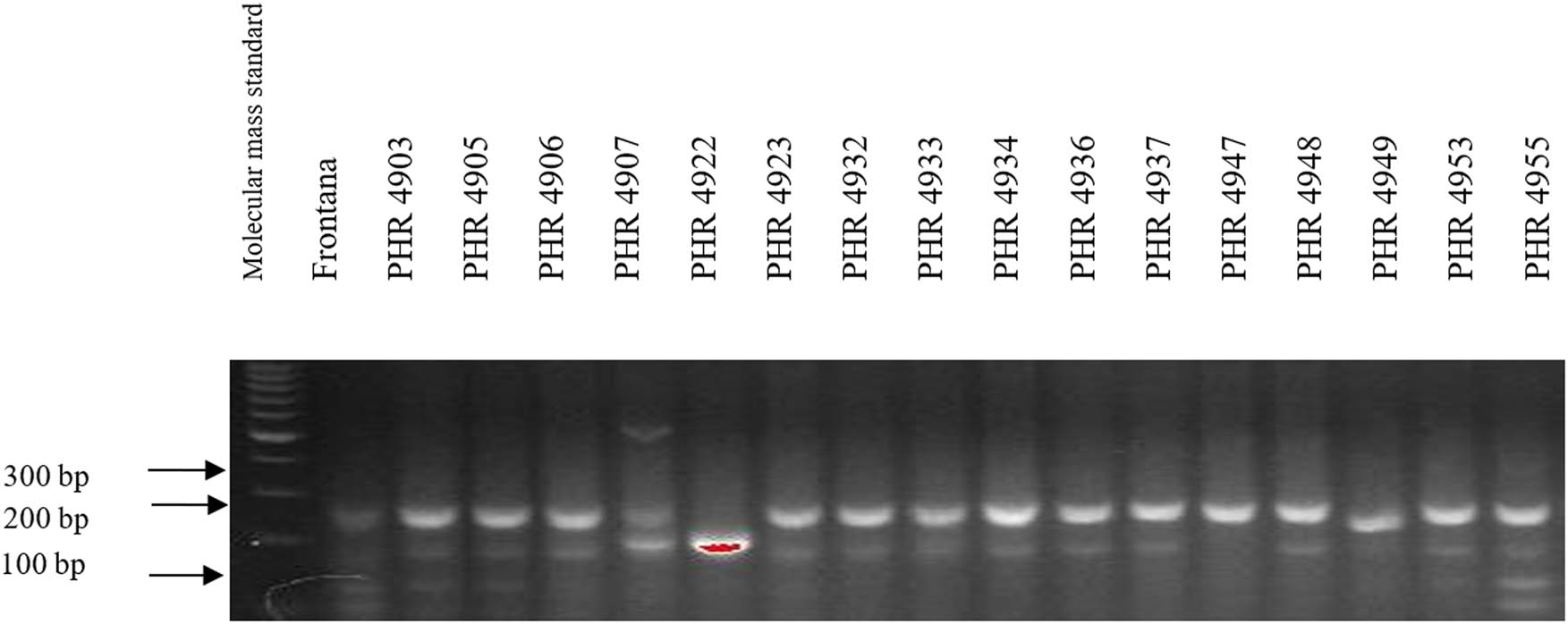
Electropherogram showing the identification of gene Lr46 marker in wheat lines of different origin (genotypes 33–48).
