Abstract
Porous molecularly imprinted polymer (MIP) microspheres were synthesized via iniferter-suspension polymerization method, employing lenacil (LA) as the template molecule and methacrylic acid (MAA) as the functional monomer. The host–guest complexes formed using LA and MAA were characterized by hydrogen nuclear magnetic resonance and ultraviolet–visible absorption spectroscopy. The obtained results showed that the interaction between LA and MAA mainly relied on hydrogen bonding. The surface morphologies and chemical structures of the MIPs were characterized by scanning electron microscopy. MIPs were spherical in shape with a relatively regular sphericity, rough surface structure, and numerous small holes, which significantly reduced the mass transfer resistance of the template molecules and exhibited excellent recognition performance for template molecules. In addition, soil samples were pretreated with solid-phase extraction columns molecularly imprinted with LA, and analyzed by high-performance liquid chromatography. The recoveries of LA, bromacil, and terbacil were up to 89.65%, 53.17%, and 44.63%, respectively. The developed method showed a minimum detection limit of 10–50 µg·mL−1. In view of the continuous increase of public requirements for pesticide residue detection, a versatile pretreatment method was developed that is green, rapid, simple, and can be miniaturized.
1 Introduction
Organophosphorus pesticides are commonly used to protect crops from pests and weeds. However, their large-scale use and abuse may lead to their residual accumulation in agricultural products, making them harmful to human health (1,2). Currently, the main methods typically used for pesticide residue detection are gas chromatography (GC) (3), high-performance liquid chromatography (HPLC) (4), GC–mass spectrometry (GC–MS) (5), liquid chromatography–mass spectrometry (LC–MS) (6), electrochemical (7), chemosensor-based methods (8), and enzyme-linked immunoassays (9). Owing to the complex matrix effects of agricultural products and trace residues of organophosphorus pesticides, the traditional sample pretreatment process poses several challenges such as elaborate steps, lack of specific selection of adsorbent materials, and slow mass transfer rate. Therefore, it is of great practical significance to prepare adsorbents with high specificities and adsorption efficiencies, develop new sample pretreatment technologies, and establish simple, highly sensitive, and low-cost concentration detection methods for monitoring organophosphorus pesticide residues in agricultural products (10).
Molecular imprinting is a bionic molecular recognition technology based on the simulation of antigen–antibody, enzyme–substrate, and other biological molecular recognition specificities (11), The molecularly imprinted polymers (MIPs) obtained by this technique have the characteristics of structure predetermination and recognition specificity (12) and offer wide application possibilities as they can specifically adsorb and identify a variety of target compounds. Owing to these remarkable properties, MIPs have been widely used for chromatographic separation (13), solid-phase extraction (SPE) (14,15), chemical sensing (16), catalysis (14,17,18), drug delivery (19), and artificial enzymes (20).
Among these applications, the SPE technology has been widely used in agricultural residue detection and food analysis owing to the advantages of requirement of few organic solvents, batch processing of samples, and enrichment and removal of impurities (21). However, in the case of complex sample matrices, impurities with similar polarities cannot be effectively removed. Molecularly imprinted SPE (MISPE) has been developed by combining molecular imprinting and SPE technologies to make full use of their individual advantages (22). This technology uses MIPs, which have a similar selectivity to immunoadsorption materials, as fillers for SPE. It not only overcomes the disadvantages of traditional SPE technologies, such as poor selectivity, but also offers the advantages of simple preparation, good stability, and reusable MIPs (23,24).
Generally, MIPs can be divided into covalent and non-covalent based on the types of interactions between the functional monomers and template molecules to form host–guest complexes (25). MIPs prepared by covalent bonding have the disadvantages of difficult template molecular elution and low specific recognition rates; therefore, the number of MIPs prepared by this method is small. Compared with covalent MIPs, non-covalent MIPs are convenient for template elution, can quickly identify the template, and are widely used owing to their high selectivity and affinity. Therefore, the degree of binding between the template molecules and functional monomers before polymerization is a crucial factor (26). The non-covalent bond forces between these two entities mainly include hydrogen bonding, metal coordination bonding, ionic bonding, hydrophobic interactions, π–π–π interactions, and van der Waals forces; therefore, only when a strong mutual interaction occurs, it is possible to rely on this force to form a stable host–guest complex through self-assembly.
