Abstract
In the blended conductive rubbers, good dispersion of conductive fillers and great interfacial bonding with the substrate are the keys to achieving excellent mechanical and electromagnetic shielding properties. It is found that compared with octyltriethoxysilane (A137), 3-methacryloxypropyl-trimethoxysilane (A174) and vinyltriethoxysilane (A151) with a double bond reduce the curing degree of the blends. The vinyl methyl silicone rubber/nickel-coated graphite (VMQ/NCG) composites modified by A137 shows poor tensile properties, while the composites modified by A174 shows inferior electrical properties. The presence of physical adsorption and chemical adsorption on the surface of NCG modified by A151, which effectively enhances the dispersibility of NCG and interfacial bonding strength with rubber, so that the material exhibits excellent comprehensive properties. When the content of A151 is 3% and modified by dry method, the tensile strength of VMQ/NCG composites can reach 1.6 MPa, the elongation at break can reach 162%, and the volume resistivity can reach 0.05 Ω·cm.
1 Introduction
Conductive silicone composites, including conductive fillers and silicone rubber, have unique characteristics such as excellent high- and low-temperature resistance, aging resistance, processability, and electrical conductivity. When used in aerospace systems, electronic chips, and other systems, they exhibit electromagnetic sealing characteristics, preventing important information leakage (1–5). Common conductive fillers include carbon (6–12) and metal fillers (13–15). Carbon fillers are generally more suitable for the preparation of conductive rubber with low conductivity. Although metal fillers have good electrical conductivity, they have high price and density. Conductive polymers often require the addition of dopants to achieve good shielding properties, which leads to relatively poor processability and mechanical properties, so they are also used as conductive fillers (16). Nowadays, it has become a new trend to adopt composite conductive fillers (17–20) with low density, good conductivity, aging resistance, and moderate cost, and to mix different conductive fillers (21–23). Among them, nickel-coated graphite (NCG) has good electrical conductivity and magnetic permeability. Moreover, silicone conductive materials with NCG, which can effectively prevent electromagnetic leakage or shield external electromagnetic interference, are widely used (24–26). However, NCG, which is an inorganic filler, has a high surface energy and hydrophilic surface, resulting in poor compatibility with rubber matrix. This causes NCG agglomeration, reduces the amount of NCG used to form conductive networks, and causes NCG direct filled silicone rubber to exhibit poor mechanical properties; hence, the surface modification of NCG is indispensable.
Silane coupling agents are first used to treat glass fiber, a reinforcing agent used in plastics. Modification using silane coupling agents is an environmentally friendly, simple, and low-cost method (27). Silane coupling agents have an organic group at one end that can be chemically bonded to a polymer or exhibit good compatibility with the polymer, and an alkoxy group at the other end. The alkoxy group of silane coupling agents is hydrolyzed, forming a silanol group, which establishes a hydrogen bond with the hydroxyl group on the surface of an inorganic material and forms a covalent bond between the coupling agent and inorganic material after dehydration. Alternatively, the alkoxy group of silane coupling agents directly condenses with the hydroxyl group on the surface of an inorganic material to form a covalent bond with the inorganic material, replacing the original van der Waals forces. Furthermore, the organic group at the other end of the coupling agent is bonded to the rubber, thereby enhancing the interfacial adhesion between the inorganic material and rubber (28). This also reduces the surface energy of the inorganic materials and the filler–filler interaction, reducing the agglomeration phenomenon, improving the filler dispersion, and effectively enhancing the performance of the composite material (29). Currently, the application of silane coupling agents has gradually extended to surface modification of nanoparticles such as silica and metal oxides, and many studies have investigated the interaction between the coupling agents and nanoparticles and discussed their effects on the properties of obtained composites (30–33). However, fewer studies examined the interaction between silane coupling agents and composite metal fillers as well as their effect on the properties of conductive rubber.
Herein, we studied the possible use of coupling agents to modify NCG and the effect of the type and loading of coupling agents as well as the treatment technique, including dry method pretreatment, wet method pretreatment, and in situ treatment (IST), on the morphology, curing characteristics, tensile properties, and electrical conductivity of silicone rubber composites filled with NCG. In addition, X-ray photoelectron spectroscopy (XPS) was used to characterize the untreated and treated NCG surfaces, which clarified the modification mechanism and provided basis for developing conductive rubber with high electromagnetic shielding characteristics and excellent tensile properties.
2 Materials and methods
2.1 Materials
Vinyl methyl silicone (VMQ) rubber (Grade KE931-U) was acquired from Shin-Etsu Chemical, Japan. NCG (average particle size of 100 μm and nickel content of 60%) was supplied by Novamet Corporation, USA. Bis (2,4-dichlorobenzoyl) peroxide (DCBP) was used as a curing agent and was obtained from Qiangsheng Chemical Engineering Company, Jiangsu, China. Vinyltriethoxysilane (A151), octyltriethoxysilane (A137), and 3-methacryloxypropyl-trimethoxysilane (A174) were used as silane coupling agents and were obtained from GE Toshiba Silicones Co., Ltd, Japan.
