Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
-
Yongqi Yang
, Yongxin Liu
, Sisi Zhang
, Zhu Cheng
Abstract
Novel superabsorbent polymers (SAPs) were synthesized by performing aqueous solution graft copolymerization of potato starch xanthate and partially hydrolyzed acrylamide using N,N′-methylene-bis-acrylamide as a crosslinker and potassium persulfate as an oxidant at 35–45°C. Various factors that influence the water absorption of SAPs were studied in detail, and the optimum formulation ratio was determined via orthogonal experiments. Several spectroscopic techniques, including scanning electron microscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis, and transmission electron microscopy, were used to determine the structure of the SAPs. In addition, adsorption experiments were carried out with various heavy metal ions, including divalent copper ions, zinc ions, nickel ions, cobalt ions, cadmium ions, and lead ions. Experiments showed that the prepared SAP has a high adsorption performance (>48 mg·g−1). Thus, these materials are expected to have important applications in the removal of heavy metal ions and the separation of dyes in aquatic environments.
Graphical abstract
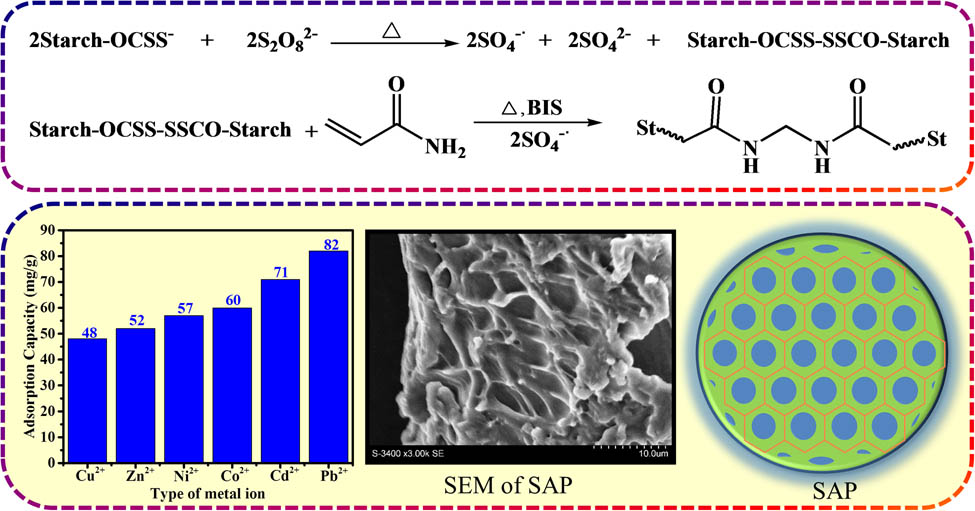
1 Introduction
Modern society relies heavily on nonrenewable petroleum resources to produce various essential items. Due to their low cost, ease of processing, high specific strength, versatility, and durability, these traditional materials are desirable. Environmental issues, particularly those related to degrading plastics and removing heavy metals from water, are pressing global concerns (1). Synthetic polymers are far superior to natural polymers in terms of thermal stability and processability. However, there are concerns about increased CO2 emissions, nonrenewable fossil energy sources, poor biodegradability, and poor biocompatibility (2). Among renewable products, starch-based biodegradable materials, such as superabsorbent polymers (SAPs) and biocomposites, have received increasing amounts of attention in recent years (3).
Due to the presence of highly hydrophilic groups, such as –NH2, –SO3H, –COOH, and –OH, and a crosslinked three-dimensional network structure, the SAP can adsorb and retain aqueous solutions up to hundreds or even thousands of times its own weight in a relatively short time (4,5). Even under some pressure, the absorbed water is difficult to remove (6). Due to their excellent properties, SAPs have attracted considerable interest and research and are widely used in horticulture (7), sanitation (8), food (9), cosmetics, wastewater treatment (10), and drug delivery (11). SAP was first identified by the United States Department of Agriculture in 1961. Scientists in Japan and Western European countries have performed various studies to improve the strength, absorption rate, and capacity of gels (12,13). Superabsorbent polyacrylonitrile fiber materials were developed in the 1980s, the fibers are mainly formed by spinning or stretching procedures, and after posttreatment, polyacrylonitrile fibers with desired properties can be obtained (11). With the continuous innovation and development of electrospinning technology (14), polyvinyl alcohol superabsorbent nanofibers are being widely used for physiological hygiene and as hemostatic and probiological materials.
Recently, there has been much focus on the production of starch-grafted acrylamide polymers, but the current process used to synthesize SAPs has many shortcomings (15). The main issues are the improvement in gel strength and water absorption, which greatly limit the promotion and application of SAPs (16). To solve the above problems, many researchers have attempted to modify starch-grafted acrylic acid or acrylamide (17,18).
Research has shown that these problems can be avoided using potato starch xanthate (PSX) graft acrylamide polymers. In this study, novel high SAPs were synthesized via aqueous solution graft copolymerization of PSX and partially hydrolyzed acrylamide using N,N′-methylene-bis-acrylamide (BIS) as a crosslinker and potassium persulfate (KPS) as an oxidant at 35–45°C. Graft copolymerization with the partially hydrolyzed monomer acrylamide and the matrix PSX may improve the hydrophilicity, number, and network structure of SAPs. The influence of the amount of acrylamide, the amount of potassium hydroxide (KOH), the amount of KPS, the amount of BIS, and the grain diameter on the properties of SAPs are discussed in this article. The best preparation conditions were determined by an orthogonal test, and adsorption experiments were performed with a variety of heavy metal ions, which demonstrated that the resin strongly adsorbed a variety of divalent heavy metal ions. A schematic representation of the process used to produce SAPs with high gel strength and good heavy metal adsorption performance is shown in Scheme 1.
