The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
Abstract
C22H14Br3N7S, H2O, triclinic, P
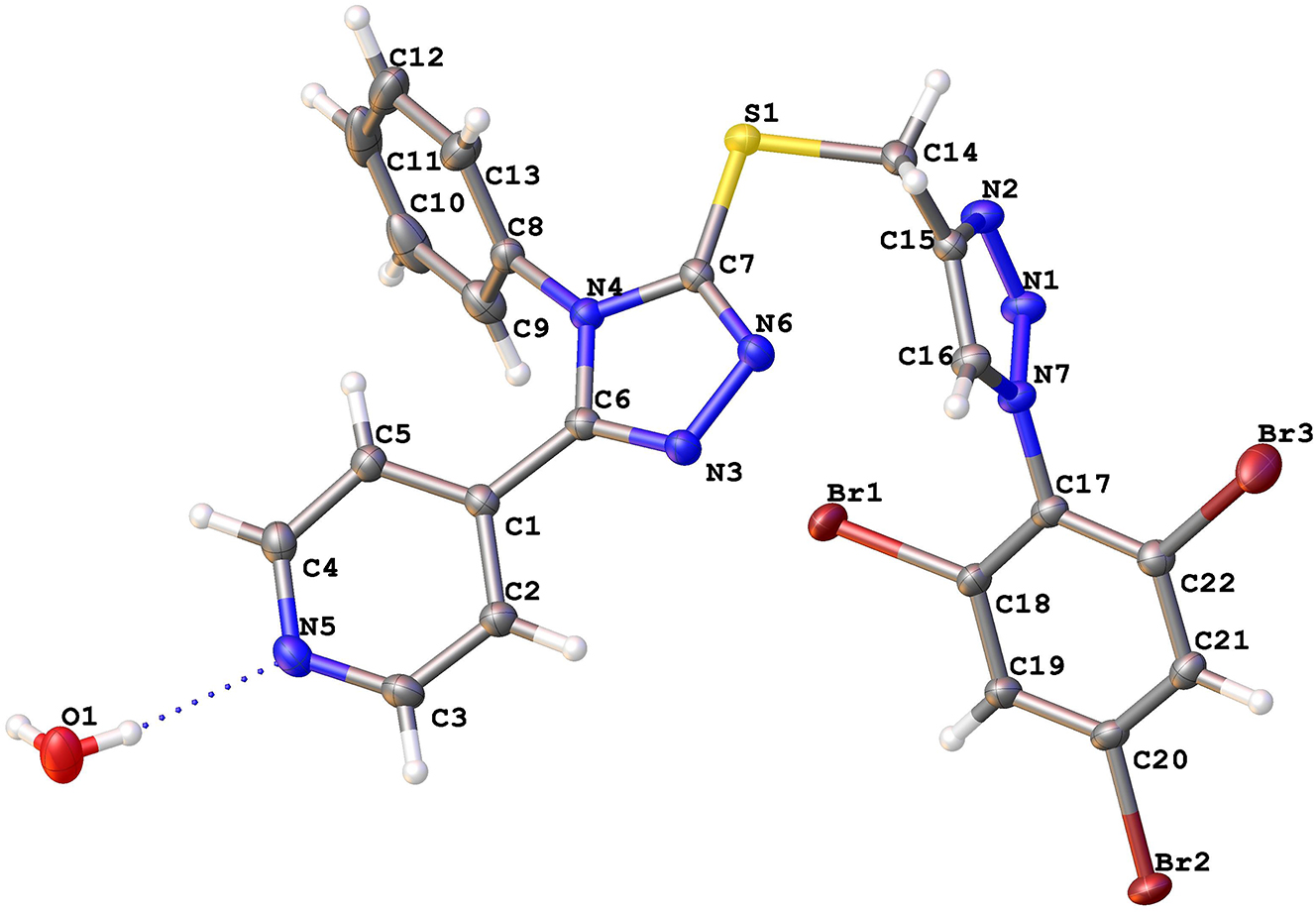
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless needle |
| Size: | 0.36 × 0.36 × 0.13 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 5.12 mm−1 |
| Diffractometer, scan mode: | Bruker D8-AVANCE, φ and ω |
| θ max, completeness: | 33.1°, >99 % |
| N(hkl) measured, N(hkl)unique, R int: | 65,262, 9136, 0.062 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 7,458 |
| N(param)refined: | 314 |
| Programs: | Bruker 1 , Olex2 2 , SHELX 3 , 4 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso */U eq |
|---|---|---|---|---|
| Br1 | 0.43362 (2) | 0.36166 (2) | 0.07683 (2) | 0.03177 (6) |
| Br2 | −0.13900 (2) | 0.28661 (2) | 0.02177 (2) | 0.02894 (5) |
| Br3 | 0.31162 (3) | −0.18387 (2) | 0.17679 (2) | 0.03681 (6) |
| S1 | 0.85811 (5) | 0.11124 (5) | 0.30978 (3) | 0.02185 (8) |
| O1 | 0.1579 (2) | 1.25720 (19) | 0.50739 (12) | 0.0405 (4) |
| H1A | 0.177 (4) | 1.173 (4) | 0.484 (2) | 0.061* |
| H1B | 0.223 (4) | 1.292 (4) | 0.482 (2) | 0.061* |
| N1 | 0.64699 (17) | −0.01594 (17) | 0.09509 (10) | 0.0229 (3) |
| N2 | 0.75981 (17) | −0.05054 (17) | 0.14230 (10) | 0.0227 (3) |
| N3 | 0.41667 (17) | 0.37427 (16) | 0.40418 (10) | 0.0208 (3) |
| N4 | 0.65443 (16) | 0.39942 (15) | 0.35044 (9) | 0.0176 (2) |
| N5 | 0.24810 (19) | 0.94764 (17) | 0.43955 (10) | 0.0256 (3) |
| N6 | 0.53061 (17) | 0.23064 (16) | 0.38249 (10) | 0.0216 (3) |
| N7 | 0.50119 (16) | 0.03971 (16) | 0.15111 (9) | 0.0193 (2) |
| C1 | 0.41213 (19) | 0.63718 (17) | 0.40209 (10) | 0.0180 (3) |