2.2 Surface treatment of NCG and preparation of VMQ/NCG composites
Two methods were used to pretreat the NCG powder using a silane coupling agent: dry method treatment (DMT) and wet method treatment (WMT). In DMT, a high-speed mixer was used to disperse pure silane coupling agent into the conductive filler. In WMT, A151, A137, or A174 was diluted with ethanol (water content = 0.5%) to a concentration of 5% and the weighed conductive fillers were then added to the silane coupling agent and ethanol mixture. Next, the mixture was stirred for 30 min and then heated in a water bath at 80°C until the ethanol solvent was completely volatilized. Finally, the treated conductive filler was baked in an air oven for 2 h at 120°C. The IST method involved sequential addition of conductive filler and coupling agent to the rubber followed by their mixing at 120°C without pretreatment.
The pretreated conductive filler was fully mixed with the rubber in a two-roll mill to provide a good dispersion of the filler without destroying the nickel coating, and then DCBP was added to the mixture to obtain the mixed rubber. The blends prepared using IST were cooled to room temperature (23℃), and DCBP was added into the masterbatch. The compositions used in this study are shown in Table 1. The mixed rubber was hot pressed at 112°C and 10 MPa for 10 min to complete the first stage of the curing reaction and then heat treated in a blast oven at 200°C for 2 h to complete the second stage of the curing reaction to obtain vulcanizates.
Compositions of conductive silicone rubber
| Ingredients | phr |
|---|---|
| VMQ | 100 |
| NCG | 200 |
| DCBP | 8 |
| Coupling agent | Variable |
2.3 Characterization
2.3.1 Environmental scanning electron microscopy (ESEM)
The morphology of the NCG powder and VMQ/NCG composites were observed using an ESEM.
2.3.2 XPS
The XPS analyses of the untreated and treated NCG were conducted using an ESCALAB 250 spectrometer (Thermo-VG Scientific, UK) with an Al Kα X-ray (1,486.6 eV) source. Survey scan spectra were acquired at binding energies of 0–1,000 eV at a step of 1 eV to examine the elemental composition of the materials, and the atomic percentages were calculated based on these spectra. In addition, a narrow scan of silicon 2p (Si2p) spectra was obtained at 90–114 eV using a step of 0.1 eV.
2.3.3 Characterization of the curing parameters
An M-3000A rotorless rheometer (High-speed Railway Testing Instrument Co., Ltd, China) was used to detect the curing curve of the VMQ/NCG composites at 112°C. For the curing characteristic analysis, the curing parameters, such as minimum torque (M L) and maximum torque (M H), were obtained based on the curing curve. T 90 is defined as the optimal curing time, which is the time corresponding to the torque of M L + (M H − M L) × 90%, whereas T 10 is the time of scorching, which is the time corresponding to the torque of M L + (M H − M L) × 10% (34). The cure rate index (CRI) represents the curing speed of the silicone rubber and can be calculated as follows (34):
2.3.4 Rubber processing analysis
The relationship between the dynamic modulus G′ and strain of mixed rubber and that between tanδ and the strain of the vulcanizates were tested using an RPA2000 rubber processing analyzer (USA) at 60°C and 1 Hz.
2.3.5 Volume resistivity
A QJ84 (Shanghai Zhengyang Instrument Factory, China) micro-ohmmeter was used for volume resistivity testing in accordance with ASTM D991 by the four-probe method.
2.3.6 Tensile properties
The tensile properties were tested using a CMT4104 universal testing machine (Shenzhen SANS Group Company, China) at a speed of 500 mm·min−1 in accordance with ASTM D412.
3 Results and discussion
3.1 Effect of the type of silane coupling agent
Figure 1 shows the ESEM micrographs of the NCG packing. It can be seen from the figure that the NCG particles are irregular flaky (Figure 1a), with an average particle size of 100 μm. The nickel coating is flat, dense, and completely encapsulates the graphite core. A very small part of the graphite core is not completely covered by the nickel coating, and the defects exist on the flat part of the graphite surface rather than at both ends (Figure 1b).

ESEM micrographs of the NCG filler.