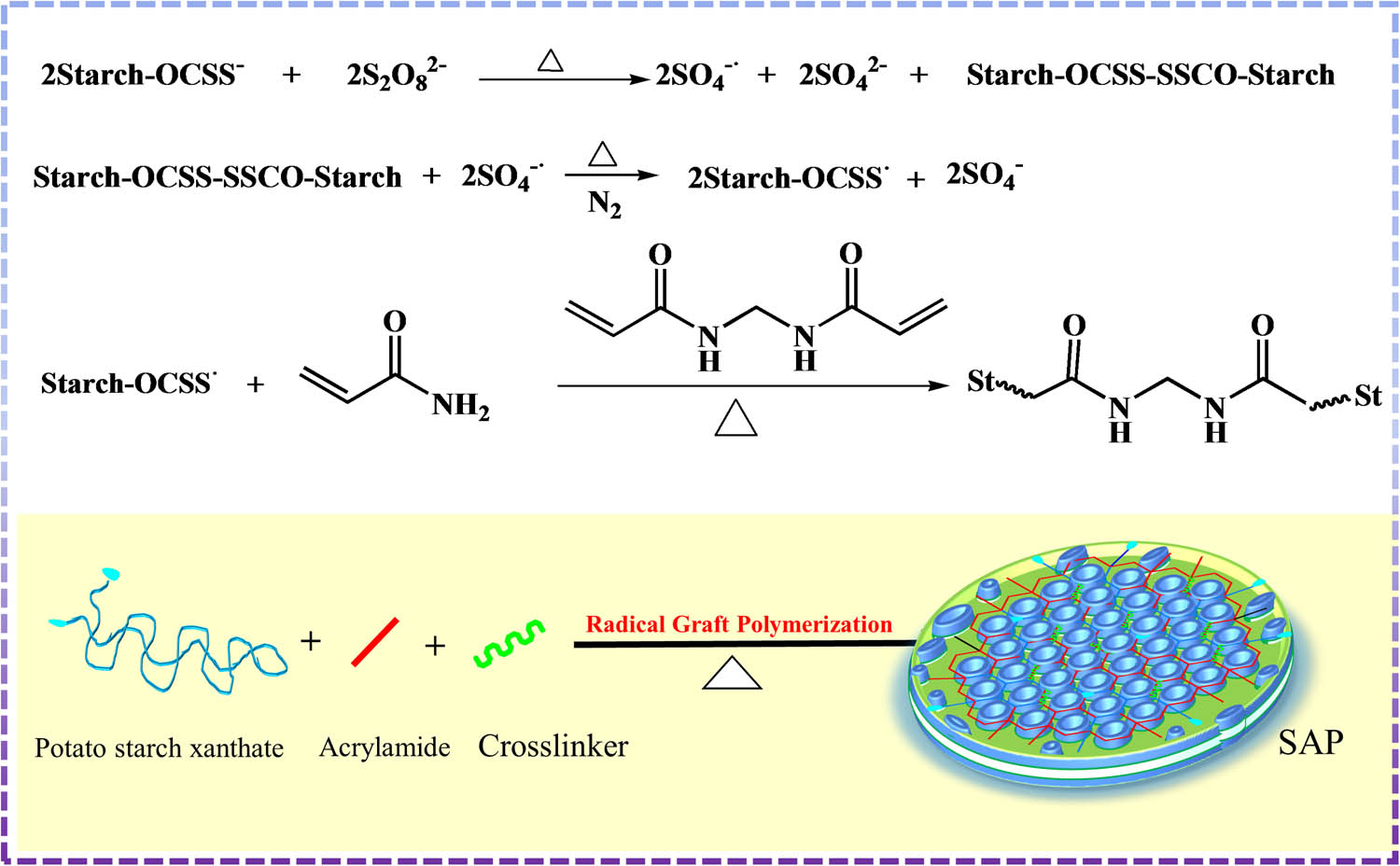
Schematic representation of the production of SAPs with high gel strength and good heavy metal adsorption performance.
2 Materials and methods
2.1 Materials
Potato starch (reagent grade) and carbon disulfide (≥99.9%) were purchased from Aladdin Chemical Reagent Co., Ltd. Sodium hydroxide (Adamas Life, ≥98%), anhydrous ethanol (Greagent, ≥99.8%), acrylamide (Adamas Life, ≥98%), KOH (Greagent, ≥90%), KPS (Adamas-beta, ≥99.5%), and BIS (Adamas-beta, ≥99%) were acquired from Shanghai Titan Co., Ltd. All other reagents used were of analytical grade.
2.2 Synthesis of PSX
Then, 50 mL of deionized water was placed in a 200 mL Erlenmeyer flask. Ten grams of accurately weighed potato starch was added, and the mixture was stirred for 15 min. Then, a 50 mL beaker was prepared, 0.28 g of sodium hydroxide and 20 mL of water were added to create a dilute solution of sodium hydroxide. The mixture was stirred until completely dissolved, and the solution was poured into a 100 mL constant-pressure dropping funnel. The constant-pressure dropping funnel was mounted on the neck of the reaction flask. After connecting the equipment, the water bath was heated to 30°C, the glass piston was opened, sodium hydroxide solution was slowly added to the starch solution, and the reaction was allowed to continue for 1 h. Then, the CS2 solution (3.5 mL) was slowly added, and the reaction was continued in a water bath for 3 h to generate the crude product. To generate the precipitate, 200 mL of anhydrous ethanol was added to a clean 1 L beaker, and the above solution of the crude product was poured into the ethanol solution. To generate pure PSX, the precipitate was centrifuged and rinsed with a small amount of deionized water. Then, the sample was reprecipitated and purified in anhydrous ethanol, freeze-dried in a lyophilizer to obtain a dry product, crushed, and passed through a 100 mesh stainless steel sieve to generate the dry matter needed for the experiment.
2.3 Synthesis of PSX graft acrylamide polymer
The procedure used to prepare SAPs was as follows: first, 2–5 g of KOH and 75 mL of DI water were weighed to formulate the solution at a specified concentration. The solution was cooled to room temperature. Then, 20–32.5 g of acrylamide was weighed and hydrolyzed with the above alkali solution in an ice-water bath to generate a partially hydrolyzed acrylamide solution. Later, 2.5 g of PSX was placed in a 500 mL plastic reaction flask, and 25 mL of DI water was added to form a disperse suspension. To form a disperse suspension, the partially hydrolyzed acrylamide solution was mixed with the PSX suspension. Then, 60–100 mg of the initiator KPS and 10–20 mg of the crosslinker BIS were added to the reaction flask. The bottle was transferred to a water bath at 30–45°C after 30 min under nitrogen. The polymerization reaction occurs when the temperature increases to 35–45°C. At this point, the solution should visibly thicken. Stirring was stopped, and the temperature of the water bath was allowed to rise rapidly to 60°C. After 1 h, the sample was removed. The sample was dried, crushed, and sieved, and other steps were performed to generate the necessary SAP size.
