Abstract
Plant diseases pose a significant threat to global food security, causing substantial crop yield losses and economic damage. Traditional diagnostic methods often lack the speed, specificity, and sensitivity required for effective disease management. The emergence of CRISPR/Cas-based detection systems offers a novel and highly efficient approach for identifying plant pathogens. This review explores the principles of CRISPR/Cas technology, its adoption for pathogen detection, and its advantages over conventional diagnostic techniques. Recent advancements in CRISPR-based detection tools, including SHERLOCK, DETECTR, and other Cas-mediated platforms, and their potential for rapid, field-deployable diagnostics have been discussed. As these technologies continue to evolve, they hold promise for transforming plant disease diagnostics and enhancing global agricultural resilience.
1 Introduction
Plant diseases are considered one of the most important threats to crop production and thus to food supply in the modern world. Since the onset of agriculture 10,000–12,000 years ago, the agro-ecosystems evolved toward high environmental homogeneity and low genetic diversity, which have driven the emergence and evolution of numerous crop pathogens and pests [1,2]. For the major crops, the global yield losses caused by pests and pathogens have been estimated in a range of 20–30%, depending on the crop and the international distribution of the production [3]. Plant diseases affect various aspects (components) of food safety both on local and global levels and may have disastrous consequences [4]; a notorious example of this is the case of the potato famine in Ireland (1845–1850) caused by Phytophthora infestans [5]. Thus, comprehensive disease control is a crucial task to maintain food safety in countries and regions of the world. The modern-day practices require a complex approach combining the utilization of crop genetic resources to increase their capability to resist diseases, direct eradication of the pathogens and their vectors, and the monitoring of the infection distribution within agricultural systems.
The crucial step in the monitoring of pathogens is their correct identification, as different infection agents require different approaches to disease management. To date, various methods with their advantages and limitations are used for plant pathogen diagnostics (Table 1). The oldest used methods of detection and diagnostics of plant pathogens are based on distinct visual features of different diseases, both on macro- and microscopic scales, as well as traditional microbiological approaches based on Koch’s postulates, when applicable [6]. The visual examination method is simple and does not require special equipment, but it is not suitable for plants with latent infections, it depends on the subjective knowledge and experience of the investigator, and it is prone to errors [7,8,9]. The limitations of such traditional approaches have led to the development of a range of more precise laboratory-based methods, from serological assays to DNA markers. The serological methods, mainly based on enzyme-linked immunosorbent assay (ELISA), are widely used due to their low cost and good performance; however, they have limitations related to their specificity in different experimental contexts [6]. The DNA detection methods based on polymerase chain reaction (PCR) and sequencing became a standard in plant pathogen diagnostics due to their exceptional sensibility and potential for customization [10,11,12]. However, despite continuous efforts to improve, this approach remains expensive, time, and labor consuming and requires a stationary laboratory environment. The demand for quick, portable, yet reliable technologies for rapid DNA- or RNA-based diagnostics has led to the development of methods of isothermal amplification: loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA), helicase-dependent amplification, etc. [13]; however, these methods have their own limitations [14].
Comparison of traditional and modern methods of plant disease diagnostics
| Method | Advantages | Disadvantages |
|---|---|---|
| Visual examination of symptoms | Does not require expensive equipment and expendables |
|
| Microbiological methods (selective medium cultivation, inoculation experiments) |
|
|
| Serology assay (ELISA, immune blotting) |
|
|
| PCR and sequencing |
|
|
| Isothermal amplification |
|
|
| CRISPR/Cas detection |
|
|
The present review is focused on CRISPR/Cas-based detection, a relatively novel but rapidly growing in popularity tool for pathogen detection, in application to plant diseases. Since the first published results in 2017 [15], the method was extensively tested for human pathogens and, later, was widely adopted for plant pathology. The growing number of studies dedicated to the diagnostics of plant pathogens indicates the increasing interest in this technology as a quick, portable, and reliable tool for plant pathology.
2 Diversity of bacterial CRISPR/Cas systems and their modifications
Since the discovery of CRISPR/Cas systems as an adaptive immune mechanism in bacteria and archaea [16], they have attracted attention both as a fundamental component of the evolution and ecology of prokaryotes [17] and as a promising tool for numerous practical applications [18]. The natural prokaryotic CRISPR/Cas systems have vast diversity. Based on available data on the composition of the systems and their evolutionary changes within various prokaryotic genomes, the current CRISPR/Cas classification includes 2 classes, 6 types, and 33 subtypes [19]. Class I systems, including CRISPR/Cas types I, III, and IV, involve multi-component protein complexes (up to 11 in Archaeoglobus fulgidus), whereas class II systems, including types II, V [20], and VI [19], contain a single multi-domain Cas protein with modules for target and crRNA recognition and target cleavage [21]. Thus, due to simplicity, CRISPR/Cas class II systems have become preferable for molecular genetic research. The CRISPR/Cas9 system of this class became a widespread tool for genetic engineering.
The first system widely applied in genetic engineering was CRISPR–Cas9 (class 2, type II). It contains several conserved domains: REC1-REC3, HNH, and a RuvC-like domain, which ensure efficient nucleic acid detection and cleavage [22]. Cas9 introduces site-specific double-strand breaks at target sites. In synthetic CRISPR–Cas9 systems, crRNA and tracrRNA are combined into an artificial tetraloop, forming a single-guide RNA (sgRNA). The target is determined by a 98-nucleotide sequence, including a 20-nucleotide spacer located at the 5′-end of the sgRNA. The specificity of the editing system is defined by the spacer sequence used [23]. Protospacer recognition depends on the presence of a PAM sequence and absolute spacer homology. Precise cleavage at the target recognition site made Cas9 a popular tool in genetic engineering for gene editing via homologous or non-homologous repair of the affected regions [24,25]. The successful application of the CRISPR–Cas9 system from Streptococcus pyogenes has spurred interest in bacterial and archaeal genomes in search of simpler and more efficient tools.
Type V systems include Cas12 effector proteins, which functionally differ from type II systems. Cas12 proteins contain only a RuvC-like domain, responsible for DNA-targeting activity [19]. Cas12a offers several advantages over Cas9 in genetic engineering and pathogen detection. First, the SpCas9 protein is larger than Cas12a. Additionally, for its nuclease activity, Cas9 requires a chimeric molecule consisting of crRNA and tracrRNA – whereas CRISPR/Cas12a is more compact and requires only a single-guide RNA, simplifying cellular delivery. Second, Cas12a cleaves DNA differently than Cas9, which is crucial for transgenesis accuracy. While Cas9 creates “blunt ends” by cutting at a single site, Cas12a cleaves both DNA strands at a small distance apart (5–7 base pairs), forming “sticky ends.” This feature enhances the precise integration of donor DNA fragments [26].
The Cas14 protein was discovered through metagenomic analysis of bacterial and archaeal databases. It is one of the smallest Cas proteins, ranging from 40 to 70 kDa, which is 400–700 amino acids smaller than other functional Cas proteins [27]. Functionally, Cas14 is more similar to type V proteins – its activity targets single-stranded DNA. However, unlike Cas9, Cas14 does not require a PAM sequence, which reduces constraints on protospacer selection. Cas14 contains an RuvC domain responsible for cleaving single-stranded DNA [27,28].
Unlike Cas9, Cas12, and Cas14, Cas13 proteins of type VI have specificity for target recognition and cleavage of RNA instead of DNA. The HEPN domain in Cas13 is essential for RNA substrate binding and nuclease activity; like type V proteins, type VI proteins process their pre-crRNA [29]. Cas13a and Cas13b have a unique property: after specific target interaction, they exhibit non-specific activity against any RNA. Due to this, Cas13 is considered a tool for genome interventions on the level of RNA, e.g., as an alternative for RNA interference for gene silencing or gene therapy [30,31].