As shown in Figure 2a, the untreated VMQ/NCG composites exhibits the highest M L, reflecting its relatively high viscosity (35). The M L of the VMQ/NCG composite decreases after the surface treatment, indicating a decrease in the viscosity and an increase in the dispersion of the NCG in the rubber matrix (Figure 2b). The ΔM (M H−M L) of the A137 sample is slightly smaller than the untreated sample, but it exhibits a smaller curing rate. It is worth noting that the ΔM and curing rate of A174 and A151 samples are smaller than the untreated sample and the A174 sample is the smallest. The probable reason is that the C═C bond of the coupling agent consumes the DCBP. Under the initiation of DCBP, the coupling agent produces free radicals that can be used for self-polymerization of coupling agent molecules or grafted to the molecular chain of VMQ, resulting in a reduced amount of free radicals used for rubber cross-linking, and exhibiting a smaller curing degree and curing rate. Moreover, compared to A151, the polar double bond of A174 has a higher activity (36) and consumes more DCBP, thus the A174 sample exhibits minimal curing degree and curing rate.

(a) Curing characteristic curves of the VMQ/NCG composite and (b) comparison of NCG dispersion before and after coupling-agent modification.
The tensile properties and conductive properties of the VMQ/NCG composites are shown in Figure 3. The untreated sample exhibits a minimum tensile strength and a maximum volume resistivity. After the surface treatment of NCG, the tensile strength of VMQ/NCG composites is improved and corresponds to their ∆M. Among them, the A137 sample exhibits the smallest elongation at break, while the A174 sample shows the highest elongation at break. Specifically, the tensile strength and elongation at break of the A151 sample are 1.2 MPa and 162%, respectively, representing an increase of 50% and 63% with the untreated sample. Moreover, the volume resistivity is 0.06 Ω·cm, which is reduced by 80%. The A151 sample exhibits a faster curing rate, the best conductivity, and moderate tensile properties, so it is the optimal surface-modification agent among the three coupling agents.

(a) Tensile properties and (b) conductive properties of the VMQ/NCG composite.
The ESEM images of the untreated VMQ/NCG sample and the A151 sample are shown in Figure 4. Numerous holes are observed on the fractured section of the untreated VMQ/NCG sample, indicating a weak interaction between the NCG powder and VMQ. No obvious holes are observed on the fractured section of the A151-modified sample, indicating that A151 improves the interaction between the NCG powder and VMQ.

ESEM micrographs of the VMQ/NCG composites (a) without A151 and (b) with A151.
3.2 Effect of the A151 content
The curing, mechanical, and conductive characteristics of the VMQ filled with NCG treated using different A151 content are listed in Table 2. With increasing A151 content, ΔM gradually decreases, indicating a decrease in the curing density of the rubber matrix (37). Moreover, with the increase of A151 content, the scorch time remains unchanged, and A151 consumes DCBP, which reduces the free radical amount during the crosslinking process of VMQ (38), thus slowing down the curing rate, showing an increase in the optimal curing time and a decrease in CRI. The tensile strength of all samples increases upon increasing the A151 content from 1% (given in mass ratio based on NCG) to 3% and decreases when the A151 content is approximately 4%. The maximum tensile strength is observed at an A151 content of 3%. Moreover, the volume resistivity of all samples decreases with the increase in the A151 content from 1% to 3% and increases at approximately 4%.
Effect of A151 content on the properties of VMQ/NCG composites
| Content of A151 (%) | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Min torque M L (dN·m) | 7.35 | 6.65 | 7.15 | 6.85 |
| Max torque M H (dN·m) | 25.66 | 22.49 | 21.25 | 18.52 |
| M H − M L (dN·m) | 18.31 | 15.48 | 14.10 | 11.67 |
| Scorch time T 10 (min) | 0.30 | 0.30 | 0.32 | 0.31 |
| Optimum curing time T 90 (min) | 13.5 | 17.0 | 18.7 | 38.0 |
| CRI (min−1) | 7.6 | 6.0 | 5.4 | 2.7 |
| Volume resistivity (Ω·cm) | 0.21 | 0.13 | 0.09 | 0.50 |
| Tensile strength (MPa) | 1.12 | 1.34 | 1.82 | 0.89 |
| Stress at 50% elongation (MPa) | 0.90 | 1.04 | 1.35 | 0.53 |
| Elongation at break (%) | 159 | 121 | 75 | 102 |
These phenomena can be attributed to two factors. The dispersion of the NCG in the rubber matrix and the filler–rubber interaction are improved with increasing A151 content, enhancing the tensile strength and conductivity of the VMQ/NCG composite (Factor I). However, the curing density of the rubber matrix decreases with increasing A151 content, leading to an increased volume resistivity and decreased tensile strength (Factor II). At an A151 content lower than 3%, the effect of Factor I on the properties of the composites is greater than that of Factor II; thus, the tensile strength and conductivity increase with the increase in the A151 content. However, when the A151 content reaches 4%, the effect of Factor II is more prominent, leading to a decrease in the curing density, a reduction in the tensile strength, and an increase in the volume resistivity. Therefore, the optimal A151 content is 3%.