In this study, the MISPE technology was used to detect the herbicide, lenacil (LA), and its structural analogs in soil samples. Specifically, LA was used as the template molecule, while porous MIP microspheres prepared using a combined iniferter (27)–suspension (28) polymerization method were used as adsorption materials for SPE to detect LA and determine its enrichment in soil samples. The interactions between the host and guest complexes (template molecules and functional monomers) were investigated by hydrogen nuclear magnetic resonance (1H NMR) and ultraviolet–visible (UV–Vis) spectroscopies, and suitable functional monomers were selected for the preparation of highly selective MIP materials. To further investigate the specific recognition performance of the generated MIPs, bromacil (BA) and terbacil (TA) were selected as disruptors for LA detection in soil samples when conducting MISPE experiments, and the sensitivity and selectivity of the method were evaluated.
2 Materials and methods
2.1 Main reagents and instruments
LA, (AR), BA, (AR), TA, (AR), methacrylic acid (MAA, AR), and polyvinyl alcohol (PVA, AR) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. N,N-diethylcarbamic acid benzyl ester (BDC) was synthesized by the literature method. Ethylene glycol dimethacrylate (EDGMA, purity is 97%) from Yantai Yunkai Chemical Co., Ltd. The remaining reagents are analytically pure and are produced by Sinopharm Group Chemical Reagent Co., Ltd.
Electron scanning microscope (Zeiss Merlin Compact, Zeiss, Germany), nuclear magnetic resonance instrument (AVANCE NEO 400 M, Bruker, Germany), ultraviolet dimethacrylate visible spectrophotometer (UV-3900, Shimadzu Corporation, Japan), high-performance liquid chromatograph (LC-2060C, Shimadzu Corporation, Japan), digital display water bath constant temperature oscillator (SHZ-C, Jintan Jiangnan Instrument Factory), ultrasonic cleaner (KQ-300DA, Kunshan Shumei Ultrasonic Instrument Co., Ltd.), Fourier transform infrared spectrometer (IRTracer-100, Shimadzu Corporation, Japan), nitrogen absorption apparatus (AutoChem III, Micromeritics, USA), and thermo gravimetric analyzer (STA200, HITATHI, Japan).
2.2 Preparation of LA MIP microspheres
The porous MIP microspheres used in this study were prepared using a combined iniferter–suspension polymerization method (29). The specific experimental steps are described below. First, PVA (0.5 g) and deionized water (50 mL) were added to a 150-mL four-port quartz flask and slowly heated to 90°C under mechanical stirring to afford a 1% PVA aqueous solution, which was obtained as a uniform and transparent solution and then stored. Subsequently, LA (0.234 g) and CHCl3 (10 mL) were added to a 50-mL single-mouth flask, treated with ultrasonic irradiation for 30 min, followed by the addition of MAA, and then placed in an ice bath at 0°C for 1 h for pre-polymerization. Then, an appropriate amount of the crosslinking agent EGDMA and iniferter photoinitiator BDC were added (Figure 1).

Schematic diagram of the LA molecular imprinting process.
A uniform transparent solution was formed after ultrasonic dispersion, which was then added to the previously prepared PVA aqueous solution and ultrasonic treatment was continued for 1 h to obtain evenly small droplets. The mixture was subjected to mechanical agitation and ultraviolet light irradiation (365 nm, 250 W high-pressure mercury lamp, 15 cm away from the flask) for 5 h under a N2 atmosphere, and the LA molecules were eluted with a methanol/acetic acid (9/1, v/v) mixture until no LA was detected in the eluent. MIP microspheres were obtained after vacuum drying.
The preparation of the non-molecularly imprinted polymer (NIP) microspheres followed a similar procedure as that described above, except that the template molecule LA was not added during the preparation process.
2.3 Preparation of the MISPE column
Dry MIPs or NIPs (500 mg) were packed in an SPE column made of polypropylene with a volume of 6 mL, which was sealed with a porous polytetrafluoroethylene sieve plate. Prior to use, the MISPE column was washed with a mixture of methanol and acetic acid (7/3, V/V) at a flow rate of 0.5 mL·min−1, and the corresponding eluent was collected until the template could not be detected by HPLC. Then, it was washed with a large amount of methanol and vacuum-dried at 50°C for further use. Before use, the column was conditioned with acetonitrile (3 mL) and deionized water (3 mL).
2.4 Methods for soil sample treatment
After centrifugation of the soil sample at 4,000 rpm for 5 min, the supernatant was collected and stored in a brown EP tube, and the sample was frozen at −16°C for future use.