3 Results and discussion
3.1 Investigate factors affecting SAP water retention
In this study, novel highly adsorptive SAPs were synthesized by aqueous solution graft copolymerization of PSX and partially hydrolyzed acrylamide using BIS as a crosslinker and KPS as an oxidant at 35–45°C. The mass ratio of the matrix PSX to the monomer acrylamide, the degree of hydrolysis of the acrylamide (i.e., the amount of KOH added), the mass of the crosslinking agent BIS, the mass of the initiator KPS, and the total amount of water in the system are reported in the literature as the main factors affecting the water retention of SAPs (19). The mass of PSX added to the system was 2.5 g, the mass of KPS was 80 mg, the mass of BIS was 15 mg, the mass of KOH was 4 g, and the total water in the system was 100 mL. The effect of the addition of 20–35 g of acrylamide on the water retention of SAPs was studied. The maximum water retention is 2,050 g·g−1. The optimum mass ratio of matrix to monomer was 1:12 (Figure 1(a)). The effect of adding KOH, KPS, and BIS and the total amount of deionized water used in the system on SAP water retention was also investigated (Figure 1(b)–(e)). The tests revealed that at a mass ratio of 1:12 and with the addition of 4 g of KOH, the maximum water retention of the SAP was 2,052 g·g−1. For the same reason, when the BIS dose was 20 mg and the KPS dose was 80 mg, SAP was found to retain more water. The best single factor was then selected to replicate a pot of SAP. This sample was then dried and ground through stainless steel sieves of various meshes. Water absorption in deionized water was studied using SAP particles with different particle size ranges. As the particle size increases, the water absorption of the SAP decreases, as shown in Figure 1(f). This may result from the mechanical action of the crusher damaging the network structure of the SAP. Therefore, with sufficient water and time for the test, the larger the particle size of the SAP, the more the water absorption. Specifically, as shown in Table S1, we then performed orthogonal experiments to determine the optimal preparation ratios.
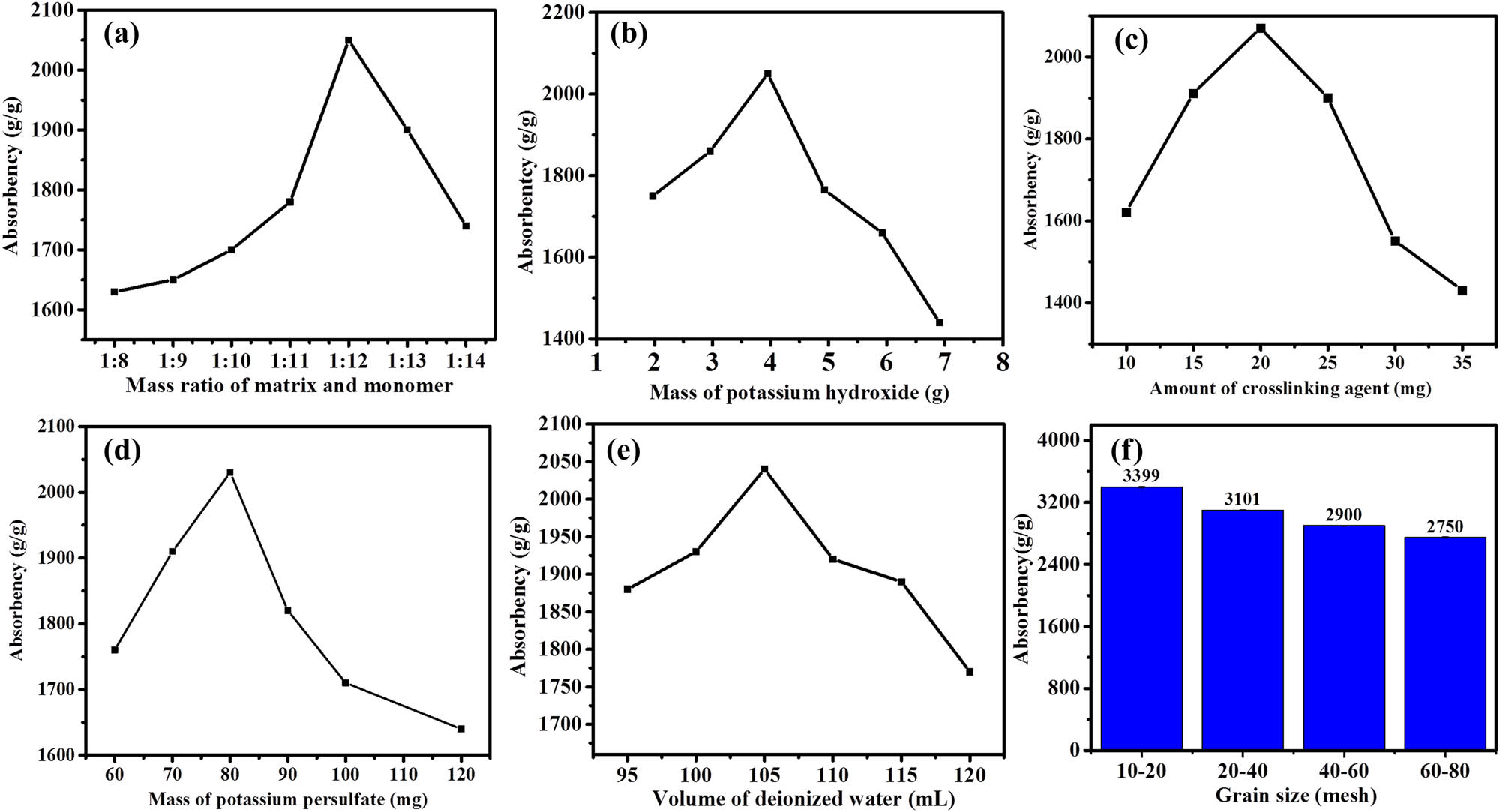
Investigation of the individual factors influencing SAP water absorption: mass ratio of the matrix and monomer (a), mass of KOH (b), amount of BIS (c), mass of KPS (d), volume of deionized water (e), and grain size (f).