3.3 Effect of filler treatment techniques
Different treatment techniques have different effects on the rubber properties. Thus, three different treatments, namely WMT, DMT, and IST, were studied. Figure 5a shows the curing curve of the VMQ/NCG composite treated using different techniques. The ΔM of the WMT sample is higher than those of the samples treated using the other two treatment techniques, suggesting that the WMT-treated VMQ/NCG composite has the highest curing level.

(a) Curing curves and (b) tanδ–strain curve of the VMQ/NCG composites treated by different techniques, (c) G′–strain curve of mixed rubber, and (d) stress–strain relationship of the VMQ/NCG composites treated using different techniques.
The loss factor (tanδ)–strain curves of VMQ/NCG composites obtained by the three different treatment methods are shown in Figure 5b. Although the WMT sample exhibits the highest curing degree, it has the highest tanδ, indicating that the energy dissipation and heat accumulation in the rubber composites are pronounced under cyclic loading conditions, reflecting weak filler–matrix interaction and/or agglomeration of some of the filler (39). According to Figure 5c, with the increase in the strain, the storage modulus of VMQ/NCG composites considerably decreases, showing an obvious Payne effect. The decrease value of G′ (∆G) in the G′–strain curve can be used to evaluate the strength of the filler network in the rubber (24). The larger the ∆G is, the stronger the filler network is (40,41). The filler network includes the interaction between the filler–filler and the interaction between the filler–rubber (42). The ∆G of the WMT sample is smaller than that of the other two samples (Figure 5c), indicating that the filler network of the VMQ/NCG composites treated with WMT is weak, i.e., the weak filler–filler interaction and/or weak filler–rubber interaction. The stress–strain curve of the WMT sample exhibits a yield point phenomenon (Figure 5d), indicating an improvement of the dispersion of the filler without agglomeration after the WMT treatment. Moreover, this phenomenon is combined with a larger tanδ and a smaller ∆G, indicating that the interfacial filler–rubber interaction is weaker than those of the samples treated with the other two methods.
The tensile properties of the samples treated using different techniques are shown in Figure 5d and conductive properties of the samples are shown in Figure 6. The WMT sample exhibits the lowest tensile strength and hardness as well as the highest elongation. Meanwhile, the WMT sample exhibits the largest volume resistivity, indicating the lowest conductivity among all treated samples. The IST sample exhibits the highest tensile strength and hardness as well as the lowest elongation. The DMT sample exhibits moderate hardness and excellent tensile properties. The IST and DMT samples exhibit similar conductivities, which is in good agreement with the results illustrated in Figure 5c. Compared with CHO-SEAL® 6371, the tensile strength and elongation at break of the DMT sample increase by 53% and 62%, respectively, and the volume resistivity decreases by 50%. DMT can improve the mechanical and electrical properties of rubber, exhibits easy implementation, high production efficiency, and easy real-time monitoring of modified quality; thus, it has potential application in the rubber industry (43).

Shore A hardness and conductive properties of the VMQ/NCG composites treated using different techniques.
3.4 Modification mechanism of A151 on the NCG powder
The A151-modified NCG pretreated using WMT is considered as an example to examine the mechanism of the A151-modified NCG. Three samples were studied to examine the surface-modification mechanism of the NCG by A151. Unmodified NCG powder is designated Sample 1#. Surface-modified NCG powder with 10% A151 pretreated using WMT is labeled Sample 2#. To prepare Sample 3#, some of Sample 2# is Soxhlet-extracted by ethanol, which is a good solvent for A151, for 24 h. After the Soxhlet extraction, the sample is air dried at 100°C for 4 h. Ni, O, and C can be detected on the surfaces of all three samples, whereas Si is only observed on the surface of Samples 2# and 3# (Figure 7). The results reveal that A151 can successfully modify the surface of NCG after WMT. After 24 h of Soxhlet extraction, physical adsorption is no longer observed on the surface of NCG, leaving only chemical adsorption (44). Table 3 provides detailed information corresponding to Figure 7a. The Si2p content in Samples 1#, 2#, and 3# are 0%, 14.94%, and 9.27%, respectively. Compared with Sample 2#, the decrease in the Si2p content in Sample 3# indicates the presence of physical and chemical adsorptions between A151 and NCG, of which chemical adsorption accounts for a large part.