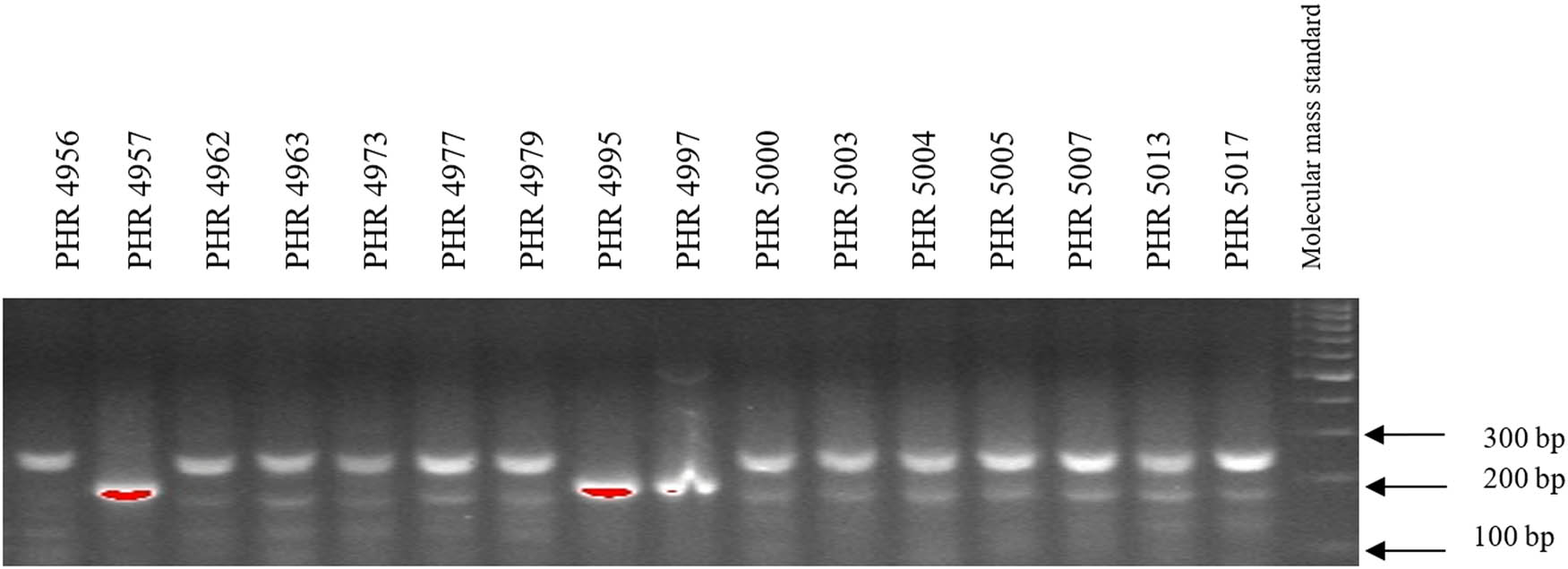
Electropherogram showing the identification of gene Lr46 marker in wheat lines of different origin (genotypes 49–64).

Electropherogram showing simultaneous identification of wmc44 and csLV markers of Lr34 and Lr36 genes in wheat lines of different origin (genotypes 1–17).
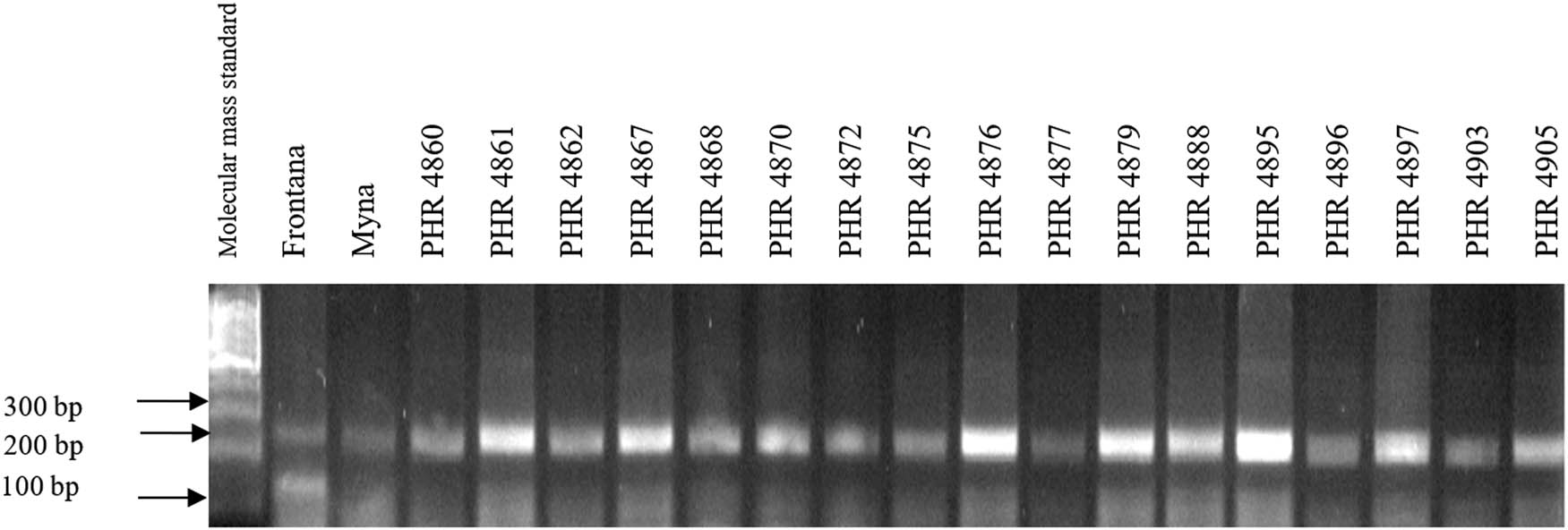
Electropherogram showing simultaneous identification of wmc44 and csLV markers of Lr34 and Lr36 genes in wheat lines of different origin (genotypes 18–34).