| C2 | 0.2410 (2) | 0.69992 (19) | 0.41646 (12) | 0.0220 (3) |
| H2 | 0.1776 | 0.6380 | 0.4142 | 0.026* |
| C3 | 0.1662 (2) | 0.8540 (2) | 0.43407 (13) | 0.0256 (3) |
| H3 | 0.0504 | 0.8956 | 0.4428 | 0.031* |
| C4 | 0.4108 (2) | 0.88549 (19) | 0.42722 (11) | 0.0234 (3) |
| H4 | 0.4707 | 0.9496 | 0.4318 | 0.028* |
| C5 | 0.4979 (2) | 0.73326 (19) | 0.40810 (11) | 0.0214 (3) |
| H5 | 0.6137 | 0.6954 | 0.3993 | 0.026* |
| C6 | 0.49144 (18) | 0.47352 (18) | 0.38505 (10) | 0.0180 (3) |
| C7 | 0.67038 (19) | 0.24937 (18) | 0.35057 (10) | 0.0183 (3) |
| C8 | 0.78093 (19) | 0.45655 (18) | 0.30950 (11) | 0.0199 (3) |
| C9 | 0.7648 (2) | 0.5382 (2) | 0.23347 (12) | 0.0273 (4) |
| H9 | 0.6695 | 0.5593 | 0.2095 | 0.033* |
| C10 | 0.8904 (3) | 0.5887 (2) | 0.19275 (15) | 0.0387 (5) |
| H10 | 0.8802 | 0.6472 | 0.1414 | 0.046* |
| C11 | 1.0302 (3) | 0.5536 (2) | 0.22733 (17) | 0.0436 (6) |
| H11 | 1.1164 | 0.5870 | 0.1989 | 0.052* |
| C12 | 1.0457 (3) | 0.4706 (3) | 0.30250 (17) | 0.0401 (5) |
| H12 | 1.1428 | 0.4464 | 0.3251 | 0.048* |
| C13 | 0.9198 (2) | 0.4218 (2) | 0.34565 (14) | 0.0288 (4) |
| H13 | 0.9286 | 0.3664 | 0.3982 | 0.035* |
| C14 | 0.7885 (2) | −0.04499 (18) | 0.29702 (12) | 0.0215 (3) |
| H14A | 0.8847 | −0.1385 | 0.2824 | 0.026* |
| H14B | 0.7228 | −0.0615 | 0.3531 | 0.026* |
| C15 | 0.68838 (19) | −0.01783 (17) | 0.22807 (11) | 0.0189 (3) |
| C16 | 0.52272 (19) | 0.04105 (19) | 0.23379 (11) | 0.0205 (3) |
| H16 | 0.4411 | 0.0752 | 0.2846 | 0.025* |
| C17 | 0.35256 (18) | 0.09539 (18) | 0.11971 (10) | 0.0187 (3) |
| C18 | 0.30365 (19) | 0.23936 (18) | 0.08337 (11) | 0.0199 (3) |