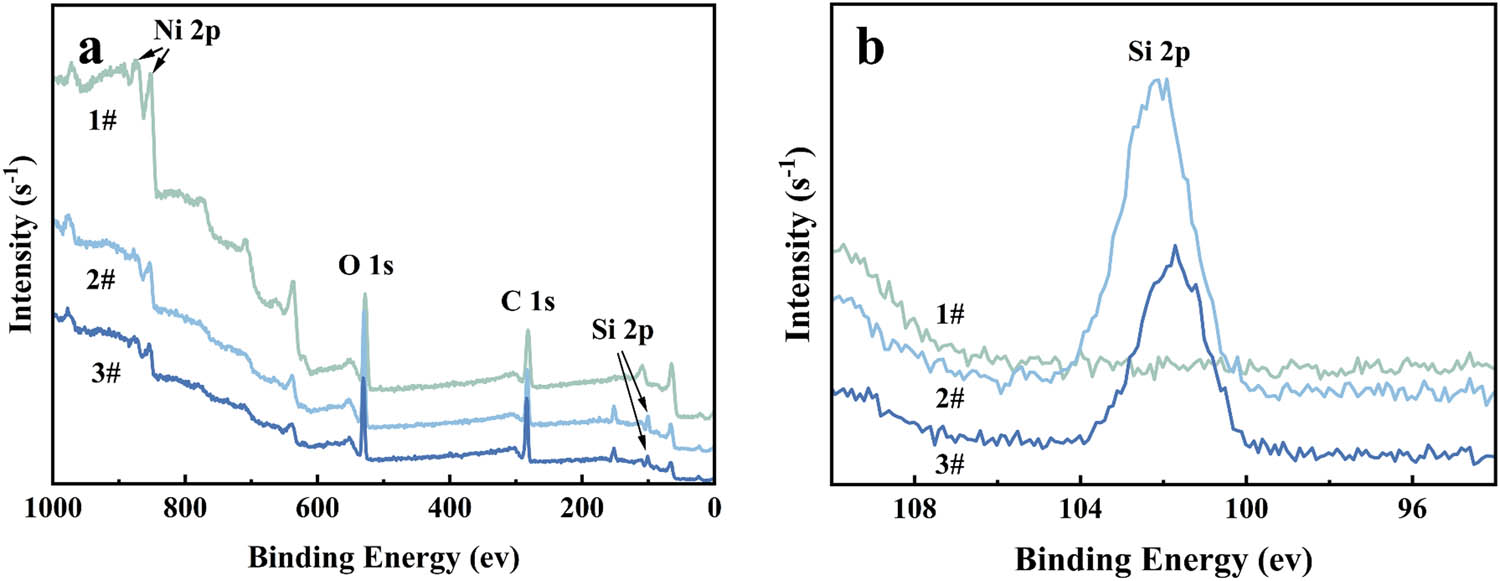
(a) XPS spectra and (b) Si 2p spectra of the composites.
Content of different surface elements in Samples 1#, 2#, and 3#
| 1# | 2# | 3# | |
|---|---|---|---|
| C1s (%) | 55.71 | 45.34 | 59.11 |
| O1s (%) | 33.14 | 34.47 | 27.85 |
| Ni2p (%) | 11.15 | 5.24 | 3.76 |
| Si2p (%) | — | 14.94 | 9.27 |
| Si/Ni (ration) | 0 | 2.85 | 2.47 |
The A151 monomer dissolved in ethanol during the WMT evaporates during the volatilization of ethanol. Thus, no A151 monomer is bound to the of NCG surface with van der Waals forces. Owing to the slow rate of hydrolysis of siloxanes under neutral conditions, the alkoxy group of A151 is not completely hydrolyzed (32,45). Oligomers can be formed by a dehydration condensation reaction between the hydroxyl groups of partially hydrolyzed A151 monomers (46). The surface treatment layer of NCG pretreated with WMT may contain the following types of physical adsorption: attachment of the A151 oligomers to the surface through van der Waals forces (Figure 8a) and attachment of the A151 monomers to the surface through hydrogen bonds (Figure 8b). In addition, A151 monomers (Figure 8c) and A151 oligomers (Figure 8d) are bound to the surface via covalent bonds.

Mechanism of the A151-modified NCG.
4 Conclusions
Herein, the effect of the surface treatment of NCG on the properties of conductive silicone rubber and the interaction between coupling agents and NCG were investigated. A151, the best modifier among the studied modifiers (A151, A137, and A174), can improve the dispersion of the filler in the rubber matrix and the interfacial bond strength between the silicone rubber and NCG. The results of Soxhlet extraction combined with XPS indicate that A151 is bound to the NCG surface through physical and chemical adsorptions, in which chemical adsorption plays the major role. The optimal A151 content, at which the best overall performance is obtained, is found to be 3%. The filler treatment exhibits a considerable effect on the rubber properties. Among the tested techniques, DMT is simple to operate and has transparent quality control process. In addition, it considerably improves the mechanical properties of the composite. Thus, it exhibits excellent potential for industrial production.