A series of MIPs and NIPs were weighed (10 mg each) and placed in a 50-mL conical bottle together with 5 mL of a methanol solution containing either LA, BA, or TA at 0.1 mg·mL−1. To the mixed aqueous soil solution (10 mL), the supernatant (5 mL) was added after high-speed centrifugation. After shock and adsorption for 10 h, the supernatant was collected as soil sample solution for further measurements after centrifugation.
The soil sample treatment method selected for this experiment was as follows. A certain quantity (50 μL) of soil sample to be tested was withdrawn and 1% acetic acid aqueous solution (15 μL) and acetonitrile (100 μL) were added to a 1.5-mL brown EP tube. Subsequently, the solution was centrifuged for 5 min at 3,000 rpm, and 50 μL of the supernatant was withdrawn. 10 μL solution was used for high-performance liquid chromatograph analysis.
3 Results
3.1 Interactions between template molecules and functional monomers
In the investigated non-imprinted polymerization systems, the molecular structure of the template molecule LA contained functional groups, such as amino and carbonyl groups (Figure 2). Under specific conditions, certain interaction forces can occur with the functional groups in the monomeric molecules (e.g., hydroxyl, carboxyl, and amino), which can give rise to a stable template molecule–functional monomer (host–guest) complex that can be polymerized and cured to remove template molecules to afford non-covalent MIPs with excellent selectivity. A detailed study was thus conducted on the interaction between the two interacting partners using UV–Vis and 1H-NMR analytical methods (30,31,32).

Chemical structures of LA, BA, and TA.
3.1.1 UV–Vis analysis
As shown in Figure 3 and Table 1, the characteristic peak of the pure LA solution is at 211 nm. When mixed with the functional monomer MAA, a peak appears at 215 nm that is redshifted by 4.5 nm, indicating a strong molecular force between LA and the functional monomer MAA. After mixing LA and acrylamide (AM), a peak is observed at 212 nm with a redshift of 1 nm, which is ascribed to a weak molecular force between LA and AM. After LA is mixed with the functional monomer acrylic acid (AA), a characteristic peak is observed at 207.5 nm with a redshift of 3.5 nm, again indicating a strong molecular interaction between LA and the functional monomer MAA. Among the three monomers studied, MAA and LA exhibit the strongest molecular interaction forces and emerge as the best monomers for these experiments.

UV–Vis diagram of LA mixed with different monomers.
UV characteristic absorption peaks of the functional monomer–LA mixture
| MAA | AM | AA | |
|---|---|---|---|
| 1:0 | 211.00 | 211.00 | 211.00 |
| 1:4 | 215.00 | 212.00 | 207.50 |
| Δλ (nm) | 4.0 | 1 | 3.5 |
During MIP preparation, the likelihood of obtaining MIPs with good selectivity and strong affinity mainly depends on whether the pre-assembled complexes formed by the template molecules and monomers are stable (33). The present study showed that the ratio between the two components was key to determining the strength of their interaction in a pre-assembled system. In this case, by keeping the concentration of the LA solution constant and gradually increasing the concentration of the functional monomer MAA (molar concentration ratio between the two ranged from 1:0 to 1:9), a series of ultraviolet absorption spectra at 190–400 nm was obtained (Figure 3).
As shown in Figure 3, in the wavelength range of 190–300 nm, as the specific performance and proportion of functional monomers increase, the absorbance of the solution also increases. When the molar ratio is 1:0, the UV absorption of the LA solution is observed, and the wavelength corresponding to the highest peak is the maximum absorption wavelength of LA. When the ratio is 1:1, both LA and MAA exist in the solution, the absorbance value of the solution sharply increases, and the rate of increase reaches a maximum, indicating that the addition of MAA affects the absorbance of LA. It can be speculated that a chemical force may be generated between the two components. With increasing concentrations of MAA, the absorbance of the preassembled system also increases, indicating that the interaction force between the two components is further enhanced. When the ratio between the two species reaches 1:4, the increasing amplitude of the absorbance value starts decreasing, whereas the amount of MAA continues to increase (1:5–1:8). Although the intensity of the absorption peak is still increasing slowly, such increase is small. For LA solutions at a certain concentration, the addition of too many or too few functional monomers does not lead to an optimal pre-assembled system. Therefore, it is necessary to ensure the strongest interactions and stability. The molar concentration ratio corresponds to the optimal proportion to form a pre-assembled complex with good selectivity and high stability only when the absorbance value increases slowly.