| C19 | 0.15813 (19) | 0.29708 (19) | 0.05335 (12) | 0.0217 (3) |
| H19 | 0.1258 | 0.3948 | 0.0282 | 0.026* |
| C20 | 0.06104 (18) | 0.20782 (19) | 0.06117 (11) | 0.0198 (3) |
| C21 | 0.10396 (19) | 0.06431 (19) | 0.09682 (11) | 0.0208 (3) |
| H21 | 0.0353 | 0.0055 | 0.1013 | 0.025* |
| C22 | 0.2510 (2) | 0.00968 (18) | 0.12574 (11) | 0.0200 (3) |
1 Source of materials
The title compound was prepared as follows: (438 mg, 1.5 mmol, 1.0 eq) from 4-(4-phenyl-5-(prop-2-yn-1-ylthio)-4H-1,2,4-triazol-3-yl)pyridine, and prepared according to the literature, 5 was dissolved and stirred in 10.0 mL of DMF. To this solution, 2-azido-1,3,5-tribromobenzene (692 mg, 1.95 mmol, 1.3 eq) along with sodium ascorbate (150 mg, 0.75 mmol) and CuSO4 ⋅ 5H2O (60 mg, 0.24 mmol) were added. The mixture was stirred overnight, and the reaction was monitored by TLC. The reaction was worked out by evaporation of the solvent under reduced pressure and the residue was subjected to column chromatography to produce the final compounds which was crystallized from methanol-dichloromethane. 5 Melting point 138 °C–139 °C. 1 H-NMR (DMSO‑d 6): δ (ppm) 8.70–8.50 (2H, m, ArH), 8.42 (1H, s, triazole H), 8.24 (2H, s, ArH), 7.65–7.55 (3H, m, ArH), 7.50–7.44 (2H, m, ArH), 7.35–7.25 (2H, m, ArH), 4.62 (2H, s, SCH2); 13 C-NMR (125 MHz, DMSO‑d 6); δ 152.82, 152.75, 150.64, 143.15, 135.48, 135.40, 134.26, 133.83, 131.05, 130.72, 128.09, 126.50, 125.67, 123.75, 27.25; Anal. Calcd. (%) for C22H14Br3N7S: C, 40.77; H, 2.18; N, 15.13; Found: C, 40.75; H, 2.15; N, 15.10. ESI-MS 646.86 (M+) Calcd. for C22H14Br3N7S.