Abbreviations
- A137
-
octyltriethoxysilane
- A151
-
vinyltriethoxysilane
- A174
-
3-methacryloxypropyl-trimethoxysilane
- DCBP
-
bis (2,4-dichlorobenzoyl) peroxide
- DMT
-
dry method treatment
- IST
-
in situ treatment
- NCG
-
nickel-coated graphite
- WMT
-
wet method treatment
Acknowledgements
The authors thank the Ministry of Education Fund for sponsoring this study.
-
Funding information: This work was funded by the Ministry of Education Fund (No. 8091B012104).
-
Author contributions: Xindi Zhuang: investigation, writing – original draft; Hua Zou: methodology, resources, supervision; Baotong Xing: formal analysis, experimental data processing; Wei Liu: writing – review and editing, visualization; Hongda Mao: writing – review and editing, formal analysis.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study may be provided by the corresponding authors upon reasonable request.
References
(1) Zhang JH, Zhang H, Wang H, Chen FB, Zhao YF. Extruded conductive silicone rubber with high compression recovery and good aging-resistance for electromagnetic shielding applications. Polym Compos. 2019;40(3):1078–86.10.1002/pc.24802Search in Google Scholar
(2) Xiao WM, Lei YP, Xia ZD, Chen X, Han Y, Nie JK. Effect of silver plating time on the properties of conductive silicone rubber filled with silver-coated carbonyl nickel powder. J Alloy Compd. 2017;724:24–8.10.1016/j.jallcom.2017.07.021Search in Google Scholar
(3) Wang R, Yang H, Wang JL, Li FX. The electromagnetic interference shielding of silicone rubber filled with nickel coated carbon fiber. Polym Test. 2014;38:54–6.10.1016/j.polymertesting.2014.06.008Search in Google Scholar
(4) Portes RC, Lopes BH, Junior MA, Florez-Vergara DE, Quirino SF, Baldan MR. Effect of granulometric distribution on electromagnetic shielding effectiveness for polymeric composite based on natural graphite. Sci Eng Compos Mater. 2019;26:531–9.10.1515/secm-2019-0037Search in Google Scholar
(5) Yang JM, Chen YJ, Wang B, Zhou YG, Chai XZ, Yan X, et al. Gradient structure silicone rubber composites for selective electromagnetic interference shielding enhancement and low reflection. Compos Sci Technol. 2022;229:109688.10.1016/j.compscitech.2022.109688Search in Google Scholar
(6) Das NC, Maiti S. Electromagnetic interference shielding of carbon nanotube/ethylene vinyl acetate composites. J Mater Sci. 2008;43:1920–5.10.1007/s10853-008-2458-8Search in Google Scholar
(7) Zaccone M, Frache A, Torre L, Armentano I, Monti M. Effect of filler morphology on the electrical and thermal conductivity of PP/carbon-based nanocomposites. J Compos Sci. 2021;5:196.10.3390/jcs5080196Search in Google Scholar
(8) Liu Z, Gao XP, Zhu B, Yuan XM. Sea urchin-like MnO2@Carbon fiber paper@carbon black modified silicone rubber interface control to improve electromagnetic properties and media factors. Chem Eng J. 2024;495:153528.10.1016/j.cej.2024.153528Search in Google Scholar
(9) Abbasi H, Antunes M, Velasco JI. Recent advances in carbon-based polymer nanocomposites for electromagnetic interference shielding. Prog Mater Sci. 2019;103:319–73.10.1016/j.pmatsci.2019.02.003Search in Google Scholar
(10) Wang G, Liao X, Yang JM, Tang WY, Zhang Y, Jiang QY, et al. Frequency-selective and tunable electromagnetic shielding effectiveness via the sandwich structure of silicone rubber/graphene composite. Compos Sci Technol. 2019;184:107847.10.1016/j.compscitech.2019.107847Search in Google Scholar
(11) Zhang J, Feng SY, Ma QY. Kinetics of the thermal degradation and thermal stability of conductive silicone rubber filled with conductive carbon black. J Appl Polym Sci. 2003;89:1548–54.10.1002/app.12277Search in Google Scholar
(12) Mondal S, Ganguly S, Rahaman M, Aldalbahi A, Chaki TK, Khastgir D, et al. A strategy to achieve enhanced electromagnetic interference shielding at low concentration with a new generation of conductive carbon black in a chlorinated polyethylene elastomeric matrix. Phys Chem Chem Phys. 2016;18:24591–9.10.1039/C6CP04274KSearch in Google Scholar PubMed