In summary, when the molar concentration ratio of LA and the functional monomer MAA is 1:4, a stable pre-assembled complex with strong interactions is formed, suggesting that, theoretically, the prepared LA MIP possesses the strongest recognition ability (Figure 4).

UV diagrams of different proportions of LA and MAA.
3.1.2 1H NMR analysis
Under the same detection conditions, Figure 5 shows that a sharp peak appears at a chemical shift of 11.13 ppm in the 1H NMR spectrum of LA; this is mainly due to the different degrees of influence of the surrounding structural factors on the amide-amino active hydrogen in the molecular structure of LA. Since such active hydrogen is conjugated with the lone pair of electrons on the N atom and the p–π orbitals of the carbonyl group, the C–N bond can act as partial double bond, giving rise to a sharp single peak. After the addition of MAA, the active hydrogenation degree of the amido group of LA shifts from 11.31 to 11.23 ppm, which may be due to the interaction between the active hydrogen on the imino group and the carboxyl group in the molecular structure of MAA (Figure 5). In contrast, a comparison of the displacement of the active hydrogen peak due to the carboxyl group in the molecular structures of MAA and LA-MAA shows that the corresponding chemical shift for MAA is 12.36 ppm, whereas the peak shifts to 12.08 ppm after complete mixing with LA. It is further proven that the amino group in the molecular structure of LA forms a hydrogen bond with the carboxyl group in the molecular structure of MAA. In the imprinting polymerization system, the nitrogen atoms in the amido group of the template molecule LA and the oxygen atoms in the carbonyl group form a stable complex with the carboxyl group of the functional monomer MAA, leading to the displacement of specific protons in the LA and MAA molecules.

1H-NMR diagrams of LA, MAA, and LA–MAA.
3.2 SEM analysis of MIPs and NIPs microspheres
SEM was used to analyze the microstructures of the MIP and NIP surfaces, and the corresponding results are shown in Figure 6. It can be clearly seen that MIP and NIP microspheres prepared by iniferter–suspension polymerization exhibit good sphericity, no surface damage, and good regularity, with an average particle size of 46.86 and 48.74 µm, respectively. The MIP diagram clearly shows that the surface is rough and numerous small holes are distributed on the surface. This may be due to the fact that the addition of the grinding plate molecules affects the growth rate of the polymeric chain, resulting in an uneven precipitation rate of the polymerization chain from the continuous phase, which affects the surface topography of the imprinted polymer and ultimately results in the formation of a relatively rough surface. In addition, the average particle size of the MIPs is lower than that of the corresponding NIPs, which may be due to the effect of the polar template on the solubility or polymerization rate of the solvent in the MIP system, resulting in a decrease in the particle size of the imprinted polymer. This phenomenon has also been observed in the case of other imprinting polymerization systems (34,35),

SEM images of MIPs (left) and NIPs (right) microspheres.
3.3 FTIR analysis of the MIPs and NIPs
Figure 7 displays the FTIR analysis spectrum, featuring the infrared spectra of MIPs and NIPs. The left image corresponds to MIPs, while the right image represents NIPs. The OH stretching vibration peaks are prominent at 3,510 and 3,450 cm−1, indicating the presence of the active hydrogen in the carboxyl group of polymethacrylic acid (33). The C═O stretching vibration peak at 1,730 cm−1 is specifically associated with the absorption peak of LA. At 1,630 cm−1, the stretching vibration peak of C═C is observed, coinciding with the special absorption peak of MAA. The peaks at 1,160 and 1,170 cm−1 indicate the stretching vibration of C═S and the stretching vibration peak of iniferter, suggesting the involvement of RAFT iniferter in the preparation of molecularly imprinted microspheres. The stretching vibration peaks at 1,250 and 1,260 cm−1 are attributed to C–O–C and the special absorption peak of EGDMA (33).

FTIR of the MIPs and NIPs.
The synthesis involves cross-linked polymerization of MAA and EGDMA. The presence of the C═S bond serves as evidence supporting the participation of iniferter in the preparation of these molecularly imprinted microspheres. These detailed findings contribute to a comprehensive understanding of the chemical structure and functional groups within the synthesized polymers.
3.4 Surface and pore size of the MIPs and NIPs
In Figure 8a, distinctive hysteresis loop is evident between the N2 adsorption and desorption curves of the MIPs, signifying a consistent and uniform porous structure on their surfaces. Under low-pressure conditions, the adsorption capacity experienced gradual increments, with N2 molecules being adsorbed in a monolayer, subsequently forming a multilayer on the inner surface of the MIP channels.