3.2 Characterization of potato starch, PSX, and SAP
Fourier transform infrared (FTIR) spectroscopy was used to confirm the structure of the functional groups of the synthesized PSX and the SAP. Figure 2(a) shows the FTIR spectra of potato starch, PSX, and SAP. The absorption bands at 3,410 and 1,640 cm−1 are the stretching and bending vibration absorption peaks of the hydroxyl groups in the potato starch molecular chain (black curve). When PSX was obtained, the intensity of the hydroxyl group in the absorption band at 1,640 cm−1 decreased, and simultaneously, a stretching vibration peak with a strong signal appeared at 1,170 cm−1. This result provided evidence for the formation of the xanthate structure (red curve). When SAP was prepared, the blue curve in Figure 2(a) indicated that the signal intensity of the absorption band was weaker at 1,640 cm−1, but the absorption band broadened, which is the carbonyl stretching vibration peak of the amide bond and carboxylate group; this evidence demonstrated that the target product was obtained. The crystalline structures of potato starch, PSX, and SAP were then analyzed using X-ray diffraction (XRD) (Figure 2(b)). The scanning range was 5°–80°. The potato starch diffraction curves showed distinct peaks at 2θ values of 15.0°, 17.2°, 22.2°, and 24.0° (black curve). The diffraction curve of PSX showed that the position of the diffraction peaks was not altered compared to that of potato starch. However, the peak intensity was weaker, indicating that the crystalline structure of potato starch was partially destroyed during the preparation of PSX (red curve). The PSX diffraction peaks disappeared, and new peaks appeared at 2θ values of 7.38° and 20.27° in the SAP diffraction curve (red curve). This result indicates that the PSX formed a complex with the lipoid and related compounds, i.e., the PSX polymerized with acrylamide and potassium acrylate to generate the SAP.
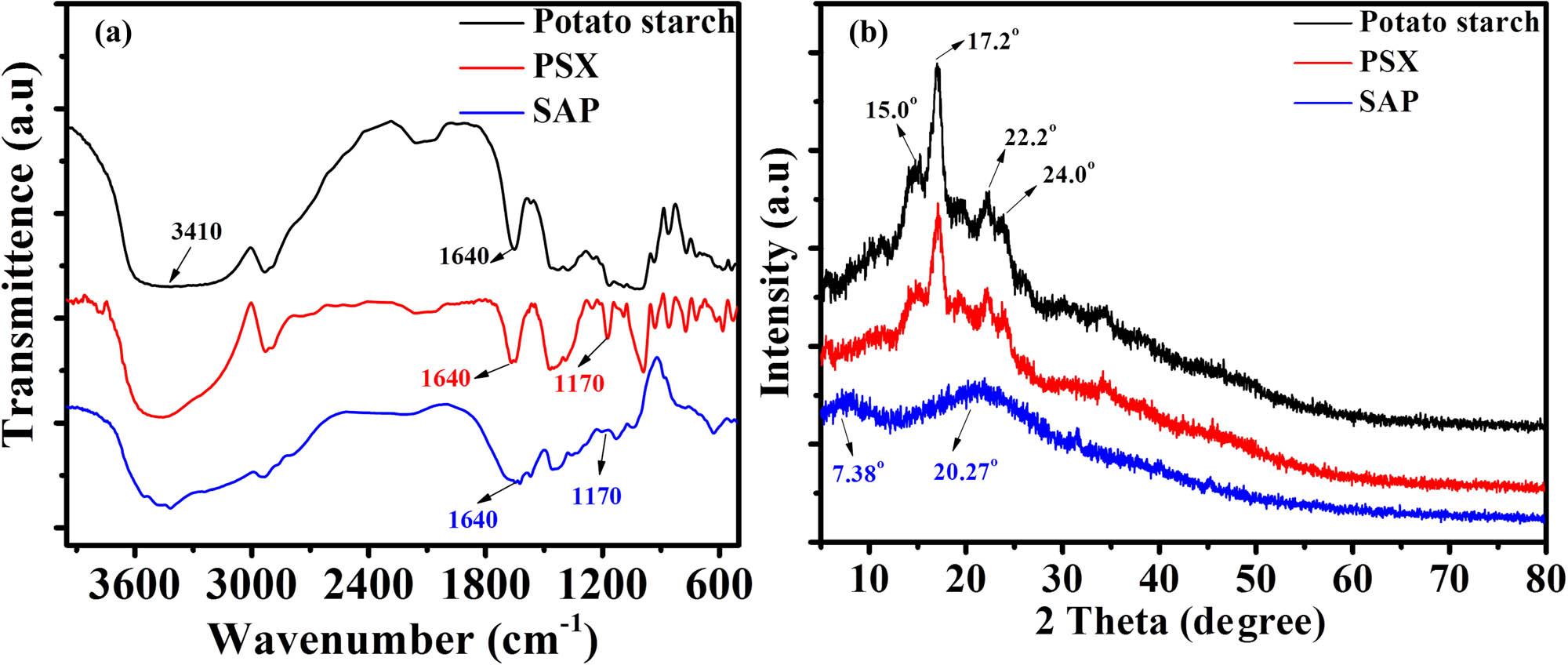
FTIR spectroscopy (a) and XRD (b) of potato starch, PSX, and SAP.
The surface structures of potato starch, PSX, and SAP, as shown in Figure 3, were examined using scanning electron microscopy. Figure 3(a) and (b) shows that the surface structure of potato starch is a smooth ellipsoid, while the surface of the modified PSXs has numerous wrinkles. The average particle sizes of the two are essentially similar, indicating that the crystal structure of the starch molecules is partially altered during the modification. The initial pleated ellipsoidal structure changed significantly to a porous three-dimensional network structure when SAP was generated (Figure 3(c) and (d)). The pore size varied, and most particle sizes were 5–10 μm in size due to the occurrence of crosslinks between molecular chains simultaneously with the graft polymerization reaction. The statistical images are shown in Figures S1–S4.