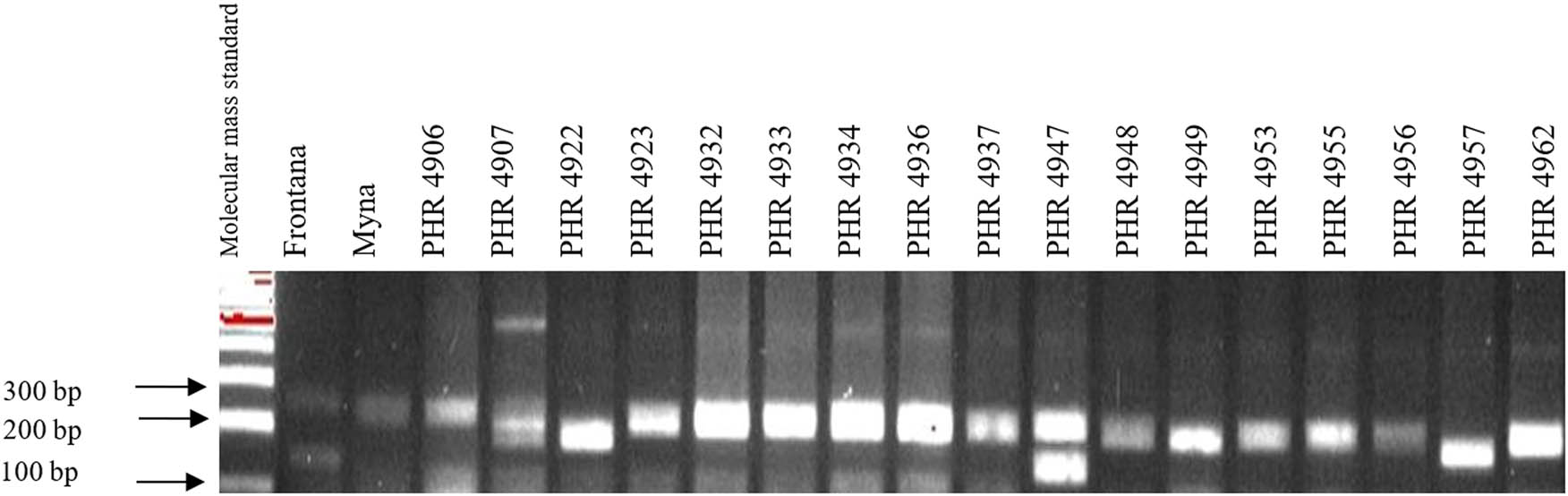
Electropherogram showing simultaneous identification of wmc44 and csLV markers of Lr34 and Lr36 genes in wheat lines of different origin (genotypes 35–51).

Electropherogram showing simultaneous identification of wmc44 and csLV markers of Lr34 and Lr36 genes in wheat lines of different origin (genotypes 52–64).
-
Funding: The authors state no funding involved.
-
Author contributions: Conceptualization: Agnieszka Tomkowiak, Sylwia Mikołajczyk, and Jerzy Nawracała; methodology: Agnieszka Tomkowiak, Julia Spychała, and Dorota Weigt; investigation: Agnieszka Tomkowiak and Roksana Skowrońska; data curation: Agnieszka Tomkowiak and Michał Kwiatek; supervision: Agnieszka Tomkowiak, Danuta Kurasiak-Popowska, and Janetta Niemann; writing – original draft: Agnieszka Tomkowiak and Przemysław Łukasz Kowalczewski; and writing – review and editing: Agnieszka Tomkowiak and Kinza Khan.