N2 adsorption analysis of the MIPs (left) and NIPs (right).
Upon reaching a relative pressure (P/P o) exceeding 0.90, the gas adsorption capacity displayed a linear increase, indicating the formation of large pores within the MIPs. These large pores play a significant role in influencing both the MIPs and their overall pore structure. Conversely, the adsorption–desorption curve of NIPs exhibited a steep rise when P/P o surpassed 0.85, accompanied by a distinct hysteresis ring. This phenomenon is likely attributed to the poor homogeneity of the pore structure in the polymer, which contains either an abundance of large pores or an accumulation of particles.
In Table 2, it is evident that the specific surface area of MIPs was determined to be notably larger at 78.05 m2·g−1 compared to NIPs at 51.02 m2·g−1, a difference likely attributed to the inclusion of the template. The imprinted polymerization system played a crucial role in enhancing the growth rate of polymer chains and influencing viscosity. During the MIPs curing, the numerous template molecules occupied positions within the polymer structure, subsequently forming cavities after elution. This process significantly increased the number of pores on the surface. The MIPs showcased not only a high specific surface area but also an expansive adsorption space, underscoring their robust adoption capabilities. Additionally, the average pore size measured at 11.23 nm surpassed the dimensions of the LA template molecule. This larger pore size facilitates the unhindered entry and exit of LA through the pore channels, resulting in low steric hindrance. Consequently, this characteristic accelerates the molecular recognition rate and imparts an exceptionally high molecular recognition ability to the MIPs.
Surface areas, pore volumes, and pore sizes of the microsphere
| Adsorbent | d p (nm) | V p (m2·g−1) | S (m2·g−1) |
|---|---|---|---|
| NIPs | 8.99 | 0.0312 | 51.02 |
| MIPs | 11.23 | 0.0524 | 78.05 |
3.5 Thermogravimetric analysis of the MIPS
The weight loss and stability of MIPs are evident from the TG analysis, as depicted in Figure 9. Notably, at approximately 100°C, there is a slight change in weight influenced by oxygen. Within the temperature range of 120–390°C, the weight of MIPs experiences a gradual reduction attributed to the decomposition of small molecules present on the MIPs. Post the 390°C mark, there is a notable decline in weight, which can be attributed to the decomposition of polymethacrylic acid induced by the elevated temperature. This abrupt decrease underscores a pivotal transformation in the composition of MIPs at higher temperatures.

Thermogravimetric analysis of the MIPS.
3.6 MISPE analysis of LA in soil samples
As shown in Figures 10 and 11, after the MISPE column treatment, the impurities detected by HPLC in the liquid soil sample as well as in standard products are low, whereas the recoveries of LA, BA, and TA are 89.65%, 53.17%, and 44.63%, respectively. The low recoveries of BA and TA in comparison to that of LA suggest that the MISPE column prepared by iniferter–suspension polymerization exhibits a strong specific recognition effect on the template molecules. In addition, the recognition of structural analogs (BA and TA) is less effective, suggesting that MIPs can specifically identify target molecules within complex environments, which is ideal for industrial applications. The detection limits of this method range from 10.0 to 50.0 µg·mL−1. Owing to the lack of imprinting holes and recognition sites matching the target molecules, the recovery efficiencies of the NIPs for LA, BA, and TA are relatively low, and only undifferentiated non-specific adsorption occurs. These results demonstrate the potential applicability of MIPs for the efficient concentration, separation, and accurate quantification of LA in complex biological samples.