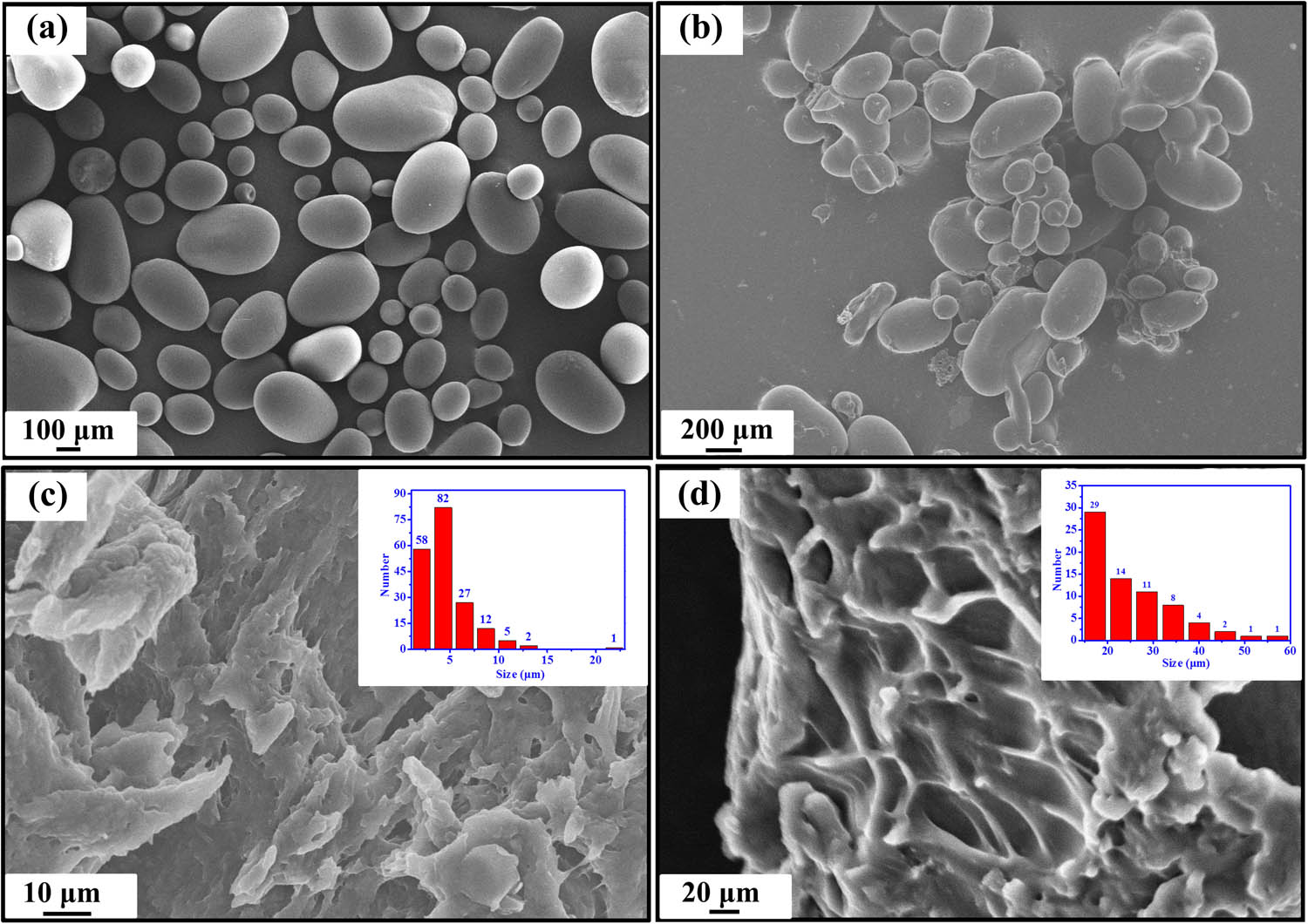
SEM images of potato starch (a), PSX (b), and SAP (c) and (d).
All the samples showed good thermal stability, as shown by the thermogravimetric analysis (TGA) curves in Figure 4. There were two distinct phases of weight loss for both PSX and SAP. Between 100°C and 210°C, both materials lost approximately 10% of their weight by evaporating water. However, in contrast to SAP, which lost 48% of its weight between 210°C and 580°C (accompanied by changes in decarboxylation and deacylation), PSX lost 60% of its weight between 210°C and 350°C, suggesting that PSX is structurally unstable and that this process is accompanied by glycosidic bond breakage. When the temperature was increased further to 800°C, the chain structure of both was completely destroyed, and the residual mass was 18%. The TGA curves also show that the crosslinked structure of the SAP exhibits greater thermal stability than that of PSX.
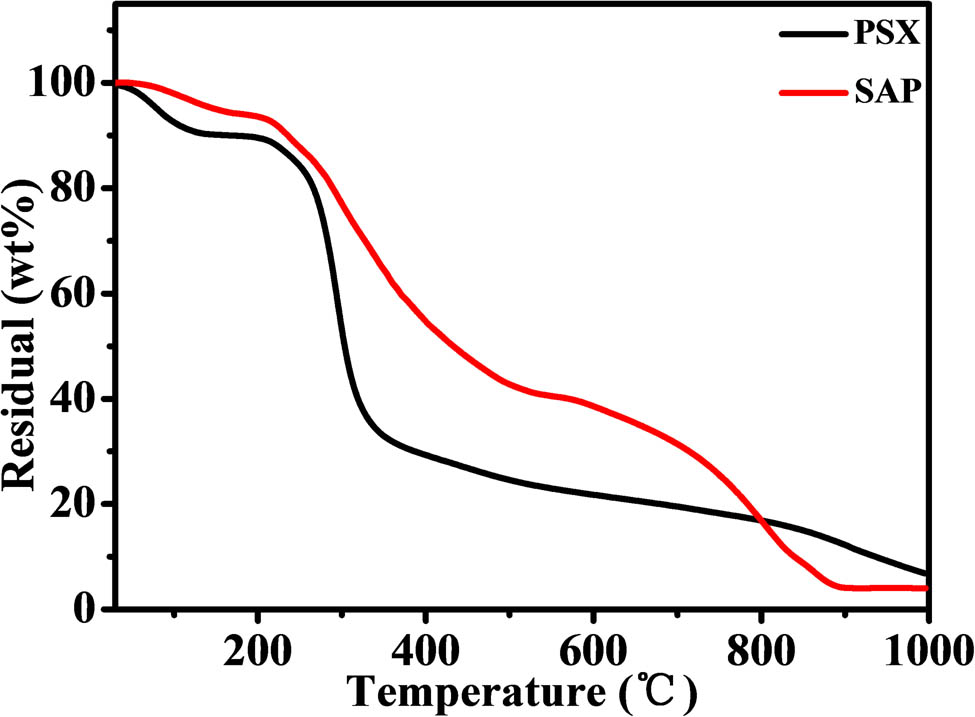
TGA curves of PSX and SAP.