3 Principles of CRISPR/Cas-based pathogen detection
The specific target binding by crRNAs laying in the basis of the function of CRISPR/Cas9 systems has raised an interest in their use for sequence recognition assays, as an alternative to traditional primers and probes. Such detection systems combine targeted recognition through specific binding of crRNA(sgRNA)/Cas9 complexes, followed by signal amplification, offering advantages over traditional methods like PCR in terms of speed, portability, and minimal equipment requirements. Earlier proposed assays were based on CRISPR/Cas9 and included diverse signal amplification methods. Different authors proposed a variety of signal detection approaches. Pardee et al. used crRNAs and Cas9 protein as part of paper-based biosensors for strain-specific detection of Zika virus [32]. SgRNA/Cas9 complex was proposed for use as a sequence-targeting agent in fluorescent in situ hybridization assay for detection of antibiotic-resistant Staphylococcus aureus [33]; the other study was focused on drug resistance in S. aureus, Acinetobacter baumannii, and Klebsiella pneumoniae [34]. Xu et al. proposed a method combining CRISPR/Cas9 target recognition with micro-bead-based ELISA and tested it for the detection of human papillomavirus [35]. Another approach based on luminescence from luciferin reporter and luciferase-linked Cas9 protein complex was tested for the detection of Mycobacterium tuberculosis [36]. An approach proposed by Huang et al. used specific target breaks made by Cas9 to trigger nicase-driven exponential circular amplification [37]. Other examples of using CRISPR/Cas9 for pathogen detection include Salmonella typhimurium [38], Orientia tsutsugamushi [39], Listeria monocytogenes, and African swine fever virus [40]. However, the use of CRISPR/Cas9 systems for nucleic acid detection has not received as much attention as their applications as genome editing tools. Although the mentioned assays demonstrated higher accuracy compared to conventional PCR, they utilized diverse reporter/biosensor systems for signal detection and amplification, which were complicated and limited widespread adoption.
The nucleic acid detection systems based on proteins Cas12 and Cas13 have been considered more promising. The specific features of these proteins are the presence of trans-cleavage activity: Cas12 and Cas13 are capable of digesting any ssDNA or RNA, respectively, upon their activation by the duplex of the target and sgRNA [41,42,43], in contrast to Cas9, which cuts DNA exclusively at the binding site [44]. When used with reporter molecules, usually labeled DNA or RNA oligonucleotides, such collateral cleavage may amplify the detection signal to sufficient levels for visual or instrumental detection (Figure 1) [45]. The SHERLOCK (Specific High-sensitivity Enzymatic Reporter UnLOCKing) assay was the first method that defined the further development of CRISPR/Cas detection systems. The proposed method utilized RNA recognition and cleavage activity of Cas13a protein (previously known as C2c2; dual ribonuclease undergoing conformational change in response to target RNA recognition which leads to cis- and trans-RNA cleavage activity [46]) coupled with RPA and reverse-transcription RPA (RT-PCR) and T7 in vitro transcription to detect DNA or RNA, correspondingly, of viral and bacterial pathogens both in culture and human samples [15]. The original protocol utilized RNA oligonucleotides labeled with a fluorescent dye and the quencher as a reporter; collateral cleavage activity of Cas13a protein led to the release of fluorescence detected and quantified by conventional plate-reading or RT-PCR tools [47]; the modified assay, SHERLOCKv2, provided further development of the reporter system including use of colorimetric lateral flow cells and multiplex detection [48]. A similar approach involving the Cas12a protein was termed DNA endonuclease-targeted CRISPR trans reporter (DETECTR) [41]. Similar to Cas13a, Cas12a undergoes activation of trans-cleavage activity upon conformation changes caused by target recognition by sgRNA; the difference is that both targeting and cleavage substrates are ssDNA [49,50]. The absence of the required in vitro transcription step made this method a convenient alternative to CRISPR/Cas13-based approaches; also, the ssDNA reporter molecules are more affordable and easier to use than RNA. As well as SHERLOCK, the combination with isothermal amplification of PCR increases sensitivity [51], and application of reverse transcription allows the use of the method also for RNA detection [52].
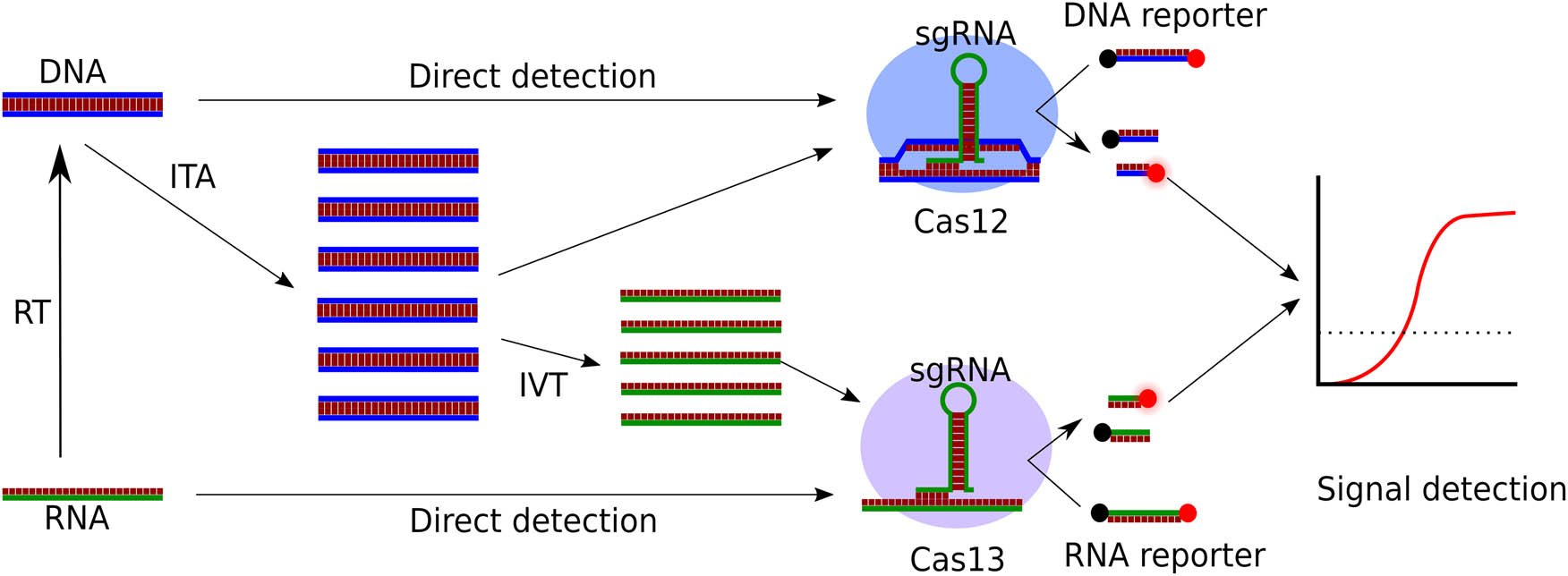
General principles of nucleic acid detection using CRISPR/Cas12-Cas13. RT – reverse transcription; ITA – isothermal amplification; IVT – in vitro transcription. Source: Created by the authors.