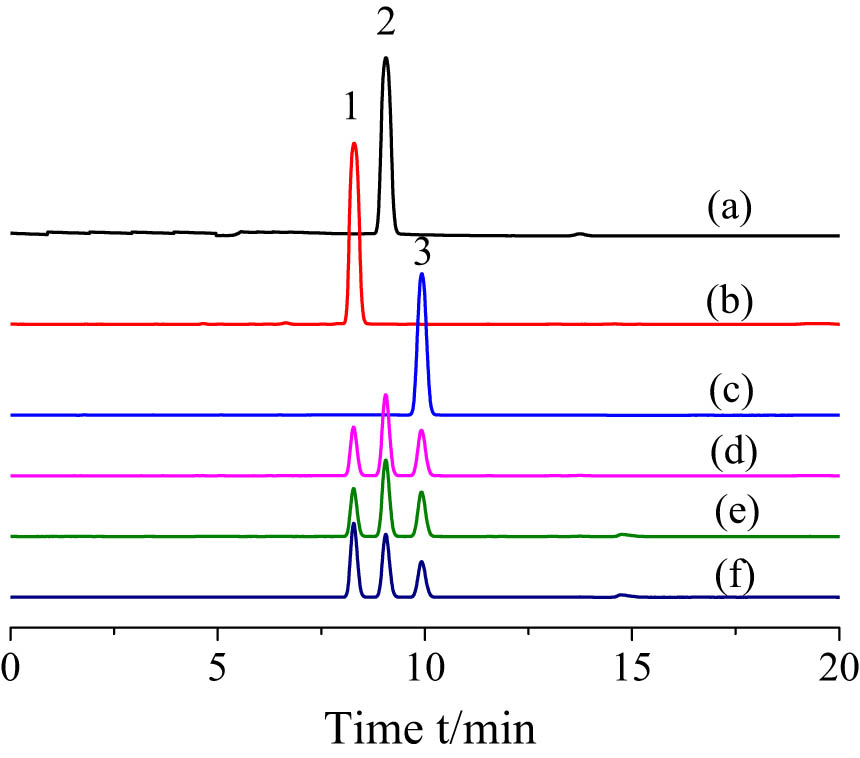
The chromatogram obtained after MIPs/NIPs-SPE pre-concentration of urine samples spiked with 10 µg·mL−1 of LA, BA, and TA, respectively. (a) TA standard solution, (b) BA standard solution, (c) LA standard solution, (d) a mixture solution of TA, BA, and LA before MISPE treatment, (e) a mixture solution of TA, BA, and LA after NISPE treatment, and (f) a mixture solution of TA, BA, and LA after MISPE treatment. Chromatographic conditions include C18 reversed-phase column: mobile phase, acetonitrile–H2O (35/65, v/v); flow rate, 0.5 mL·min−1; UV detector wavelength, 211 nm; column temperature, 30°C.

Recoveries of TA, BA, and LA on MISPE and NISPE cartridges.
4 Conclusions
In summary, porous MIP microspheres were prepared using a combined iniferter–suspension polymerization method and the interactions between functional monomers and template molecules were analyzed during the preparation process. This method allowed for the effective preparation and selection of MIP materials with excellent performance. Moreover, accurate determination of ultra-trace LA residues in samples from complex environments was achieved. The public requirements for pesticide residue detection continue to expand, and pretreatment methods shall be developed in the direction of being green, fast, simple, and miniaturization. There is still a wide scope for the study of environmental sample pretreatments.
-
Funding information: This research was funded by The Anhui Provinical Natural Science Foundation of China (2008085ME174), the Natural Science Foundation of the Anhui Higher Education Institutions of China (2023AH052934, 2023AH040362), Bengbu University Funded Project of Scientific Research Startup for High-level Talents (BBXY2018KYQD20), Anhui Province Excellent Young Teacher Training Project (2023, Study on Polylactic Acid Catalyst), and Anhui Provinical College Students’ Innovative Entrepreneurship Training Program (2022170, 2022097).
-
Author contributions: Conceptualization, writing – original draft preparation, X.Y. and R.S.; methodology, J.X. and B.Y.; formal analysis, K.C. and H.Y.; writing – review and editing, X.Y. All authors have read.
-
Institutional review board statement: Not applicable.
-
Conflict of interest: Authors state no conflict of interest.