3.3 Adsorption properties of SAP
The absorbance was determined as shown in Figure 5(a) by weighing 0.5 g of SAP of the same particle size and placing it in 3,000 mL of deionized water, 500 mL of piped water, physiological saline (0.9 wt% NaCl solution), homemade blood, and homemade urine for 12 h. Adsorption tests were carried out in accordance with the literature natural filtration method (20). Based on the amount adsorbed, its capacity for absorbing liquids is much greater than that reported in the literature (21). Subsequently, 0.1 g of SAP was added to 100 mL of Zn(NO3)2, Ni(NO3)2, Co(NO3)2, Cd(NO3)2, Pb(NO3)2, or Cu(NO3)2 solution at a concentration of 2 mmol·L−1. The amount of heavy metals adsorbed was then measured after 12 h. Surprisingly, the adsorption was greater than 48 mg·g−1, providing new research ideas for the study of heavy metal ion adsorption (Figure 5(b)). To closely observe the adsorption of heavy metal ions by SAPs, we carried out transmission electron microscope (TEM) tests of divalent lead ions (Figure 5(c) and (d) and Figure S5) and observed the scanning electron microscopic energy dispersive X-ray spectroscopy energy spectra before and after the adsorption of heavy metal ions (Figures S6–S10). Characterization revealed the adsorption of heavy metal ions both in the interior and on the surface of the SAP, with a broad spectrum of adsorption not reported in the literature.
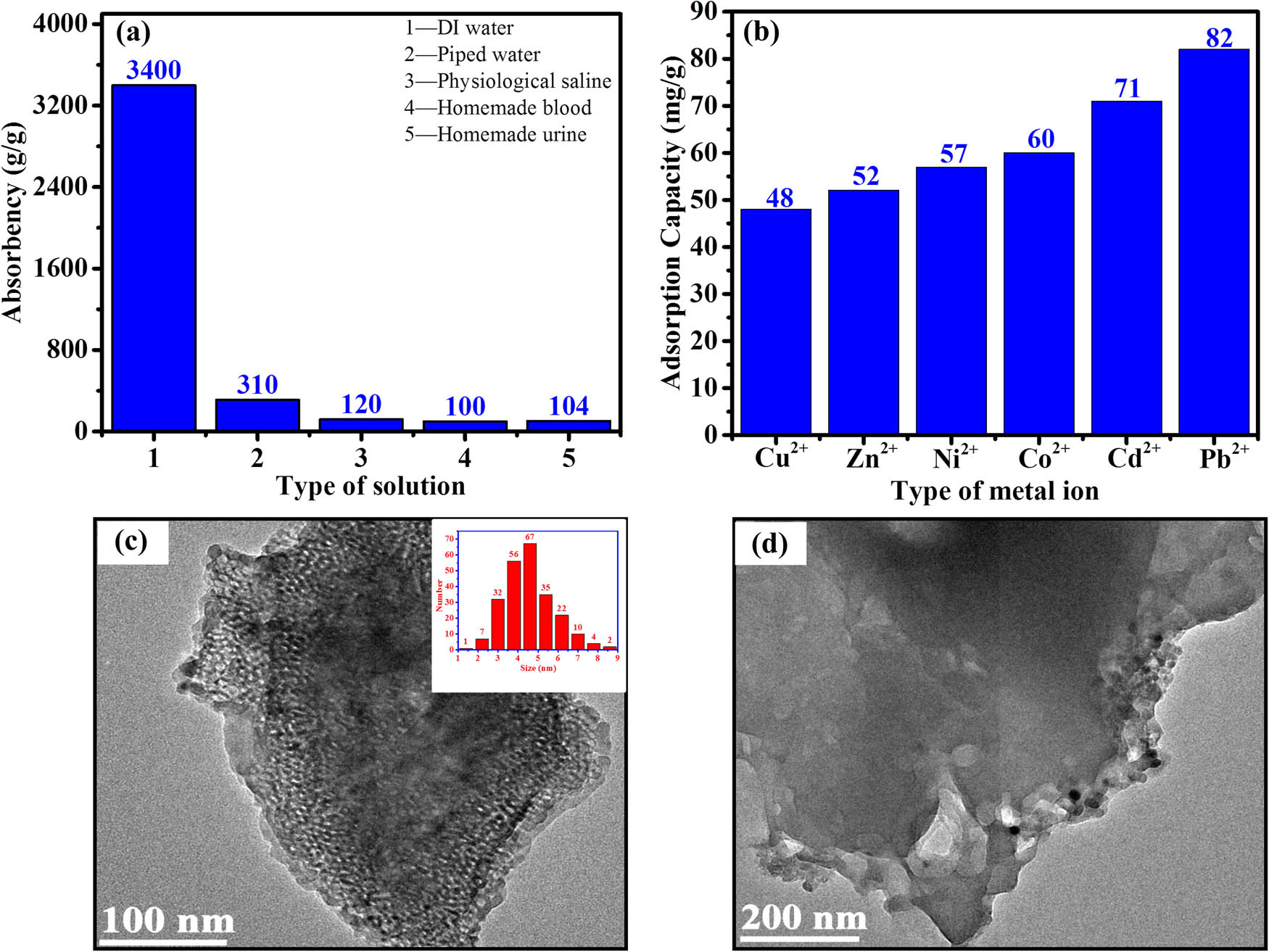
Characterization of the SAP adsorption properties: adsorption properties in different solutions (a), adsorption properties in different heavy metal ion solutions (b), and representative TEM images after adsorption of Pb2+ (c) and (d).