2 Experimental details
All chemicals and solvents were used as purchased without further purifications. The uncorrected melting point was determined using an electrothermal digital melting-point apparatus. The NMR spectra were recorded at room temperature in DMSO‑d 6 solution on a Bruker Avance 500 MHz NMR spectrometer. Single crystal of C22H14Br3N7S was obtained through crystallization of the pure compound from slow evaporation of methanol-dichloromethane. The collected frames were integrated with the Bruker SAINT software package using a narrow-frame algorithm. Data corrections were performed for absorption effects using the multi-scan method (SADABS). The structure was solved and refined using the Bruker SHELXTL 3 software package. Using Olex2, 2 the structure was further refined with the with the ShelXL 4 refinement package using least squares minimization. All H atoms bonded to C atoms were refined as riding, with C–H distances of 0.93 Å (for aromatic ring).
3 Comment
Significant attention in synthetic chemistry was attributed to heterocyclic system with poly nitrogen containing compounds. 6 , 7 , 8 Among these heterocyclic compounds, 1,2,4 and 1,2,3-triazole systems had proven to be of special interest due to the established biological activities and their existence in many modern drugs such as ribavirin, fluconazole, and tazobuctum which are currently used as antiviral, antifungals, and anti-bacterial activities, respectively. 9 Diverse compounds containing these two moieties are proven to have anticancer activities. 10 , 11 In a continuation of our group interest in design and synthesize new biologically active system, 12 , 13 , 14 , 15 , 16 we report the modification of both 1,2,4 and 1,2,3-triazole moieties tethered by the sulfur atom, 5 the construction of the 1,2,3-triazole was successfully achieved via Cu(I) catalyzed click chemistry of the tribromophenylazide, which was prepared from the aniline derivative according to the published work, 17 and the propargyl group constructed on the 1,2,4-triazole moiety prepared as described. 5 Herein, we are reporting on the X-ray structure of the title compound.
The measurement shows that the crystal structure consists of the C22H14Br3N7S monomeric molecule with one molecule of lattice water. The asymmetric unit shows one molecular unit, in which all bond lengths and angles are in normal ranges. 18 The compound composed of five unsaturated rings, triazole and phenyl rings, twice each, and one pyridyl ring. The phenyl and pyridyl substituents on the 1,2,4-triazole ring are about perpendicular (68.37(5)°) to each other due to steric repulsion between rings. The pyridyl group shows angle of 19.09(7)° with the normal plane of the 1,2,4-triazole ring. On the other hand, the phenyl ring, attached to N4, shows an angle of 66.45(7)° with the normal plane the 1,2,4-triazole ring. Conversely, in the 1,2,3-triazole ring the substituted phenyl ring, attached to N7 forms an angle of 79.66(6)° with the normal plane of the 1,2,3-triazole. The N–N [N3–N6: 1.390(2), N1–N2: 1.306, N1–N7: 1.362 Å] and C–N [C6–N4: 1.382, C6–N3: 1.315, C7–N4: 1.370, C7–N6: 1.313, C15–N2: 1.372, C16–N7: 1.351 Å] bond lengths of both triazole rings are within the values reported for N–N and C–N in triazole rings. The substituents on sulfur atom (C7–S1–C14) show a normal bent angle of 99.29(8)°. Lattice water molecule link two moieties of the compound via hydrogen bonds with the pyridyl nitrogen (N4) with a distance of 2.115(4) Å and with the N3 (+x, 1 + y, +z) with a distance of 2.243 Å. Furthermore, the molecular packing of the title compound shows interaction between C20–Br2⋯H19 (−x, 1 − y, −z) with a distance 3.0913(7) Å to form centrosymmetric dimers.
Acknowledgments
ME acknowledge University of Sharjah, for financial support (grant No. 21021440109). Part of this work has been carried out during sabbatical leave granted to MAK from the University of Jordan during the academic year 2021–2022.
-
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: University of Sharjah (grant no. 21021440109).