References
(1) Liu G, Li L, Huang X, Zheng S, Xu X, Liu Z, et al. Adsorption and removal of organophosphorus pesticides from environmental water and soil samples by using magnetic multi-walled carbon nanotubes @ organic framework ZIF-8. J Mater Sci. 2018;53(15):10772. 10.1007/s10853-018-2352-y.Search in Google Scholar
(2) Dorneles AL, de Souza Rosa A, Blochtein B. Toxicity of organophosphorus pesticides to the stingless bees Scaptotrigona bipunctata and Tetragonisca fiebrigi. Apidologie. 2017;48(5):612. 10.1007/s13592-017-0502-x.Search in Google Scholar
(3) Lobato A, Fernandes VC, Pacheco JG, Delerue-Matos C, Gonçalves LM. Organochlorine pesticide analysis in milk by gas-diffusion microextraction with gas chromatography-electron capture detection and confirmation by mass spectrometry. J Chromatogr A. 2021;1636461797. 10.1016/j.chroma.2020.461797.Search in Google Scholar PubMed
(4) Lv X, Wang F, Cui Y, Fan B, Kong Z, Yan T, et al. Modification and validation of the simultaneous detection of 38 pesticide residues method by ultra-high-performance liquid chromatography/tandem mass spectrometry with QuEChERS extraction in different oil crops and products. Rapid Commun Mass Spectrom. 2022;36(12):e9284. 10.1002/rcm.9284.Search in Google Scholar PubMed
(5) Ioime P, Piva E, Pozzebon M, Pascali JP. Automated sample preparation and analysis by gas chromatography tandem mass spectrometry (GC-MS/MS) for the determination of 3- and 2-monochloropropanediol (MCPD) esters and glycidol esters in edible oils. J Chromatogr A. 2021;1650462253. 10.1016/j.chroma.2021.462253.Search in Google Scholar PubMed
(6) Shuai W, Xiang-nan L, Kai-yu W, Sheng-nan H, Sheng-qian H. Determination of 33 kinds of pesticide residues in wine by GC- MS. Sci Technol Indus. 2015;17:305. 10.13386/j.issn1002-0306.2015.17.054.Search in Google Scholar
(7) Govindasamy M, Rajaji U, Chen S-M, Kumaravel S, Chen T-W, Al-Hemaid FMA, et al. Detection of Pesticide Residues (Fenitrothion) in Fruit Samples Based On Niobium Carbide@Molybdenum Nanocomposite: An Electrocatalytic Approach. Analy Chim Acte. 2018;103052. 10.1016/j.aca.2018.05.044.Search in Google Scholar PubMed
(8) Umapathi R, Ghoreishian SM, Sonwal S, Rani GM, Huh YS. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord Chem Rev. 2022;453214305. 10.1016/j.ccr.2021.214305.Search in Google Scholar
(9) Li X, Liu X, Jia Z, Wang T, Zhang H. Screening of estrogenic endocrine-disrupting chemicals in meat products based on the detection of vitellogenin by enzyme-linked immunosorbent assay. Chemosphere. 2021;263128251. 10.1016/j.chemosphere.2020.128251.Search in Google Scholar PubMed
(10) Galani YJH, Houbraken M, Wumbei A, Djeugap JF, Fotio D, Gong YY, et al. Monitoring and dietary risk assessment of 81 pesticide residues in 11 local agricultural products from the 3 largest cities of Cameroon. Food Control. 2020;118107416. 10.1016/j.foodcont.2020.107416.Search in Google Scholar
(11) Mintz Hemed N, Leal-Ortiz S, Zhao ET, Melosh NA. On-Demand, Reversible, Ultrasensitive Polymer Membrane Based on Molecular Imprinting Polymer. ACS Nano. 2023;17(6):5632. 10.1021/acsnano.2c11618.Search in Google Scholar PubMed PubMed Central
(12) Dong C, Shi H, Han Y, Yang Y, Wang R, Men J. Molecularly imprinted polymers by the surface imprinting technique. Euro Polym J. 2021;145110231. 10.1016/j.eurpolymj.2020.110231.Search in Google Scholar
(13) Wang X, Zhu Y, Zhou L, Zhao P, Xiong Z, Yu J. Magnetic solid-phase extraction based on zirconium-based metal–organic frameworks for simultaneous enantiomeric determination of eight chiral pesticides in water and fruit juices. Food Chem. 2022;370131056. 10.1016/j.foodchem.2021.131056.Search in Google Scholar PubMed
(14) Ying X, He J, Li X. Molecularly imprinted electrospun fiber membrane for colorimetric detection of hexanoic acid. e-Polymers. 2021;21(1):500. 10.1515/epoly-2021-0049.Search in Google Scholar
(15) Wu F, Zhang Z, Liu W, Liu Y, Chen X, Liao P, et al. Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples. e-Polymers. 2022;22(1):488. 10.1515/epoly-2022-0034.Search in Google Scholar
(16) Fuchs Y, Soppera O, Mayes AG, Haupt K. Holographic molecularly imprinted polymers for label-free chemical sensing. Adv Mater. 2013;25(4):566. 10.1002/adma.201203204.Search in Google Scholar PubMed