-
Conflict of interest: Przemysław Łukasz Kowalczewski, who is the co-author of this article, is a current Editorial Board member of Open Life Sciences. This fact did not affect the peer-review process.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Mancuso T, Verduna T, Blanc S, Di Vita G, Brun F. Environmental sustainability and economic matters of commercial types of common wheat. Agric Econ (Zemědělská Ekon). 2019;65(4):194–202. 10.17221/172/2018-AGRICECON.Search in Google Scholar
[2] Saharan MS. Current status of resistant source to Fusarium head blight disease of wheat: a review. Indian Phytopathol. 2020;73(1):3–9. 10.1007/s42360-019-00186-x.Search in Google Scholar
[3] Torres AM, Palacios SA, Yerkovich N, Palazzini JM, Battilani P, Leslie JF, et al. Fusarium head blight and mycotoxins in wheat: prevention and control strategies across the food chain. World Mycotoxin J. 2019;12(4):333–55. 10.3920/WMJ2019.2438.Search in Google Scholar
[4] Tomkowiak A, Skowrońska R, Buda A, Kurasiak-Popowska D, Nawracała J, Kowalczewski PŁ, et al. Identification of leaf rust resistance genes in selected wheat cultivars and development of multiplex PCR. Open Life Sci. 2019;14(1):327–34. 10.1515/biol-2019-0036.Search in Google Scholar PubMed PubMed Central
[5] Pietrusińska A. The use of molecular markers for introduction of leaf rust (Puccinia recondita f. sp. tritici) and powdery mildew (Blumeria graminis f. sp. tritici) resistance genes in winter wheat (Triticum aestivum). Bull Plant Breed Acclim Inst. 2010;256:31–44.10.37317/biul-2010-0030Search in Google Scholar
[6] Strzembicka A, Czajowski G, Karska K. Characteristic of the winter wheat breeding materials in respect of resistance to leaf rust Puccinia triticina. Bull Plant Breed Acclim Inst. 2013;268:7–14.Search in Google Scholar
[7] Singh RP, Huerta-Espino J. Effect of leaf rust resistance gene Lr34 on components of slow rusting at seven growth stages in wheat. Euphytica. 2003;129(3):371–6. 10.1023/A:1022216327934.Search in Google Scholar
[8] Martínez F, Niks RE, Singh RP, Rubiales D. Characterization of Lr46, a gene conferring partial resistance to wheat leaf rust. Hereditas. 2004;135(2–3):111–4. 10.1111/j.1601-5223.2001.00111.x.Search in Google Scholar PubMed
[9] Agarwal S, Saini RG. Undescribed wheat gene for partial leaf rust and stripe rust resistance from Thatcher derivatives RL6058 and 90RN249 carryingLr34. J Appl Genet. 2009;50(3):199–204. 10.1007/BF03195673.Search in Google Scholar PubMed
[10] Kolmer JA, Singh RP, Garvin DF, Viccars L, William HM, Huerta-Espino J, et al. Analysis of the Lr34/Yr18 rust resistance region in wheat germplasm. Crop Sci. 2008;48(5):1841–52. 10.2135/cropsci2007.08.0474.Search in Google Scholar
[11] Kolmer JA, Lagudah ES, Lillemo M, Lin M, Bai G. The Lr46 Gene Conditions partial adult- plant resistance to stripe rust, stem rust, and powdery mildew in Thatcher wheat. Crop Sci. 2015;55(6):2557–65. 10.2135/cropsci2015.02.0082.Search in Google Scholar
[12] McIntosh RA. Breeding wheat for resistance to biotic stresses. Euphytica. 1998;100(1):19–34. 10.1023/A:1018387402918.Search in Google Scholar
[13] Dyck PL. The association of a gene for leaf rust resistance with the chromosome 7D suppressor of stem rust resistance in common wheat. Genome. 1987;29(3):467–9. 10.1139/g87-081.Search in Google Scholar
[14] Spielmeyer W, McIntosh RA, Kolmer J, Lagudah ES. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor Appl Genet. 2005;111(4):731–5. 10.1007/s00122-005-2058-9.Search in Google Scholar PubMed
[15] William M, Singh RP, Huerta-Espino J, Islas SO, Hoisington D. Molecular marker mapping of leaf rust resistance gene Lr46 and its association with stripe rust resistance gene Yr29 in wheat. Phytopathology®. 2003;93(2):153–9. 10.1094/PHYTO.2003.93.2.153.Search in Google Scholar PubMed
[16] Skowrońska R, Kwiatek M, Tomkowiak A, Nawracała J. Development of multiplex PCR to detect slow rust resistance genes Lr34 and Lr46 in wheat. J Appl Genet. 2019;60(3–4):301–4. 10.1007/s13353-019-00520-z.Search in Google Scholar PubMed PubMed Central