References
1. Bruker. APEX4, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2021.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8. https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. El-Naggar, M.; Hasan, K.; Khanfar, M.; Shehadi, I. A.; El-Awady, R.; Negm El-Dein, A.; Abdelmonsef, A. H.; Al-Qawasmeh, R. A. Synthesis, Biological Assessment and Molecular Docking Study of New Sulfur-Linked 1,2,4-Triazole and 1,2,3-Triazole Hybrid Derivatives as Potential DNA Gyrase Inhibitors. Z. Naturforsch. 2024, 79, 419–429.10.1515/znb-2024-0012Search in Google Scholar
6. Opsomer, T.; Dehaen, W. Triazoles. In Comprehensive Heterocyclic Chemistry IV; Black, D. S.; Cossy, J.; Stevens, C. V., Eds.; Elsevier: Oxford, 2022; pp. 78–121.10.1016/B978-0-12-409547-2.14854-1Search in Google Scholar
7. Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M. L.; Prim, D. Recent Advances in the Chemistry of 1,2,4-Triazoles: Synthesis, Reactivity and Biological Activities. Tetrahedron Lett. 2021, 86, 153518. https://doi.org/10.1016/j.tetlet.2021.153518.Search in Google Scholar
8. Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E. A.; Cravotto, G. Benefits Applications of Microwave-Assisted Synthesis of Nitrogen Containing Heterocycles in Medicinal Chemistry. RSC Adv. 2020, 10, 14170–14197. https://doi.org/10.1039/d0ra01378a.Search in Google Scholar PubMed PubMed Central
9. Amjad, H.; Abbasi, M. A.; Siddiqui, S. Z.; Iqbal, J.; Rasool, S.; Ashraf, M.; Hussain, S.; Shah, S. A. A.; Imran, S.; Shahid, M.; Rasool, A.; Rehman, M. T.; Rehman, A. U. In Vitro And In Silico Assessment of Bioactivity Properties and Pharmacokinetic Studies of New 3,5-Disubstituted-1,2,4-Triazoles. J. Mol. Struct. 2023, 1275, 134720. https://doi.org/10.1016/j.molstruc.2022.134720.Search in Google Scholar
10. Bozorov, K.; Zhao, J.; Aisa, H. A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. https://doi.org/10.1016/j.bmc.2019.07.005.Search in Google Scholar PubMed PubMed Central
11. Gonnet, L.; Baron, M.; Baltas, M. Synthesis of Biologically Relevant 1,2,3- and 1,3,4-Triazoles: From Classical Pathway to Green Chemistry. Molecules 2021, 26, 5667. https://doi.org/10.3390/molecules26185667.Search in Google Scholar PubMed PubMed Central
12. Al-Qawasmeh, R. A.; Huthail, B. B.; Sinnokrot, M. O.; Semreen, M. H.; Odeh, R. A.; Abu-Zarga, M. H.; Tarazi, H.; Yousef, I. A.; Al-Tel, T. H. Design, Synthesis and Qualitative Structure Activity Relationship Evaluations of Quinoline-Based Bisarylimidazoles as Antibacterial Motifs. Med. Chem. 2016, 12, 563–573. https://doi.org/10.2174/1573406412666160518142441.Search in Google Scholar PubMed