Both original SHERLOCK and DETECTR protocols have been registered as trademarks by The Broad Institute, Inc., Cambridge, USA (USA registration number 6295441, Mar. 16, 2021) and Mammoth Biosciences, Inc., Brisbane, California, USA (USA registration number 6473033, Aug. 31 2021), respectively, and patented; main patents include US12037639B2 (USA), JP2023011606A (Japan), ES2927463T3 (Spain), EP4119663B1 (European Union), WO2018107129A1 (WIPO), AU2017371324B2 (Australia) for SHERLOCK and US20210102242A1, US11174470B2 (USA), EP3830301B1 (European Union) for DETECTR. The development of novel modifications of basic SHERLOCK/DETECTR assays continues to expand them for newer applications, improve their features, and avoid limitations. The protocol HOLMES (an one-HOur Low-cost Multipurpose highly Efficient System) was developed to allow quantitative detection of the target molecules [53,54]. As proof of concept, the use of Cas14 protein instead of Cas12a was proposed for nucleic acid detection with potentially higher fidelity [28].
Due to their versatility, accessibility, and customization potential, SHERLOCK and DETECTR-like methodologies became a standard for CRISRP/Cas-based detection of nucleic acid. A high speed of analysis and potential portability are usually considered the most important advantages of these tools, and the new protocols are developed to further improve these qualities.
Whereas the requirements for the basic procedure of CRISPR/Cas detection are limited by a thermostat and single tube reaction mixture, the methods proposed for the nucleic acid cleavage signal detection vary in their affordability (Figure 2). The most common approach described above utilizes fluorescent probes [48,53] and thus requires equipment for fluorescence detection, such as plate readers and real-time thermal cyclers; however, it was shown that the visual detection of fluorescence is also possible and provides qualitative test outcomes using only a UV light source [55]. An alternative approach is the colorimetric method utilizing gold or iron nanoparticles as reporters [56,57,58]. In case of visual detection, similar to isothermal amplification methods (LAMP, RPA), which are usually used in conjunction with CRISPR/Cas, the methodology can be deployed using a minimal set of portable equipment like mini-centrifuges and solid-body thermostats [59]. Lateral flow elements [60] and biosensors [61] have been proposed to further increase the availability of express detection directly in the field. To exclude a step of isolation of the nucleic acids, a combination of SHERLOCK with the HUDSON (Heating Unextracted Diagnostic Samples to Obliterate Nucleases) method was proposed for direct detection of viruses in biological samples [62]. On the other hand, the implementation of new complicated techniques for precise signal detection paves the way for higher sensitivity and fidelity of nucleic acid detection [63]. Use of microchamber-based array was shown to increase sensitivity by excluding the amplification step and thus reducing possible amplification errors [64]. Similarly, the possibility of direct quantitative detection of RNA using a digital droplet platform without amplification was shown [65]. Thus, the CRISPR/Cas detection systems can be deployed at various scales, from express detection at the points of care (fields and gardens, in case of crop disease) to more complicated laboratory environments with different levels of precision and sensibility, depending on the goals. Therefore, the methodology of CRISPR/Cas detection has significant potential as both a rapid, portable diagnostic tool and a reliable and efficient tool for well-equipped laboratories.
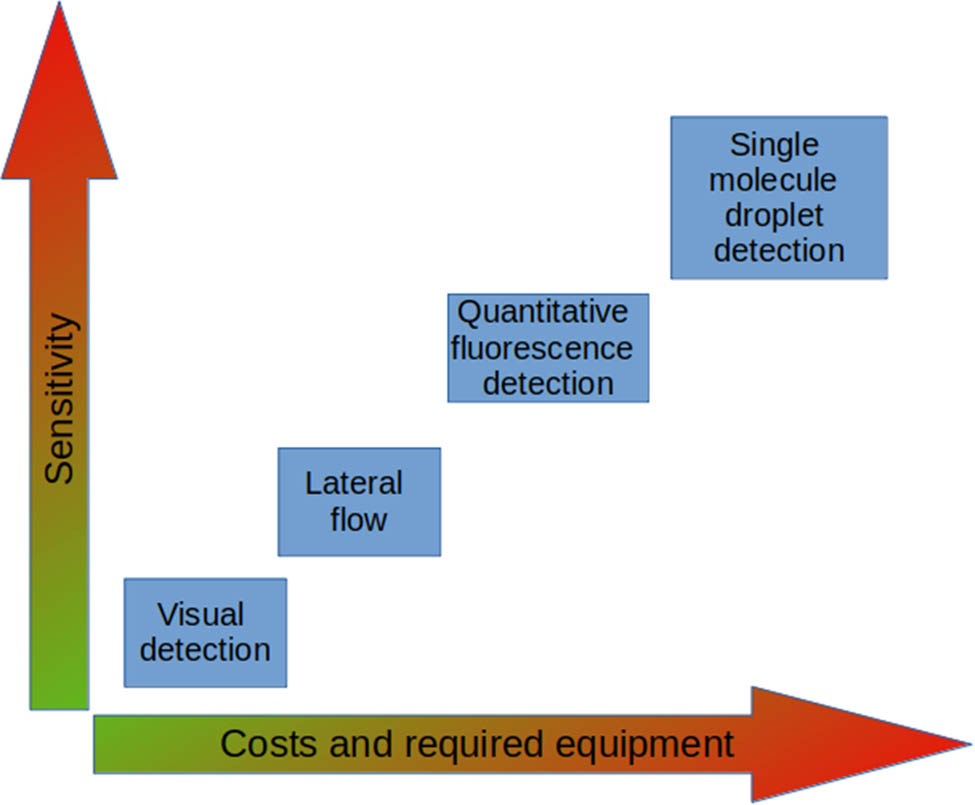
CRISPR/Cas signal detection methods ordered by their sensitivity and cost.
Compared to the conventional methods based on PCR, the CRISPR/Cas has several advantages (Table 2). CRISPR/Cas demonstrated sensitivity similar to or greatly surpassing PCR; e.g., for Staphilococcus auresus, the CRISPR/Cas system was able to detect 1.2 CFU whereas PCR detected 60 CFU [66]. Both methods have high specificity as they rely on specific oligonucleotide matching; however, CRISPR/Cas is more sensitive to the mismatches than PCR primers and thus allows precise identification of particular sequences and their variants [66,67]: even single mismatches in the target have been shown to reduce cleavage efficiency [56]. This, as well as potentially low costs, demands for time and labor, and portability, as discussed above, makes CRISPR/Cas-based detection systems a tool of choice for many applications currently relying on PCR, including detection of pathogens of human [68], animals [69], and plants [70].
Comparison of CRISPR/Cas detection and PCR
| Feature | PCR | CRISPR/Cas-based detection |
|---|---|---|
| Sensitivity | High | Ultra-high (can surpass PCR/qPCR) |
| Specificity | High | High (can discriminate single nucleotides) |
| Speed | Hours | 15–60 min |
| Equipment | Expensive, requires thermocycler | Simple, often isothermal |
| Cost | Higher | Potentially lower |
| Quantification | Excellent (qPCR) | Good |
| Practicality | Lab-based, skilled personnel | Point-of-care potential, user-friendly |
3.1 Use of CRISPR/Cas for detection of plant pathogens: current status and future perspectives
As described above, the key field of development and application of CRISPR/Cas-based pathogen detection and diagnostics is medicine. However, this technology has also been adopted for the demands of plant pathology and epidemiology. Similar to the primer- and probe-based method, the core of the methodology is versatile and can potentially be applied to any research object using custom-designed gRNA sequences. The early example is the use of CRISPR/Cas9 coupled with LAMP and gold nanoparticles to detect Phytophthora infestans, the common pathogen of vegetable crops [56]. Tripathi et al. adopted the CRISPR/Cas9-nicase method [71] to detect banana bunchy top virus and banana streak virus in banana plants. However, the later studies focused on the systems based on Cas12 or Cas13 proteins, as their convenience and versatility have been proven by the use of systems like SHERLOCK and DETECTR for medical purposes. The cases of use of Cas13a protein with SHERLOCK-like protocols for plant pathogen detection include identification of tomato spotted wilt virus [72] and fungus Verticillium dahliae in smoke tree [73]. Hak et al. proposed an express-protocol for direct detection of tomato brown rugose fruit virus (TBRFV) by CRISPR/Cas13a without steps of RNA isolation, RT and amplification, and in vitro transcription; although sensitivity of the assay was lower comparing methods combined with amplification, high portability and rapid execution have made it very promising as in field plant pathogen diagnostic tool [74].