Kinetic modeling was also performed for divalent lead ion solutions, and it was found that the R 2 of the fitted pseudo-first-order kinetic equation for the adsorption process was significantly smaller than that of the pseudo-second-order kinetic equation, while the theoretical maximum adsorption amount q e derived from the fitted pseudo-first-order rate law was much less than the actual value (Figure 6(a) and (b)). Therefore, the pseudo-second-order kinetic equation is more consistent with the adsorption process of metal ions by the SAP (22,23).
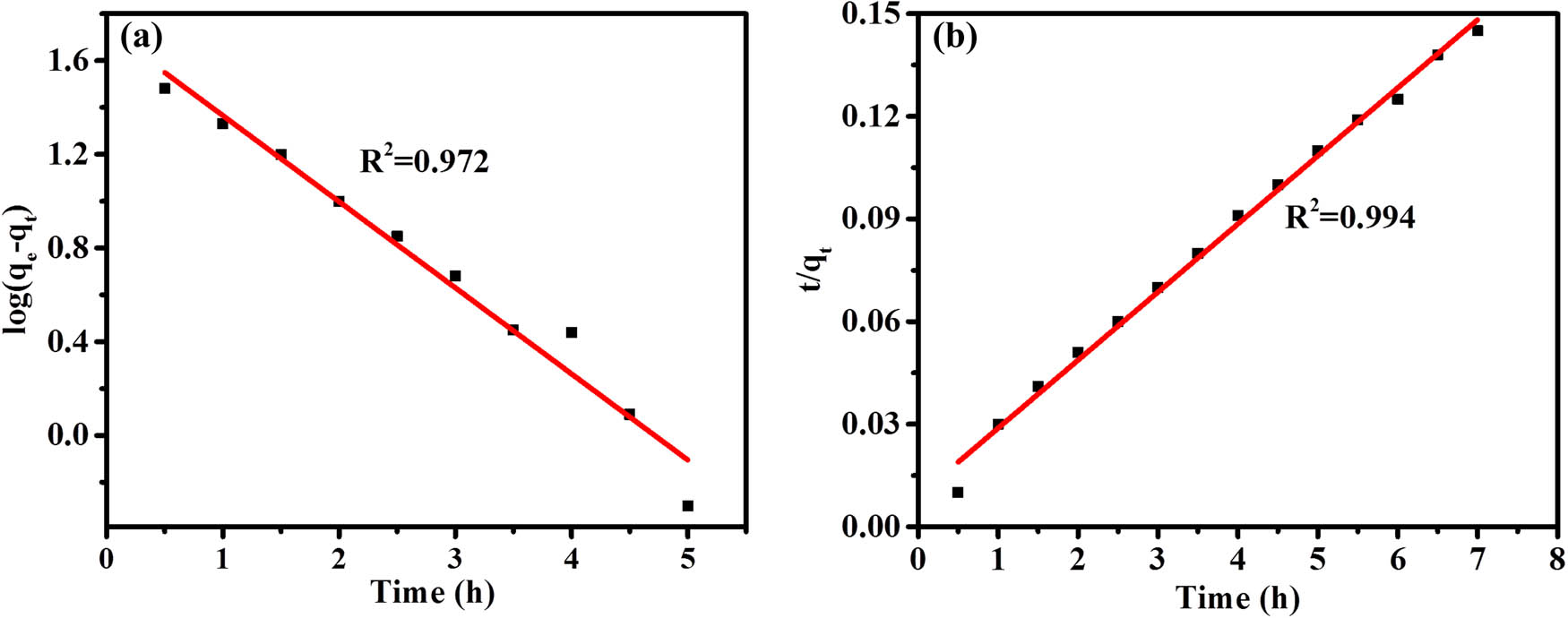
Pseudo-first-order kinetics plot (a) and pseudo-second-order kinetics plot (b) for SAP adsorption of Pb2+.
4 Conclusions
In this work, from the SAP orthogonal experimental data, the highest water absorption of 3,160 g·g−1 is obtained when the mass ratio of the matrix PSX to the monomer acrylamide (AM) is 1:12, the AM hydrolysis is 17%, the initiator is 2.8% by mass of PSX, and the crosslinking agent is 0.8% by mass of PSX. Heavy metal adsorption experiments of SAPs were also carried out. The adsorption pattern conformed to a pseudo-second-order kinetic curve, and the adsorption amounts of the various heavy metals were greater than 48 mg·g−1. The experiments showed that the prepared SAP exhibited high water absorption, high gel strength, and extensive heavy metal adsorption. In the future, electrospinning and other advanced techniques will make it possible to convert SPAs into fibers, films, nanoparticles, and other composites for use in hygiene, hemostasis, and environmental restoration applications.
Acknowledgements
The financial support of Shandong Provincial Natural Science Foundation (ZR2020QB084 and ZR2022MB118) and Weifang University of Science and Technology (KJRC2019009) are greatly appreciated. We thank professor Zesheng An of Jilin University and professor Guohua Wen of Inner Mongolia University for their guidance and advice on experimental design. All individuals included in this section have consented to the acknowledgement.
-
Funding information: This research was funded by the financial support of Shandong Provincial Natural Science Foundation, grant numbers ZR2020QB084 and ZR2022MB118 and Weifang University of Science and Technology, grant number KJRC2019009.
-
Author contributions: Yongqi Yang, Yongxin Liu, Sisi Zhang, and Zhu Cheng: designed this research; Youjun Yan, Jun Liu, and Meng Lian: conducted this research; Yongqi Yang, Sisi Zhang, and Fangfang Liu: analyzed the draft data; Yongqi Yang and Zhu Cheng: wrote the original draft; Yongxin Liu: modified the article; Yongqi Yang, Yongxin Liu, and Sisi Zhang: edited the whole manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data are contained within the article.