13. Hersi, F.; Omar, H. A.; Al-Qawasmeh, R. A.; Ahmad, Z.; Jaber, A. M.; Zaher, D. M.; Al-Tel, T. H. Design and Synthesis of New Energy Restriction Mimetic Agents: Potent Anti-Tumor Activities of Hybrid Motifs of Aminothiazoles and Coumarins. Sci. Rep. 2020, 10, 2893. https://doi.org/10.1038/s41598-020-59685-x.Search in Google Scholar PubMed PubMed Central
14. Al-Aboudi, A.; Al-Qawasmeh, R. A.; Shahwan, A.; Mahmood, U.; Khalid, A.; Ul-Haq, Z. In-Silico Identification of the Binding Mode of Synthesized Adamantyl Derivatives inside Cholinesterase Enzymes. Acta Pharmacol. Sin. 2015, 36, 879–886. https://doi.org/10.1038/aps.2014.173.Search in Google Scholar PubMed PubMed Central
15. Al-Qawasmeh, R. A.; Abadleh, M. M.; Zahra, J. A.; El-Abadelah, M. M.; Albashiti, R.; Zani, F.; Incerti, M.; Vicini, P. Design Synthesis and Antibacterial Activity Studies of New Thiadiazoloquinolone Compounds. J. Enzyme Inhib. Med. Chem. 2014, 29, 777–785. https://doi.org/10.3109/14756366.2013.855925.Search in Google Scholar PubMed
16. Shehadi, I. A.; Delmani, F. A.; Jaber, A. M.; Hammad, H.; AlDamen, M. A.; Al-Qawasmeh, R. A.; Khanfar, M. A. Synthesis, Characterization and Biological Evaluation of Metal Adamantyl 2-Pyridylhydrazone Complexes. Molecules 2020, 25, 2530. https://doi.org/10.3390/molecules25112530.Search in Google Scholar PubMed PubMed Central
17. Saber, S. W.; Al-Qawasmeh, R. A.; Abu-Qatouseh, L.; Shtaiwi, A.; Khanfar, M. A.; Al-Soud, Y. A. Novel Hybrid Motifs of 4-Nitroimidazole-Piperazinyl Tagged 1,2,3-Triazoles: Synthesis, Crystal Structure, Anticancer Evaluations, and Molecular Docking Study. Heliyon 2023, 9, e19327. https://doi.org/10.1016/j.heliyon.2023.e19327.Search in Google Scholar PubMed PubMed Central
18. Sun, G.-X.; Min, L.-J.; Han, L.; Liu, X.-H. Crystal Structure of 2-((4-Phenyl-5-(pyridin-4-Yl)-4h-1,2,4-triazol-3-Yl)thio)acetonitrile, C15H11N5S. Z. Kristallogr. N. Cryst. Struct. 2022, 237, 199–200. https://doi.org/10.1515/ncrs-2021-0431.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of 3-nitrophenol-2,1,3-benzoselenadiazole (1/1), C12H9N3O3Se
- Crystal structure of diaqua-(hydroxido)-{μ-[2-(hydroxy)-5-[(4-nitrophenyl)diazenyl]benzoato]}-{2-hydroxy-5-[(4-nitrophenyl)diazenyl]benzoato}-(1,10-phenanthroline)-diterbium hydrate, C38H27.4N8O12.2Tb
- Crystal structure of poly[bis(μ3-3-fluoro-4-(1H-1,2,4-triazol-1-yl)benzoato-κ3 O:O′:N)cadmium(II)] – dimethylformamide (1/1), C21H17CdF2N7O5
- The crystal structure of 2-amino-N-(pyridin-2-yl)benzamide, C12H11N3O
- The crystal structure of 2,3-di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O
- Crystal structure of 2-chloro-4-fluorobenzyl (R)-2-(6-methoxynaphthalen-2-yl)propanoate, C21H18ClFO3
- Crystal structure of [1-(4-carboxyphenyl)-4-oxo-1,4-dihydropyridazine-3-carboxylic acid]-(methylsulfinyl)methane, C15H16N2O6S