The most used approach is the use of Cas12a protein, due to its compatibility with DNA and RNA targets (via reverse transcription) without the necessary step of in vitro transcription. A system for the direct detection of tomato mosaic virus (Tobamovirus tomatotessellati [1]) and TBRFV (Tobamovirus fructirugosum) in tomato without a preliminary isothermal amplification step was developed and shown to differentially detect two viruses from 15 to 30 ng of PCR product [76]; another system for TBRFV has shown sensibility similar to conventional real-time PCR and has been recommended as suitable at the points-of-care [77]. Another tomato viruses, tomato yellow leaf curl virus (Begomovirus coheni) and tomato leaf curl New Delhi virus (Begomovirus solanumdelhiense), have been successfully detected using Cas12a with LAMP [78]. Even in case of RNA viruses, the use of CRISPR/Cas12a combined with RT-RPA or RT-LAMP was considered preferable and tested for detection of beet necrotic yellow vein virus (Benyvirus necrobetae) in sugarbeet [79], tobacco mosaic virus (Tobamovirus tabaci), potato virus X (Potexvirus ecspotati), potato virus Y (Potyvirus yituberosi) in tobacco [80], maize chlorotic mottle virus (Machlomovirus zeae) in maize [81], apple necrotic mosaic virus (Ilarvirus ApNMV), apple stem pitting virus (Foveavirus mali), apple stem grooving virus (Capillovirus mali), apple chlorotic leaf spot virus (Trichovirus mali), apple scar skin viroid (Apscaviroid cicatricimali) in apple tree [82], citrus tristeza virus (Closterovirus tristezae) [83], and brassica yellows virus (Polerovirus TUYV) [84]. Although Cas13a could be used for direct detection of viral RNA, as shown by Hak et al. [74] and Marqués et al. [85], Cas12a detection combined with reverse transcription and isothermal amplification remains preferable due to higher sensitivity. Typical targets used for the detection of plant viruses include ORFs encoding coat (capsid) protein, as in the case of PCR-based or other sequence-specific assays.
CRISPR/Cas12a assay got extensively used for the diagnostics of fungal diseases in plants. The list of the successfully tested pathogens includes, but not limited by Alternaria solani in potato [57], Sporisorium scitamineum and Colletotrichum falcatum in sugar cane [86], Diaporthe helianthi in sunflower [87], Sclerotium rolfsii in cassava [88], Verticillium dahliae [89], Venturia carpophila in peach [90], Fusarium circinatum in pines [91], F. asiaticum in rice [92], F. graminearum in wheat [93], Leptosphaeria maculans in Brassica [94], Magnaporthe grisea (syn. Magnapothe oryzae) in rice [95], Diaporthe aspalathi and D. caulivora in soybean [96], Elsinoё fawcettii [97] and Alternaria spp. [98] in Citrus, etc. Systems for the detection of oomycetes Phytophthora ramorum [99], P. sojae [100], and P. cambivora [101], deleterious pathogens of wild range of plant species. The typical target loci used for the identification of fungal species are 16S rRNA gene [86], ITS [87,88,94], Cal gene, EF1-α [87], GAPDH [89], CYP51C [92], etc. The bacterial pathogens for which CRISPR/Cas-based diagnostic systems have been proposed include Acidovorax citrulli [102], Xylella fastidiosa [103], Xanthomonas albilineans [86], Candidatus Liberibacter asiaticus [104], and Candidatus Phytoplasma trifolii [105]; ITS and 16S rRNA gene sequences were used as a target to identify bacterial species.
The listed studies report detection sensibility from attogram to nanogram amounts of the target, depending on whether isothermal pre-amplification has been used or not. With such sensibility comparable or surpassing conventional PCR and real-time PCR-based assays [106], and taking into account costs, time, and labor demands, interest in the application of CRISPR/Cas-based detection systems in plant pathology is growing, as shown by the available publications. As the existing studies show, the prevailing approach is the use of CRISPR/Cas12a in conjunction with isothermal amplification methods (LAMP, RPA). In this direction, this technology may be considered an upgrade of isothermal amplification, allowing it to overcome its limitations (like lower specificity compared to PCR) at a low cost of additional manipulations and time. On the other hand, CRISPR/Cas detection protocols can be further developed as an independent, amplification-free tool. For plant viruses, which are predominantly RNA-based, a direct detection with CRISPR/Cas13 without steps of reverse transcription and amplification can be a potential game-changer, significantly increasing speed and portability of the assay. However, due to higher sensitivity, amplification-coupled protocols remain prevailing. The accumulated data lead to the development of common protocols, e.g., for the detection of viruses [107], which will make the wider adoption of the technology to crops and their pathogens easier. The applicability range of the CRISPR/Cas assays varies from express systems for rapid in field and as part of a sophisticated laboratory environment, with the corresponding scaling of sensitivity and fidelity of the detection. Thus, CRISPR/Cas detection systems are considered a promising alternative to PCR-based methods, both as is and as an extension of isothermal amplification assays.
4 Conclusion
The pathogen detection methods based on CRISPR/Cas have proven themselves a promising and reliable alternative to traditional PCR-based approaches. The main features include high specificity and sensibility, rapid preparation and result reading, versatility in various research and practical cases, and, of particular importance, low demand for equipment and potentially high portability. As was shown by numerous studies on human pathogen tests, CRISPR/Cas systems proved their viability and so pose interest not only for medicine but also for plant pathology. The portability and accessibility of the technology make it suitable for the express testing of infected plants in the field. The available research data indeed show the growing interest in CRISPR/Cas detection for a wide range of plant pathogens, and so development and improvement of the technology is a promising field of study and practical use in plant pathology and epidemiology.
-
Funding information: The study was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan within the framework of a targeted funding program BR21882269 “Using genome editing technology to increase the productivity of economically important crop plants.”