References
(1) Lin WH, Wang HY, Kuo J, Lo SL. Adsorption and desorption characteristics of heavy metals onto conventional and biodegradable plastics. Chemosphere. 2023;333:138920. 10.1016/j.chemosphere.2023.138920.Search in Google Scholar PubMed
(2) Zhou J, Hsu TG, Wang J. Mechanochemical degradation and recycling of synthetic polymers. Angew Chem Int Ed. 2023;62:e202300768. 10.1002/anie.202300768.Search in Google Scholar PubMed
(3) Wang S, Zhang P, Li Y, Li J, Li X, Yang J, et al. Recent advances and future challenges of the starch-based bio-composites for engineering applications. Carbohydr Polym. 2023;307:120627. 10.1016/j.carbpol.2023.120627.Search in Google Scholar PubMed
(4) Schröfl C, Erk KA, Siriwatwechakul W, Wyrzykowski M, Snoeck D. Recent progress in superabsorbent polymers for concrete. Cem Concr. 2022;151:106648. 10.1016/j.cemconres.2021.106648.Search in Google Scholar
(5) Venkatachalam D, Kaliappa S. Superabsorbent polymers: a state-of-art review on their classification, synthesis, physicochemical properties, and applications. Rev Chem Eng. 2023;39(1):127–71. 10.1515/revce-2020-0102.Search in Google Scholar
(6) Yang J, Sun Z, De Belie N, Snoeck D. Self-healing ability of cracks in alkali-activated slag systems incorporating superabsorbent polymers. Cem Concr. 2023;170:107183. 10.1016/j.cemconres.2023.107183.Search in Google Scholar
(7) Beigi S, Azizi M, Iriti M. Application of super absorbent polymer and plant mucilage improved essential oil quantity and quality of ocimum basilicum var. keshkeni luvelou. Molecules. 2020;25(11):2503. 10.3390/molecules25112503.Search in Google Scholar PubMed PubMed Central
(8) Nangan S, Okhawilai M, Senthil Kumar P, Verma D, Rajendran K, Qin J, et al. Baby diaper’s super absorbent polymer derived carbon templated NiCuP@NiCu nanostructures for green hydrogen production. Int J Hydrogen Energy. 2024;52:401–11. 10.1016/j.ijhydene.2023.02.069.Search in Google Scholar
(9) Berton SBR, De Jesus GAM, Sabino RM, Monteiro JP, Venter SAS, Bruschi ML, et al. Properties of a commercial κ-carrageenan food ingredient and its durable superabsorbent hydrogels. Carbohydr Res. 2020;487:107883. 10.1016/j.carres.2019.107883.Search in Google Scholar PubMed
(10) Situ Y, Huang C, Yang Y, Liao Z, Mao X, Chen X. Synthesis and application of super absorbent polymers synthesized with ammonia solution and diatomaceous earth with low toxic residues. Environ Technol Innov. 2023;32:103371. 10.1016/j.eti.2023.103371.Search in Google Scholar
(11) Yang Y, Liang Z, Zhang R, Zhou S, Yang H, Chen Y, et al. Research advances in superabsorbent polymers. Polymers. 2024;16(4):501. 10.3390/polym16040501.Search in Google Scholar PubMed PubMed Central
(12) Ma X, Wen G. Development history and synthesis of super-absorbent polymers: a review. J Polym Res. 2020;27(6):136. 10.1007/s10965-020-02097-2.Search in Google Scholar
(13) Chen J, Wu J, Raffa P, Picchioni F, Koning CE. Superabsorbent polymers: from long-established, microplastics generating systems, to sustainable, biodegradable and future proof alternatives. Prog Polym Sci. 2022;125:101475. 10.1016/j.progpolymsci.2021.101475.Search in Google Scholar
(14) Yu D, Gong W, Zhou J, Liu Y, Zhu Y, Lu X. Engineered shapes using electrohydrodynamic atomization for an improved drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2024;16(3):e1964. 10.1002/wnan.1964.Search in Google Scholar PubMed
(15) Noordergraaf IW, Witono JR, Heeres HJ. Grafting starch with acrylic acid and Fenton’s initiator: the selectivity challenge. Polymers. 2024;16(2):255. 10.3390/polym16020255.Search in Google Scholar PubMed PubMed Central
(16) Pellá MCG, Simão AR, Mauricio MR, Estrada RA, Pereira GM, da Silva R, et al. A comparative study of starch-g-(glycidyl methacrylate)/synthetic polymer-based hydrogels. Carbohydr Polym. 2023;307:120614. 10.1016/j.progpolymsci.2021.101475.Search in Google Scholar
(17) Gou P, Ye L, Zhao X. Fabrication of all-starch-based hydrogels as eco-friendly water-absorbent resin: structure and swelling behaviors. Int J Biol Macromol. 2023;253:127646. 10.1016/j.ijbiomac.2023.127646.Search in Google Scholar PubMed
(18) Avalos Belmontes F, Castañeda-Flores ME, González FJ, Garcia-Lobato MA, Téllez-Rosas MM. Biodegradable acrylic polymers and nanocomposites. In green-based nanocomposite materials and applications. Cham: Springer International Publishing; 2023. p. 141–71. 10.1007/978-3-031-18428-4-8.Search in Google Scholar
(19) Kim YJ, Hong SJ, Shin WS, Kwon YR, Lim SH, Kim JS, et al. Preparation and performance of superabsorbent polymer with cellulose additives. Fibers Polym. 2020;21:2448–55. 10.1007/s12221-020-1198-5.Search in Google Scholar
(20) Teli MD, Waghmare NG. Synthesis of superabsorbent from carbohydrate waste. Carbohydr Polym. 2009;78:492–6. 10.1007/s00289-023-04877-4.Search in Google Scholar
(21) Kenawy ER, Elnaby HH, Azaam MM. Synthesis of superabsorbent composite based on chitosan-g-poly(acrylamide)/attapulgite. Polym Bull. 2024;81(4):3527–43. 10.1016/j.carbpol.2009.05.006.Search in Google Scholar
(22) Rao MM, Ramana DK, Seshaiah K, Wang MC, Chang SW. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J Hazard Mater. 2009;166(2–3):1006–13. 10.1016/j.jhazmat.2008.12.002.Search in Google Scholar PubMed
(23) El-Hamshary H, Fouda MMG, Moydeen M, Al-Deyab S. Removal of heavy metal using poly (N-vinylimidazole)-grafted-carboxymethylated starch. Int J Biol Macromol. 2014;66:289–94. 10.1016/j.ijbiomac.2014.02.047.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