- The crystal structure of 2-ethyl-1,1-dimethyl-1H-benzo[e]indole, C16H17N
- The crystal structure of (Z)-5-amino-N ′-hydroxy-1H-pyrazole-4-carboximidamide, C4H7N5O
- The crystal structure of 2,2,5-trimethyl-3-(4-(4-(5-phenyl-4,5-dihydroisoxazol-3-yl)thiazol-2-yl)phenyl)imidazolidin-4-one, C24H24N4O2S
- The crystal structure of tetrakis(μ2-acetato-κ2 O:O′)-bis[(4′-phenyl-4,2′:6′,4″-terpyridine-κ1 N)dicopper(II)], C25H21CuN3O4
- Crystal structure of poly(3-thiophenecarboxylato-κ 3 O,O′:O′)-(methanol-κO)cadmium(II), C11H10O5S2Cd
- The crystal structure of dichloridobis[4′-(p-methoxylphenyl)-4,2′:6′,4″-terpyridine-κN] zinc(II), C44H34Cl2N6O2Zn
- The crystal structure of 1-(2-carboxyethyl)-1H-imidazole 3-oxide
- Crystal structure of 1,1′,1″-(nitrilotris(ethane-2,1-diyl))tris(3-(4-(((E)-pyridin-2-ylmethylene)amino)phenyl)urea), C45H47N13O4
- Crystal structure of a (E)-4-bromo-N-(4-(diethylamino)-2-hydroxybenzylidene) benzenaminium acetate ─ 4-bromoaniline (1/1)
- Crystal structure of 2,2′-(iminobis(methylene))bis(benzimidazolium) bis(p-toluenesulfonate), C30H31N5O6S2
- The crystal structure of alogliptinium meta-chlorobenzoate
- Crystal structure of 4-bromobenzyl 2-(6-methoxy-naphthalen-2-yl)propanoate, C21H19BrO3
- The hydrated double salt structure of (E)-4-(2-benzylidenehydrazine-1-carbonyl)pyridin-1-ium cation with 2-hydroxybenzoate and benzoate anions
- Crystal structure of (R)(R)-5-chloro-3-((S,1E,3E)-3,5-dimethyl-hepta-1,3-dien-1-yl)-7-methyl-6,8-dioxo-2,6,7,8-tetrahydroisoquinolin-7-yl acetate, C21H24ClNO4
- The crystal structure of bis(3-oxo-1,3-diphenylprop-1-en-1-olato-κ 2 O:O′)-bis(1,4-dioxane-κ 1 O)nickel(II), C38H38O8Ni
- Crystal structure of poly[aqua-(pyridine-3-carboxylato-κ1 N)(pyridine-3-carboxylato-κ2 O,O′) cadmium(II)] dihydrate, C12H14N2O7Cd
- The crystal structure of 4-(4-phenyl-5-(((1-(2,4,6-tribromophenyl)-1H-1,2,3-triazol-4-yl)methyl)thio)-4H-1,2,4-triazol-3-yl)pyridine, C22H14Br3N7S
- The crystal structure of N-benzylquinoline-2-carbothioamide, C17H14N2S
- Crystal structure of bis(3-isopropylphenyl)-4,4′-bipyridinium dichloride dihydrate, C28H30N2⋅2Cl⋅2H2O
- The crystal structure of ethyl 2-amino-4-(cyanophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C19H18N2O4
- Crystal structure of (4R,10S)-6-hydroxy-7-isopropyl-4,10-dimethyl-1,2,3,5-hexahydro-6,10-epoxyazulen-9-one, C15H22O3
- The crystal structure of (E)-(2-(2-hydroxy-3-methoxybenzylidene)aminophenyl)arsonic acid, C14H14AsNO5
- The crystal structure of poly[(μ 2-2-aminoisophthalato-κ4O,O′:O″:O″′)-(N-methylpyrrolidone κ1O)-dioxido-uranium(VI)], C13H14N2O7U
- The crystal structure of the co-crystal isonicotinamide · terephthalic acid, C8H6O4·2(C6H6N2O)
- The crystal structure of (E)-1-phenyl-3-(p-tolylthio)but-2-en-1-one, C17H16OS