[17] Dong OX, Ronald PC. Genetic engineering for disease resistance in plants: recent progress and future perspectives. Plant Physiol. 2019;180(1):26–38. 10.1104/pp.18.01224.Search in Google Scholar PubMed PubMed Central
[18] Yin K, Qiu J-L. Genome editing for plant disease resistance: applications and perspectives. Philos Trans R Soc B Biol Sci. 2019;374(1767):20180322. 10.1098/rstb.2018.0322.Search in Google Scholar PubMed PubMed Central
[19] Yang X, Pan Y, Singh PK, He X, Ren Y, Zhao L, et al. Investigation and genome-wide association study for Fusarium crown rot resistance in Chinese common wheat. BMC Plant Biol. 2019;19(1):153. 10.1186/s12870-019-1758-2.Search in Google Scholar PubMed PubMed Central
[20] Shi C, Zheng Y, Geng J, Liu C, Pei H, Ren Y, et al. Identification of herbicide resistance loci using a genome-wide association study and linkage mapping in Chinese common wheat. Crop J. 2020;8(4):666–75. 10.1016/j.cj.2020.02.004.Search in Google Scholar
[21] Herrera-Foessel SA, Singh RP, Huerta-Espino J, Rosewarne GM, Periyannan SK, Viccars L, et al. Lr68: a new gene conferring slow rusting resistance to leaf rust in wheat. Theor Appl Genet. 2012;124(8):1475–86. 10.1007/s00122-012-1802-1.Search in Google Scholar PubMed
[22] Peksa K, Bankina B. Characterization of puccinia recondita, the causal agent of brown rust: a review. Res Rural Dev. 2019;2:70–6. 10.22616/rrd.25.2019.051.Search in Google Scholar
[23] Reynolds MP, Borlaug NE. Impacts of breeding on international collaborative wheat improvement. J Agric Sci. 2006;144(1):3–17. 10.1017/S0021859606005867.Search in Google Scholar
[24] Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, et al. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science (80). 2009;323(5919):1360–3. 10.1126/science.1166453.Search in Google Scholar PubMed
[25] Peng FY, Yang RC. Prediction and analysis of three gene families related to leaf rust (Puccinia triticina) resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2017;17(1):108. 10.1186/s12870-017-1056-9.Search in Google Scholar PubMed PubMed Central
[26] Prasad P, Savadi S, Bhardwaj SC, Gupta PK. The progress of leaf rust research in wheat. Fungal Biol. 2020;124(6):537–50. 10.1016/j.funbio.2020.02.013.Search in Google Scholar PubMed
[27] Charpe A, Koul S, Gupta SK, Singh A, Pallavi JK, Prabhu K. Marker assisted gene pyramiding of leaf rust resistance genes Lr9, Lr24 and Lr28 in a bread wheat cultivar HD 2329. J Wheat Res. 2012;4:20–8.Search in Google Scholar
[28] Lagudah ES, Krattinger SG, Herrera-Foessel S, Singh RP, Huerta-Espino J, Spielmeyer W, et al. Gene-specific markers for the wheat gene Lr34/Yr18/Pm38 which confers resistance to multiple fungal pathogens. Theor Appl Genet. 2009;119(5):889–98. 10.1007/s00122-009-1097-z.Search in Google Scholar PubMed
[29] Lagudah ES, McFadden H, Singh RP, Huerta-Espino J, Bariana HS, Spielmeyer W. Molecular genetic characterization of the Lr34/Yr18 slow rusting resistance gene region in wheat. Theor Appl Genet. 2006;114(1):21–30. 10.1007/s00122-006-0406-z.Search in Google Scholar PubMed
[30] Singh RP, Huerta‐Espino J. Effect of leaf rust resistance gene Lr34 on grain yield and agronomic traits of spring wheat. Crop Sci. 1997;37(2):390–5. 10.2135/cropsci1997.0011183X003700020014x.Search in Google Scholar
[31] Risk JM, Selter LL, Krattinger SG, Viccars LA, Richardson TM, Buesing G, et al. Functional variability of the Lr34 durable resistance gene in transgenic wheat. Plant Biotechnol J. 2012;10(4):477–87. 10.1111/j.1467-7652.2012.00683.x.Search in Google Scholar PubMed
[32] Wellings CR. Global status of stripe rust: a review of historical and current threats. Euphytica. 2011;179(1):129–41. 10.1007/s10681-011-0360-y.Search in Google Scholar
[33] Singh RP, Mujeeb-Kazi A, Huerta-Espino J. Lr46: a gene conferring slow-rusting resistance to leaf rust in wheat. Phytopathology®. 1998;88(9):890–4. 10.1094/PHYTO.1998.88.9.890.Search in Google Scholar PubMed
[34] El Orabey WM, Shahin SI. Relationship between partial resistance and inheritance of adult plant resistance gene Lr46 of leaf rust in six bread wheat varieties. Adv Crop Sci Technol. 2014;3(1):161. 10.4172/2329-8863.1000161.Search in Google Scholar
© 2021 Agnieszka Tomkowiak et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