(13) Lee HH, Chou KS, Shih ZW. Effect of nano-sized silver particles on the resistivity of polymeric conductive adhesives. Int J Adhes Adhes. 2005;25:437–41.10.1016/j.ijadhadh.2004.11.008Search in Google Scholar
(14) Jia LC, Jia XX, Sun WJ, Zhang YP, Xu L, Yan DX, et al. Stretchable liquid metal-based conductive textile for electromagnetic interference shielding. ACS Appl Mater Inter. 2020;12:53230–8.10.1021/acsami.0c14397Search in Google Scholar PubMed
(15) Peng F, Zhu WB, Fang Y, Fu BC, Chen HT, Ji HJ, et al. Ultralight and highly conductive silver nanowire aerogels for high performance electromagnetic interference shielding. ACS Appl Mater Inter. 2023;15:4284–93.10.1021/acsami.2c16940Search in Google Scholar PubMed
(16) Rashid IA, Hamza A, Asim S, Zubair K, Shakir HF, Afzal A, et al. Achieving enhanced electromagnetic shielding by novel flexible rubber based nanocomposite with the incorporation of nickel spinal ferrites and polyaniline. Synth Met. 2023;295:117339.10.1016/j.synthmet.2023.117339Search in Google Scholar
(17) Liang TX, Guo WL, Yan YH, Tang CH. Electroless plating of silver on graphite powders and the study of its conductive adhesive. Int J Adhes Adhes. 2007;28:55–8.10.1016/j.ijadhadh.2007.03.006Search in Google Scholar
(18) Shao XM, He L, Chen Y, Yao QY, Hao MZ, Tian M, et al. Preparation of antisalt spray conductive silver-plated carbon fiber via tannic acid/metal ion complex inhibitors for electromagnetic interference shielding. Ind Eng Chem Res. 2024;63:2231–43.10.1021/acs.iecr.3c03725Search in Google Scholar
(19) Yang JM, Yang YQ, Duan HJ, Zhao GZ, Liu YQ. Light-weight epoxy/nickel coated carbon fibers conductive foams for electromagnetic interference shielding. J Mater Sci-Mater Electron. 2017;28:5925–30.10.1007/s10854-016-6266-7Search in Google Scholar
(20) Mao HD, Zou H, Liu W, Zhuang XD, Xing BT. Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance. E-Polymers. 2024;24:20240018.10.1515/epoly-2024-0018Search in Google Scholar
(21) Mei SQ, Wang J, Wan JT, Wu XC. Preparation methods and properties of CNT/CF/G carbon-based nano-conductive silicone rubber. Appl Sci (Basel). 2023;13:6726.10.3390/app13116726Search in Google Scholar
(22) Wang GX, Yu QZ, Hu YM, Zhao GY, Chen JW, Li H, et al. Influence of the filler dimensionality on the electrical, mechanical and electromagnetic shielding properties of isoprene rubber-based flexible conductive composites. Compos Commun. 2020;21:100417.10.1016/j.coco.2020.100417Search in Google Scholar
(23) Xue JX, Hao YJ, Qu SQ, Wang CS, Li L. Exploring the reinforcement and conductivity mechanism of K2FeO4-modified multiwalled carbon nanotubes in carbon black/natural rubber composites. Polym Compos. 2024;45(7):6252–63.10.1002/pc.28193Search in Google Scholar
(24) Zou H, Zhang LQ, Tian M, Wu SZ, Zhao SH. Study on the structure and properties of conductive silicone rubber filled with nickel-coated graphite. J Appl Polym Sci. 2010;115:2710–7.10.1002/app.29901Search in Google Scholar
(25) Zou H, Yan SN, Sun YB, Zhang LQ, Tian M. Conducting stability of nickel-coated graphite/methyl vinyl silicone rubber composites. J Appl Polym Sci. 2012;125:3456–62.10.1002/app.36532Search in Google Scholar
(26) Wu LR, Yang HT, Cheng JX, Hu CQ, Wu ZW, Feng Y. Review in preparation and application of nickel-coated graphite composite powder. J Alloy Compd. 2021;862:158014.10.1016/j.jallcom.2020.158014Search in Google Scholar
(27) Shokoohi S, Arefazar A, Khosrokhavar R. Silane coupling agents in polymer-based reinforced composites: a review. J Reinf Plast Compos. 2008;27:473–85.10.1177/0731684407081391Search in Google Scholar
(28) Liu Q, Ding J, Chambers DE, Debnath S, Wunder SL, Baran GR. Filler-coupling agent–matrix interactions in silica/polymethylmethacrylate composites. J Biomed Mater Res. 2001;57:384–93.10.1002/1097-4636(20011205)57:3<384::AID-JBM1181>3.0.CO;2-FSearch in Google Scholar
(29) Aziz T, Ullah A, Fan H, Jamil MI, Khan FU, Ullah R, et al. Recent progress in silane coupling agent with its emerging applications. J Polym Environ. 2021;29:3427–43.10.1007/s10924-021-02142-1Search in Google Scholar