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. Writing – original draft preparation, A.P., V.K., K.A., and A.T.; writing – review and editing, A.P. and D.G.; supervision, D.G.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Stukenbrock EH, McDonald BA. The origins of plant pathogens in agro-ecosystems. Annu Rev Phytopathol. 2008;46:75–100. 10.1146/annurev.phyto.010708.154114.Search in Google Scholar PubMed
[2] McDonald BA, Stukenbrock EH. Rapid emergence of pathogens in agro-ecosystems: global threats to agricultural sustainability and food security. Philos Trans R Soc B: Biol Sci. 2016;371:20160026. 10.1098/rstb.2016.0026.Search in Google Scholar PubMed PubMed Central
[3] Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A. The global burden of pathogens and pests on major food crops. Nat Ecol Evol. 2019;3:430–9. 10.1038/s41559-018-0793-y.Search in Google Scholar PubMed
[4] Savary S, Bregaglio S, Willocquet L, Gustafson D, Mason D’Croz D, Sparks A, et al. Crop health and its global impacts on the components of food security. Food Secur. 2017;9:311–27. 10.1007/s12571-017-0659-1.Search in Google Scholar
[5] Fraser EDG. Social vulnerability and ecological fragility: building bridges between social and natural sciences using the irish potato famine as a case study. Conserv Ecol. 2003;7:art9. 10.5751/ES-00534-070209.Search in Google Scholar
[6] Trippa D, Scalenghe R, Basso MF, Panno S, Davino S, Morone C, et al. Next-generation methods for early disease detection in crops. Pest Manag Sci. 2024;80:245–61. 10.1002/ps.7733.Search in Google Scholar PubMed
[7] Riley MB, Williamson MR, Maly O. Plant disease diagnosis. The American Phytopathological Society; 2002. https://www.apsnet.org/edcenter/apsnetfeatures/Pages/PDDiagnosis.aspx, accessed March 28, 2025.10.1094/PHI-I-2002-1021-01Search in Google Scholar
[8] Mutka AM, Bart RS. Image-based phenotyping of plant disease symptoms. Front Plant Sci. 2015;5:734. 10.3389/fpls.2014.00734.Search in Google Scholar PubMed PubMed Central
[9] Khakimov A, Salakhutdinov I, Omolikov A, Utaganov S. Traditional and current-prospective methods of agricultural plant diseases detection: A review. IOP Conf Ser: Earth Environ Sci. 2022;951:012002. 10.1088/1755-1315/951/1/012002.Search in Google Scholar
[10] Vincelli P, Tisserat N. Nucleic acid–based pathogen detection in applied plant pathology. Plant Dis. 2008;92:660–9. 10.1094/PDIS-92-5-0660.Search in Google Scholar PubMed
[11] Martinelli F, Scalenghe R, Davino S, Panno S, Scuderi G, Ruisi P, et al. Advanced methods of plant disease detection. A review. Agron Sustain Dev. 2015;35:1–25. 10.1007/s13593-014-0246-1.Search in Google Scholar
[12] Henson JM, French RC. The polymerase chain reaction and plant disease diagnosis. Annu Rev Phytopathol. 1993;31:81–109.10.1146/annurev.py.31.090193.000501Search in Google Scholar PubMed
[13] Ivanov AV, Safenkova IV, Zherdev AV, Dzantiev BB. The potential use of isothermal amplification assays for in-field diagnostics of plant pathogens. Plants. 2021;10:2424. 10.3390/plants10112424.Search in Google Scholar PubMed PubMed Central
[14] Motamedi MK, Saghafinia M, Karami A, Gill P. A review of the current isothermal amplification techniques: Applications, advantages and disadvantages. J Glob Infect Dis. 2011;3:293–302.10.4103/0974-777X.83538Search in Google Scholar
[15] Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science. 2017;356:438–42. 10.1126/science.aam9321.Search in Google Scholar PubMed PubMed Central
[16] Barrangou R, Marraffini LA. CRISPR-Cas systems: prokaryotes upgrade to adaptive immunity. Mol Cell. 2014;54:234–44. 10.1016/j.molcel.2014.03.011.Search in Google Scholar PubMed PubMed Central
[17] Westra ER, Buckling A, Fineran PC. CRISPR–Cas systems: beyond adaptive immunity. Nat Rev Microbiol. 2014;12:317–26. 10.1038/nrmicro3241.Search in Google Scholar PubMed
[18] Barrangou R, Horvath P. A decade of discovery: CRISPR functions and applications. Nat Microbiol. 2017;2:1–9. 10.1038/nmicrobiol.2017.92.Search in Google Scholar PubMed
[19] Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns S, et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18:67–83. 10.1038/s41579-019-0299-x.Search in Google Scholar PubMed PubMed Central
[20] Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, et al. An updated evolutionary classification of CRISPR–Cas systems. Nat Rev Microbiol. 2015;13:722–36. 10.1038/nrmicro3569.Search in Google Scholar PubMed PubMed Central
[21] Koonin EV, Makarova KS. Origins and evolution of CRISPR-Cas systems. Philos Trans R Soc B. 2019;374:20180087. 10.1098/rstb.2018.0087.Search in Google Scholar PubMed PubMed Central
[22] Jiang F, Doudna JA. CRISPR–Cas9 structures and mechanisms. Annu Rev Biophys. 2017;46:505–29. 10.1146/annurev-biophys-062215-010822.Search in Google Scholar PubMed
[23] Jiang F, Zhou K, Ma L, Gressel S, Doudna JA. A Cas9–guide RNA complex preorganized for target DNA recognition. Science. 2015;348:1477–81. 10.1126/science.aab1452.Search in Google Scholar PubMed
[24] Bortesi L, Zhu C, Zischewski J, Perez L, Bassié L, Nadi R, et al. Patterns of CRISPR/Cas9 activity in plants, animals and microbes. Plant Biotechnol J. 2016;14:2203–16. 10.1111/pbi.12634.Search in Google Scholar PubMed PubMed Central
[25] Nidhi S, Anand U, Oleksak P, Tripathi P, Lal JA, Thomas G, et al. Novel CRISPR–Cas systems: an updated review of the current achievements, applications, and future research perspectives. Int J Mol Sci. 2021;22:3327. 10.3390/ijms22073327.Search in Google Scholar PubMed PubMed Central
[26] Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163:759–71. 10.1016/j.cell.2015.09.038.Search in Google Scholar PubMed PubMed Central
[27] Burstein D, Harrington LB, Strutt SC, Probst AJ, Anantharaman K, Thomas BC, et al. New CRISPR–Cas systems from uncultivated microbes. Nature. 2017;542:237–41. 10.1038/nature21059.Search in Google Scholar PubMed PubMed Central
[28] Harrington LB, Burstein D, Chen JS, Paez-Espino D, Ma E, Witte IP, et al. Programmed DNA destruction by miniature CRISPR-Cas14 enzymes. Science. 2018;362:839–42. 10.1126/science.aav4294.Search in Google Scholar PubMed PubMed Central
[29] Koonin EV, Makarova KS, Zhang F. Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol. 2017;37:67–78. 10.1016/j.mib.2017.05.008.Search in Google Scholar PubMed PubMed Central
[30] Hillary VE, Ceasar SA. A review on the mechanism and applications of CRISPR/Cas9/Cas12/Cas13/Cas14 proteins utilized for genome engineering. Mol Biotechnol. 2023;65:311–25. 10.1007/s12033-022-00567-0.Search in Google Scholar PubMed PubMed Central
[31] Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, et al. RNA editing with CRISPR-Cas13. Science. 2017;358:1019–27. 10.1126/science.aaq0180.Search in Google Scholar PubMed PubMed Central