- The crystal structure of 4,5-bis((Z)-chloro(hydroxyimino)methyl)-1H-imidazol-3-ium chloride monohydrate
- The crystal structure of 1,2-bis(4-(dimethylamino)phenyl)ethane-1,2-dione. C18H20N2O2
- Crystal structure of 2-chloro-4-fluorobenzyl 2-acetoxybenzoate, C16H12ClFO4
- Crystal structure of methyl 1-phenyl-9H-pyrido[3,4-b]indole-3-carboxylate, C19H14N2O2
- Crystal structure of (3-(dimethoxymethyl)-5-methoxy-1H-indol-1-yl)(5-fluoro-2-iodophenyl)methanone, C19H17FINO4
- Crystal structure of tetrachlorido-bis(1-[(1H-triazole-1-yl)methyl]-1H-benzotriazole-κ2 N:N′)dicopper, C36H32Cu2N24Cl4
- Crystal structure of 2-(2,3-bis(4-methoxyphenyl)-1H-pyrrolo[2,3-b]quinoxalin-1-yl)anilin, C30H24N4O2
- Crystal structure of 5,7-dihydroxy-2-phenyl-4H-chromen-4-one–N,N-dimethylformamide(1/1), C18H17NO5
- The crystal structure of bis(μ 2-biphenyl-2,2′-dicarboxylato)-diaqua-bis(nitrato)-bis(2,2′:6′,2′′-terpyridine)dineodymium(III), C46H32I2N8Nd2O16
- Crystal structure of (Z)-4-amino-N ′-((4-chlorophenyl)(phenyl)methylene)benzohydrazide, C20H16ClN3O
- Crystal structure of (E)-6,8-dimethoxy-4-(4-morpholinobenzylidene)-3,4-dihydro-1-benzoxepin-5(2H)-one, C23H25NO5
- Crystal structure of (R)-2-((3-(3-aminopiperidin-1-yl)-6-methyl-5-oxo-1,2,4-triazin-4(5H)-yl) methyl)-4-fluorobenzonitrile benzoate monohydrate, C24H27FN6O4
- The crystal structure of [triaqua-(8-carboxymethoxy-quinoline-2-carboxylato-κ 3 N,O,O)copper(II)]monohydrate, C12H15NO9Cu
- Crystal structure of (((4-chlorophenyl)sulfonyl)glycinato-κ 2 N,O)bis(1,10-phenanthroline-κ 2 N,N′)cobalt(II) tetrahydrate, C32H30ClCoN5O8S
- Crystal structure of (((3-nitrophenyl)sulfonyl)-β-alaninato-κO)bis(2,2′-bipyridine-κ 2 N, N′)copper(II) 3-nitrobenzenesulfonate, C35H29CuN7O11S2
- Crystal structure of 3-phenoxybenzyl 2-(6-methoxynaphthalen-2-yl)propanoate, C27H24O4
- 6-(2′,3′-Dihydroxy-3′-methylbutyl)-7-methoxy-8-(3″-methylbut-2″-en-1″-yl)-2H-chromen-2-one, C20H26O5
- Crystal structure of bromido-(2,2′:6′,2″-terpyridine-4′-onato-κ3N)palladium(II) methanol solvate
- The crystal structure of ethyl 2-amino-(4-nitrophenyl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carboxylate, C20H22N2O6
- Crystal structure of (1E,3E,5E)-1,6-bis(4-(pentyloxy)phenyl)hexa-1,3,5-triene, C28H36O2
- The crystal structure of tris(2-bromo-4-methylphenyl)amine, C21H18Br3N
- The crystal structure of 3-(2,5-dimethylanilino)-1-(2,5-dimethylphenyl)-4-methyl-1H-pyrrole-2,5-dione, C21H22N2O2
- Crystal structure of dicarbonyl (μ2-indole-2-carboxylato κ2 O:O′)tris(triphenylarsine-κAs)dirhodium(I) acetone solvate, C68H56As3NO5Rh2
- The crystal structure of 4-chloro-2-formylphenyl 4-methylbenzenesulfonate, C14H11ClO4S
- Crystal structure of 4-iodobenzyl 2-(6-methoxynaphthalen-2-yl) propanoate, C21H19IO3