(30) Han DS, Pan Y, Xue JX, Yu BH, Yan G, Wang CS, et al. Effect of adding different silane coupling agents on metal friction and wear in mixing process. J Appl Polym Sci. 2021;138:51408.10.1002/app.51408Search in Google Scholar
(31) Lee SY, Kim JS, Lim SH, Jang SH, Kim DH, Park NH, et al. The investigation of the silica-reinforced rubber polymers with the methoxy type silane coupling agents. Polymers. 2020;12:3058.10.3390/polym12123058Search in Google Scholar PubMed PubMed Central
(32) Ahangaran F, Navarchian AH. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: a review. Adv Colloid Interface. 2020;286:102298.10.1016/j.cis.2020.102298Search in Google Scholar PubMed
(33) Li JX, Zhang SY, Fan YQ, Wang AS, Miao Z, Cheng P, et al. Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultrahigh-filled polypropylene composites. E-Polymers. 2022;22:585–94.10.1515/epoly-2022-0056Search in Google Scholar
(34) Liu W, Zou H, Xing BT, Wang SQ, Mao HD, Zhang JY. Influence of the molecular structure of phenylamine antioxidants on anti-migration and anti-aging behavior of high-performance nitrile rubber composites. E-Polymers. 2023;23:20230090.10.1515/epoly-2023-0090Search in Google Scholar
(35) Ismail H, Nordin R, Noor AM. Cure characteristics, tensile properties and swelling behaviour of recycled rubber powder-filled natural rubber compounds. Polym Test. 2002;21:565–9.10.1016/S0142-9418(01)00125-8Search in Google Scholar
(36) Choi W, Kim H, Kang S, Lee YS, Han JH, Kim B. Mechanical interfacial adhesion of carbon fibers-reinforced polarized-polypropylene matrix composites: effects of silane coupling agents. Carbon Lett. 2016;17(1):79–84.10.5714/CL.2016.17.1.079Search in Google Scholar
(37) Surya I, Siswarni MZ. Crosslink density and rheometric behaviour of natural rubber/chloroprene rubber blends. Iop Conf Ser-Mat Sci. 2019;505:012113.10.1088/1757-899X/505/1/012113Search in Google Scholar
(38) Stricher AM, Rinaldi RG, Barrès C, Ganachaud F, Chazeau L. How I met your elastomers: from network topology to mechanical behaviours of conventional silicone materials. RSC Adv. 2015;5:53713–25.10.1039/C5RA06965CSearch in Google Scholar
(39) Kazemi H, Mighri F, Park KW, Frikha S, Rodrigue D. Natural rubber biocomposites reinforced with cellulose nanocrystals/lignin hybrid fillers. Polym Compos. 2022;43:5442–53.10.1002/pc.26846Search in Google Scholar
(40) Hao Z, Lu XF, Shen Z, Shen JQ, Sheng X, Luo Z, et al. Processing rheological behavior of functionalized silica/natural rubber composites. Mater Res Express. 2019;6:085303.10.1088/2053-1591/ab1c17Search in Google Scholar
(41) Phuhiangpa N, Ponloa W, Phongphanphanee S, Smitthipong W. Performance of nano- and microcalcium carbonate in uncrosslinked natural rubber composites: new results of structure–properties relationship. Polymers. 2020;12:2002.10.3390/polym12092002Search in Google Scholar PubMed PubMed Central
(42) Fu W, Wang L. Variation of the payne effect in natural rubber reinforced by graft-modified carbon black. J Macromol Sci B. 2017;56:53–63.10.1080/00222348.2016.1261593Search in Google Scholar
(43) Zhu DH, Nai XY, Lan SJ, Bian SJ, Liu X, Li W. Surface modification of magnesium hydroxide sulfate hydrate whiskers using a silane coupling agent by dry process. Appl Surf Sci. 2016;390:25–30.10.1016/j.apsusc.2016.08.033Search in Google Scholar
(44) Yamazaki R, Karyu N, Noda M, Fujii S, Nakamura Y. Quantitative measurement of physisorbed silane on a silica particle surface treated with silane coupling agents by thermogravimetric analysis. J Appl Polym Sci. 2016;133:43256.10.1002/app.43256Search in Google Scholar
(45) Wang Z, Liu MC, Chang ZY, Li HB. Study on the graft modification mechanism of macroporous silica gel surface based on silane coupling agent vinyl triethoxysilane. RSC Adv. 2021;11:25158–69.10.1039/D1RA04296CSearch in Google Scholar
(46) Khurana N, Arora P, Pente AS, Pancholi KC, Kumar V, Kaushik CP, et al. Surface modification of zinc oxide nanoparticles by vinyltriethoxy silane (VTES). Inorg Chem Commun. 2021;124:108347.10.1016/j.inoche.2020.108347Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