[32] Pardee K, Green AA, Takahashi MK, Braff D, Lambert G, Lee JW, et al. Rapid, low-cost detection of zika virus using programmable biomolecular components. Cell. 2016;165:1255–66. 10.1016/j.cell.2016.04.059.Search in Google Scholar PubMed
[33] Guk K, Keem JO, Hwang SG, Kim H, Kang T, Lim EK, et al. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens Bioelectron. 2017;95:67–71. 10.1016/j.bios.2017.04.016.Search in Google Scholar PubMed
[34] Kim H, Lee S, Seo HW, Kang B, Moon J, Lee KG, et al. Clustered regularly interspaced short palindromic repeats-mediated surface-enhanced raman scattering assay for multidrug-resistant bacteria. ACS Nano. 2020;14:17241–53. 10.1021/acsnano.0c07264.Search in Google Scholar PubMed
[35] Xu X, Luo T, Gao J, Lin N, Li W, Xia X, et al. CRISPR-Assisted DNA Detection: A Novel dCas9-Based DNA Detection Technique. CRISPR J. 2020;3:487–502. 10.1089/crispr.2020.0041.Search in Google Scholar PubMed
[36] Zhang Y, Qian L, Wei W, Wang Y, Wang B, Lin P, et al. Paired design of dCas9 as a Systematic platform for the detection of featured nucleic acid sequences in pathogenic strains. ACS Synth Biol. 2017;6:211–6. 10.1021/acssynbio.6b00215.Search in Google Scholar PubMed
[37] Huang M, Zhou X, Wang H, Xing D. Clustered regularly interspaced short palindromic repeats/cas9 triggered isothermal amplification for site-specific nucleic acid detection. Anal Chem. 2018;90(3):2193–200. 10.1021/acs.analchem.7b04542.Search in Google Scholar PubMed
[38] Wang T, Zhang H, Zhu H. CRISPR technology is revolutionizing the improvement of tomato and other fruit crops. Hortic Res. 2019;6:77. 10.1038/s41438-019-0159-x.Search in Google Scholar PubMed PubMed Central
[39] Koo B, Kim DE, Kweon J, Jin CE, Kim SH, Kim Y, et al. CRISPR/dCas9-mediated biosensor for detection of tick-borne diseases. Sens Actuators B: Chem. 2018;273:316–21. 10.1016/j.snb.2018.06.069.Search in Google Scholar PubMed PubMed Central
[40] Wang X, Xiong E, Tian T, Cheng M, Lin W, Wang H, et al. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay. ACS Nano. 2020;14:2497–508. 10.1021/acsnano.0c00022.Search in Google Scholar PubMed
[41] Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360:436–9. 10.1126/science.aar6245.Search in Google Scholar PubMed PubMed Central
[42] Zhao L, Qiu M, Li X, Yang J, Li J. CRISPR-Cas13a system: A novel tool for molecular diagnostics. Front Microbiol. 2022;13:1060947. 10.3389/fmicb.2022.1060947.Search in Google Scholar PubMed PubMed Central
[43] Feng W, Zhang H, Le XC. Signal amplification by the trans-cleavage activity of CRISPR-cas systems: kinetics and performance. Anal Chem. 2023;95:206–17. 10.1021/acs.analchem.2c04555.Search in Google Scholar PubMed PubMed Central
[44] Gunitseva N, Evteeva M, Borisova A, Patrushev M, Subach F. RNA-dependent RNA targeting by CRISPR-Cas systems: Characterizations and applications. Int J Mol Sci. 2023;24:6894. 10.3390/ijms24086894.Search in Google Scholar PubMed PubMed Central
[45] Petri K, Pattanayak V. SHERLOCK and DETECTR open a new frontier in molecular diagnostics. CRISPR J. 2018;1:209–11. 10.1089/crispr.2018.29018.kpe.Search in Google Scholar PubMed
[46] Liu L, Li X, Ma J, Li Z, You L, Wang J, et al. The molecular architecture for RNA-Guided RNA cleavage by Cas13a. Cell. 2017;170(714–726):e10. 10.1016/j.cell.2017.06.050.Search in Google Scholar PubMed
[47] Kellner MJ, Koob JG, Gootenberg JS, Abudayyeh OO, Zhang F. SHERLOCK: nucleic acid detection with CRISPR nucleases. Nat Protoc. 2019;14:2986–3012. 10.1038/s41596-019-0210-2.Search in Google Scholar PubMed PubMed Central
[48] Gootenberg JS, Abudayyeh OO, Kellner MJ, Joung J, Collins JJ, Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360:439–44. 10.1126/science.aaq0179.Search in Google Scholar PubMed PubMed Central
[49] Nguyen LT, Smith BM, Jain PK. Enhancement of trans-cleavage activity of Cas12a with engineered crRNA enables amplified nucleic acid detection. Nat Commun. 2020;11:4906. 10.1038/s41467-020-18615-1.Search in Google Scholar PubMed PubMed Central
[50] Kim H, Lee S, Yoon J, Song J, Park HG. CRISPR/Cas12a collateral cleavage activity for simple and rapid detection of protein/small molecule interaction. Biosens Bioelectron. 2021;194:113587. 10.1016/j.bios.2021.113587.Search in Google Scholar PubMed
[51] Zeng D, Jiao J, Mo T. Combination of nucleic acid amplification and CRISPR/Cas technology in pathogen detection. Front Microbiol. 2024;15:15. 10.3389/fmicb.2024.1355234.Search in Google Scholar PubMed PubMed Central
[52] Kim S, Ji S, Koh HR. CRISPR as a diagnostic tool. Biomolecules. 2021;11:1162. 10.3390/biom11081162.Search in Google Scholar PubMed PubMed Central
[53] Li S-Y, Cheng Q-X, Wang J-M, Li XY, Zhang ZL, Gao S, et al. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018;4:1–4. 10.1038/s41421-018-0028-z.Search in Google Scholar PubMed PubMed Central
[54] Li L, Li S, Wu N, Wu J, Wang G, Zhao G, et al. HOLMESv2: A CRISPR-Cas12b-assisted platform for nucleic acid detection and DNA methylation quantitation. ACS Synth Biol. 2019;8:2228–37. 10.1021/acssynbio.9b00209.Search in Google Scholar PubMed
[55] Xu T, Yang X, Feng X, Luo H, Luo C, Jia MA, et al. Sensitive and visual detection of brassica yellows virus using reverse transcription loop-mediated isothermal amplification-coupled CRISPR-Cas12 assay. Phytopathology®. 2024;114:474–83. 10.1094/PHYTO-06-23-0195-R.Search in Google Scholar PubMed
[56] Chang W, Liu W, Liu Y, Zhan F, Chen H, Lei H, et al. Colorimetric detection of nucleic acid sequences in plant pathogens based on CRISPR/Cas9 triggered signal amplification. Microchim Acta. 2019;186:243. 10.1007/s00604-019-3348-2.Search in Google Scholar PubMed
[57] Guo H, Zhang Y, Kong F, Wang C, Chen S, Wang J, et al. A Cas12a-based platform combined with gold nanoparticles for sensitive and visual detection of Alternaria solani. Ecotoxicol Environ Saf. 2023;263:115220. 10.1016/j.ecoenv.2023.115220.Search in Google Scholar PubMed
[58] Pal T, Liu Z, Chen J. CIMNE-CRISPR: A novel amplification-free diagnostic for rapid early detection of African Swine Fever Virus. Biosens Bioelectron. 2025;273:117154. 10.1016/j.bios.2025.117154.Search in Google Scholar PubMed PubMed Central
[59] Asiello PJ, Baeumner AJ. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip. 2011;11:1420–30. 10.1039/c0lc00666a.Search in Google Scholar PubMed
[60] Bai J. Cas12a-based on-site and rapid nucleic acid detection of African swine fever. Front Microbiol. 2019;10:2830. 10.3389/fmicb.2019.02830.Search in Google Scholar PubMed PubMed Central
[61] Zheng F, Chen Z, Li J, Wu R, Zhang B, Nie G, et al. A highly sensitive CRISPR-empowered surface plasmon resonance sensor for diagnosis of inherited diseases with femtomolar-level real-time quantification. Adv Sci. 2022;9:2105231. 10.1002/advs.202105231.Search in Google Scholar PubMed PubMed Central
[62] Myhrvold C, Freije CA, Gootenberg JS, Abudayyeh OO, Metsky HC, Durbin AF, et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science. 2018;360:444–8. 10.1126/science.aas8836.Search in Google Scholar PubMed PubMed Central
[63] Li H, Xie Y, Chen F, Bai H, Xiu L, Zhou X, et al. Amplification-free CRISPR/Cas detection technology: challenges, strategies, and perspectives. Chem Soc Rev. 2023;52:361–82. 10.1039/D2CS00594H.Search in Google Scholar
[64] Shinoda H, Taguchi Y, Nakagawa R, Makino A, Okazaki S, Nakano M, et al. Amplification-free RNA detection with CRISPR–Cas13. Commun Biol. 2021;4:1–7. 10.1038/s42003-021-02001-8.Search in Google Scholar PubMed PubMed Central
[65] Wang K, Yin H, Li S, Wan Y, Xiao M, Yuan X, et al. Quantitative detection of circular RNA and microRNA at point-of-care using droplet digital CRISPR/Cas13a platform. Biosens Bioelectron. 2025;267:116825. 10.1016/j.bios.2024.116825.Search in Google Scholar PubMed
[66] Cheng W-C, Horn T, Zayats M, Rizk G, Major S, Zhu H, et al. Ultra-sensitive and rapid detection of nucleic acids and microorganisms in body fluids using single-molecule tethering. Nat Commun. 2020;11:4774. 10.1038/s41467-020-18574-7.Search in Google Scholar PubMed PubMed Central
[67] Yuan B, Yuan C, Li L, Long M, Chen Z. Application of the CRISPR/Cas system in pathogen detection: A Review. Molecules. 2022;27:6999. 10.3390/molecules27206999.Search in Google Scholar PubMed PubMed Central
[68] Khambhati K, Bhattacharjee G, Singh V. Current progress in CRISPR‐based diagnostic platforms. J Cell Biochem. 2019;120:2721–5. 10.1002/jcb.27690.Search in Google Scholar PubMed PubMed Central
[69] Zhang X. Development of CRISPR-mediated nucleic acid detection technologies and their applications in the livestock industry. Genes. 2022;13:2007. 10.3390/genes13112007.Search in Google Scholar PubMed PubMed Central
[70] Shanmugaraj C, Kumar HMA, Jaiganesh V, Biswas MK, Gangaraj R. CRISPR-Cas technology: an emerging opportunity for precise identification of diseases in plants. J Plant Dis Prot. 2025;132:39. 10.1007/s41348-024-01025-6.Search in Google Scholar
[71] Tripathi L, Ntui VO, Tripathi JN, Kumar PL. Application of CRISPR/Cas for diagnosis and management of viral diseases of banana. Front Microbiol. 2021;11:609784. 10.3389/fmicb.2020.609784.Search in Google Scholar PubMed PubMed Central
[72] Zhang W, Jiao Y, Ding C, Shen L, Li Y, Yu Y, et al. Rapid detection of tomato spotted wilt virus with Cas13a in tomato and frankliniella occidentalis. Front Microbiol. 2021;12:745173. 10.3389/fmicb.2021.745173.Search in Google Scholar PubMed PubMed Central
[73] Chen Q, Wu J, Tang C, Wang Y. CRISPR-based platforms for the specific and dual detection of defoliating/nondefoliating strains of. Pest Manag Sci. 2024;80:2042–52. 10.1002/ps.7940.Search in Google Scholar PubMed
[74] Hak H, Ostendorp S, Reza A, Ishgur Greenberg S, Pines G, Kehr J, et al. Rapid on-site detection of crop RNA viruses using CRISPR/Cas13a. J Exp Bot. 2024;erae495. 10.1093/jxb/erae495.Search in Google Scholar PubMed
[75] Lefkowitz EJ, Dempsey DM, Hendrickson RC, Orton RJ, Siddell SG, Smith DB. Virus taxonomy: the database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018;46:D708–17. 10.1093/nar/gkx932.Search in Google Scholar PubMed PubMed Central
[76] Alon DM, Hak H, Bornstein M, Pines G, Spiegelman Z. CRISPR-cas12a assay for detecting ToMV and ToBRFV. Plants. 2021;10:1256. 10.3390/plants10061256.Search in Google Scholar PubMed PubMed Central
[77] Bernabé-Orts JM, Hernando Y, Aranda MA. Toward a CRISPR-based point-of-care test for tomato brown rugose fruit virus detection. PhytoFrontiersTM. 2022;2:92–100. 10.1094/PHYTOFR-08-21-0053-TA.Search in Google Scholar
[78] Mahas A, Hassan N, Aman R, Marsic T, Wang Q, Ali Z, et al. LAMP-coupled CRISPR–Cas12a module for rapid and sensitive detection of plant DNA viruses. Viruses. 2021;13:466. 10.3390/v13030466.Search in Google Scholar PubMed PubMed Central
[79] Ramachandran V, Weiland JJ, Bolton MD. CRISPR-based isothermal next-generation diagnostic method for virus detection in sugarbeet. Front Microbiol. 2021;12:12. 10.3389/fmicb.2021.679994.Search in Google Scholar PubMed PubMed Central
[80] Aman R. Efficient, rapid, and sensitive detection of plant RNA viruses with one-pot RT-RPA–CRISPR/Cas12a assay. Front Microbiol. 2020;11:610872. 10.3389/fmicb.2020.610872.Search in Google Scholar PubMed PubMed Central
[81] Lei R, Kuang R, Peng X, Jiao Z, Zhao Z, Cong H, et al. Portable rapid detection of maize chlorotic mottle virus using RT-RAA/CRISPR-Cas12a based lateral flow assay. Front Plant Sci. 2023;14:1088544. 10.3389/fpls.2023.1088544.Search in Google Scholar PubMed PubMed Central
[82] Jiao J, Kong K, Han J, Song S, Bai T, Song C, et al. Field detection of multiple RNA viruses/viroids in apple using a CRISPR/Cas12a-based visual assay. Plant Biotechnol J. 2021;19:394–405. 10.1111/pbi.13474.Search in Google Scholar PubMed PubMed Central
[83] Kim H-J, Cho I-S, Choi S-R, Jeong RD. Identification of an isolate of citrus tristeza virus by nanopore sequencing in korea and development of a CRISPR/Cas12a-based assay for rapid visual detection of the virus. Phytopathology®. 2024;114:1421–8. 10.1094/PHYTO-10-23-0354-R.Search in Google Scholar PubMed
[84] Xu T, Yang X, Feng X, Luo H, Luo C, Jia MA, et al. Sensitive and visual detection of brassica yellows virus using reverse transcription loop-mediated isothermal amplification-coupled CRISPR-Cas12 assay. Phytopathology. 2024;114:474–83. 10.1094/PHYTO-06-23-0195-R.Search in Google Scholar PubMed
[85] Marqués M-C, Sánchez-Vicente J, Ruiz R, Montagud-Martínez R, Márquez-Costa R, Gómez G, et al. Diagnostics of infections produced by the Plant Viruses TMV, TEV, and PVX with CRISPR-Cas12 and CRISPR-Cas13. ACS Synth Biol. 2022;11:2384–93. 10.1021/acssynbio.2c00090.Search in Google Scholar PubMed PubMed Central
[86] Zhu L, Di R, Huang Z, Lu M, Yin L, Huang Y, et al. Cas-mCfLAMP: A multiplex rapid visualization assay for sugarcane pathogens based on labeled LAMP and CRISPR/Cas12a. Microchem J. 2024;199:109993. 10.1016/j.microc.2024.109993.Search in Google Scholar
[87] Kuang R, Lei R, Li M, Sun X, Duan W, Yang Y, et al. Rapid diagnosis of Diaporthe helianthi in sunflower using RPA/CRISPR-Cas12 and lateral flow assay. Phytopathol Res. 2025;7:17. 10.1186/s42483-024-00310-4.Search in Google Scholar
[88] Changtor P, Jaroenpol W, Buddhachat K, Wattanachaiyingcharoen W, Yimtragool N. Rapid detection of Sclerotium rolfsii causing dry stem and root rot disease in cassava by recombinase polymerase amplification technique (RPA) combined with CRISPR/Cas12a. Crop Prot. 2023;172:106340. 10.1016/j.cropro.2023.106340.Search in Google Scholar
[89] Wang Q, Qin M, Coleman JJ, Shang W, Hu X. Rapid and sensitive detection of Verticillium dahilae from complex samples using CRISPR/Cas12a technology combined with RPA. Plant Dis. 2023;107:1664–9. 10.1094/PDIS-08-22-1790-SC.Search in Google Scholar PubMed
[90] Hu J-J, Liu D, Cai M-Z, Zhou Y, Yin W-X, Luo C-X. One-pot assay for rapid detection of benzimidazole resistance in Venturia carpophila by combining RPA and CRISPR/Cas12a. J Agric Food Chem. 2023;71(3):1381–90. 10.1021/acs.jafc.2c06549.Search in Google Scholar PubMed
[91] Chen Z, Yang X, Xia H, Wu C, Yang J, Dai T. A frontline, rapid, nucleic acid-based Fusarium circinatum detection system Using CRISPR/Cas12a combined with recombinase polymerase amplification. Plant Dis. 2023;107:1902–10. 10.1094/PDIS-05-22-1234-RE.Search in Google Scholar PubMed
[92] Zhang J, Liang X, Zhang H, Ishfaq S, Xi K, Zhou X, et al. Rapid and sensitive detection of toxigenic Fusarium asiaticum integrating recombinase polymerase amplification, CRISPR/Cas12a, and lateral flow techniques. Int J Mol Sci. 2023;24:14134. 10.3390/ijms241814134.Search in Google Scholar PubMed PubMed Central
[93] Mu Y, Zhang C, Li T, Jin FJ, Sung YJ, Oh HM, et al. Development and applications of CRISPR/Cas9-based genome editing in Lactobacillus. Int J Mol Sci. 2022;23:12852. 10.3390/ijms232112852.Search in Google Scholar PubMed PubMed Central
[94] Lei R, Li Y, Li L, Wang J, Cui Z, Ju R, et al. A CRISPR/Cas12a-based portable platform for rapid detection of Leptosphaeria maculans in Brassica crops. Front Plant Sci. 2022;13. 10.3389/fpls.2022.976510.Search in Google Scholar PubMed PubMed Central
[95] Zhang Y, Zhang Y, Xie K. Evaluation of CRISPR/Cas12a-based DNA detection for fast pathogen diagnosis and GMO test in rice. Mol Breed. 2020;40:11. 10.1007/s11032-019-1092-2.Search in Google Scholar
[96] Sun X, Lei R, Zhang H, Chen W, Jia Q, Guo X, et al. Rapid and sensitive detection of two fungal pathogens in soybeans using the recombinase polymerase amplification/CRISPR-Cas12a method for potential on-site disease diagnosis. Pest Manag Sci. 2024;80:1168–81. 10.1002/ps.7847.Search in Google Scholar PubMed
[97] Shin K, Kwon S-H, Lee S-C, Moon YE. Sensitive and rapid detection of citrus scab using an RPA-CRISPR/Cas12a system combined with a lateral flow assay. Plants. 2021;10:2132. 10.3390/plants10102132.Search in Google Scholar PubMed PubMed Central
[98] Liu Y, Ma L, Liu W, Xie L, Wu Q, Wang Y, et al. RPA-CRISPR/Cas12a combined with rolling circle amplification-enriched dnazyme: a homogeneous photothermal sensing strategy for plant pathogens. J Agric Food Chem. 2023;71(11):4736–44. 10.1021/acs.jafc.2c07965.Search in Google Scholar PubMed
[99] Guo Y, Xia H, Dai T, Liy T, Shamoun SF, Cui-Ping W. CRISPR/Cas12a-based approaches for efficient and accurate detection of Phytophthora ramorum. Front Cell Infect Microbiol. 2023;13:1218105. 10.3389/fcimb.2023.1218105.Search in Google Scholar PubMed PubMed Central
[100] Guo Y, Xia H, Dai T, Liu T. RPA-CRISPR/Cas12a mediated isothermal amplification for visual detection of Phytophthora sojae. Front Cell Infect Microbiol. 2023;13:1208837. 10.3389/fcimb.2023.1208837.Search in Google Scholar PubMed PubMed Central
[101] Zhou L, Kong K, Han J, Song S, Bai T, Song C, et al. CRISPR-Cas12a-based detection of Phytophthora cambivora in chestnut trees. Plant Pathol. 2023;18(2):394–405. 10.1111/pbi.13474.Search in Google Scholar PubMed PubMed Central
[102] Wang Z, Cheng W, Dong Z, Yao X, Deng X, Ou C. A CRISPR/LbCas12a-based method for detection of bacterial fruit blotch pathogens in watermelon. Microbiol Spectr. 2024;12(3):e03846–23. 10.1128/spectrum.03846-23.Search in Google Scholar PubMed PubMed Central
[103] Farrall T, Abeynayake SW, Webster W, Fiorito S, Dinsdale A, Whattam M, et al. Development of a rapid, accurate, and field deployable LAMP-CRISPR-Cas12a integrated assay for Xylella fastidiosa detection and surveillance. Australas Plant Pathol. 2024;53:115–20. 10.1007/s13313-023-00954-4.Search in Google Scholar
[104] Wheatley MS, Duan Y-P, Yang Y. Highly sensitive and rapid detection of citrus huanglongbing pathogen (‘Candidatus Liberibacter asiaticus’) Using Cas12a-based methods. Phytopathology®. 2021;111:2375–82. 10.1094/PHYTO-09-20-0443-R.Search in Google Scholar PubMed
[105] Wheatley MS, Wang Q, Wei W, Bottner-Parker KD, Zhao Y, Yang Y. Cas12a-based diagnostics for potato purple top disease complex associated with infection by ‘Candidatus Phytoplasma trifolii’-related strains. Plant Dis. 2022;106:2039–45. 10.1094/PDIS-09-21-2119-RE.Search in Google Scholar PubMed
[106] Arnaout R, Lee RA, Lee GR, Callahan C, Cheng A, Yen CF, et al. The limit of detection matters: the case for benchmarking severe acute respiratory syndrome coronavirus 2 testing. Clin Infect Dis. 2021;73:e3042–6. 10.1093/cid/ciaa1382.Search in Google Scholar PubMed PubMed Central
[107] Rather M, Wani S, Rashid S, Iralu N, Roy A, Pappu HR, et al. Detection of plant viruses by CRISPR-Cas13a system. In: Hamid A, Ali G, Shikari A, editors. Detection of plant viruses: Advanced techniques. New York, NY: Springer US; 2025. p. 199–204. 10.1007/978-1-0716-4390-7_30.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Optimizing carrageenan–citric acid synergy in mango gummies using response surface methodology
- The strategic role of agricultural vocational training in sustainable local food systems
- Agricultural planning grounded in regional rainfall patterns in the Colombian Orinoquia: An essential step for advancing climate-adapted and sustainable agriculture
- Perspectives of master’s graduates on organic agriculture: A Portuguese case study
- Developing a behavioral model to predict eco-friendly packaging use among millennials
- Government support during COVID-19 for vulnerable households in Central Vietnam
- Citric acid–modified coconut shell biochar mitigates saline–alkaline stress in Solanum lycopersicum L. by modulating enzyme activity in the plant and soil
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society
Articles in the same Issue
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Optimizing carrageenan–citric acid synergy in mango gummies using response surface methodology
- The strategic role of agricultural vocational training in sustainable local food systems
- Agricultural planning grounded in regional rainfall patterns in the Colombian Orinoquia: An essential step for advancing climate-adapted and sustainable agriculture
- Perspectives of master’s graduates on organic agriculture: A Portuguese case study
- Developing a behavioral model to predict eco-friendly packaging use among millennials
- Government support during COVID-19 for vulnerable households in Central Vietnam
- Citric acid–modified coconut shell biochar mitigates saline–alkaline stress in Solanum lycopersicum L. by modulating enzyme activity in the plant and soil
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society