(17) Wei W, Zhou T, Wu S, Shen X, Zhu M, Li S. An enzyme-like imprinted-polymer reactor with segregated quantum confinements for a tandem catalyst. RSC Adv. 2018;8(3):1610. 10.1039/C7RA12320E.Search in Google Scholar
(18) Hong Y, Wang X, Fu D, Wang G, Zhao L, Cheng H. Synthesis of cadmium(ii) ion-imprinted composite membrane with a pyridine functional monomer and characterization of its adsorption performance. e-Polymers. 2023;23(1). 10.1515/epoly-2022-8103.Search in Google Scholar
(19) Sellergren B, Allender CJ. Molecularly imprinted polymers: A bridge to advanced drug delivery. Adv Drug Deli Rev. 2005;57(12):1733. 10.1016/j.addr.2005.07.010.Search in Google Scholar PubMed
(20) Díaz-Díaz G, Diñeiro Y, Menéndez MI, Blanco-López MC, Lobo-Castañón MJ, Miranda-Ordieres AJ, et al. Molecularly imprinted catalytic polymers with biomimetic chloroperoxidase activity. Polymer. 2011;52(12):2468. 10.1016/j.polymer.2011.04.004.Search in Google Scholar
(21) Mavumengwana-Khanyile B, Katima Z, Songa EA, Okonkwo JO. Recent advances in sorbents applications and techniques used for solid-phase extraction of atrazine and its metabolites deisopropylatrazine and deethylatrazine: a review. Inter J Envir Analy Chem. 2019;991017.10.1080/03067319.2019.1597866Search in Google Scholar
(22) Han F, Zhou D-B, Song W, Hu Y-Y, Lv Y-N, Ding L, et al. Computational design and synthesis of molecular imprinted polymers for selective solid phase extraction of sulfonylurea herbicides. J Chromatogr A. 2021;1651462321. j.chroma.2021.462321.Search in Google Scholar
(23) Al-Degs YS, Abu-Surrah AS, Ibrahim KA. Preparation of highly selective solid-phase extractants for Cibacron reactive dyes using molecularly imprinted polymers. Anal Bioanal Chem. 2009;393(3):1055. 10.1007/s00216-008-2502-1.Search in Google Scholar PubMed
(24) Yousaf H, Khalid I, Barkat K, Mehmood Y, Badshah SF, Anjum I, et al. Development of polymeric IPN hydrogels by free radical polymerization technique for extended release of letrozole: Characterization and toxicity evaluation. e-Polymers. 2023;23(1). 10.1515/epoly-2023-0033.Search in Google Scholar
(25) Han S, Leng Q, Teng F, Ding Y, Yao A. Preparation of mesh covalent organic framework Tppa-2-based adsorption enhanced magnetic molecularly imprinted composite for selective extraction of tetracycline residues from animal-derived foods. Food Chem. 2022;384132601. 10.1016/j.foodchem.2022.132601.Search in Google Scholar PubMed
(26) Hung C-Y, Huang Y-T, Huang H-H, Hwang C-C. Synthesis and molecular recognition of molecularly imprinted polymer with ibuprofen as template. J Chin Chem Soc. 2006;53(5):1173. 10.1002/jccs.200600155.Search in Google Scholar
(27) Song R, Hu X, Li J, Guan P. Synthesis of surface imprinted polymer microspheres with ultrathin polymer shells via surface-initiated iniferter polymerization. Adv Mater Res. 2013;70268. 10.4028/AMR.702.68.Search in Google Scholar
(28) Lai J-P, Lu X-Y, Lu C-Y, Ju H-F, He X-W. Preparation and evaluation of molecularly imprinted polymeric microspheres by aqueous suspension polymerization for use as a high-performance liquid chromatography stationary phase. Analy Chim Acte. 2001;442(1):105. 10.1016/S0003-2670(01)01115-1.Search in Google Scholar
(29) Shen J, Wu X, Yu J, Yin F, Hao L, Lin C, et al. Hydrogen bonding interactions between arsenious acid and dithiothreitol/dithioerythritol at different pH values: a computational study with an explicit solvent model. N J Chem. 2021;45(43):20181. 10.1039/D1NJ03191K.Search in Google Scholar
(30) Perez M, Concu R, Ornelas M, Cordeiro MNDS, Azenha M, Fernando Silva A. Measurement artifacts identified in the UV–vis spectroscopic study of adduct formation within the context of molecular imprinting of naproxen. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2016;153661. 10.1016/j.saa.2015.10.005.Search in Google Scholar PubMed
(31) Zhang Z, Fang H. Uncovering the effects of different substituents on excited state hydrogen-bonding interaction and fluorescent behavior in BTN system: a TD-DFT insight. Theor Chem Acc. 2023;142(11):109. 10.1007/s00214-023-03054-0.Search in Google Scholar
(32) Wang Y, Cao F, Ge M, Lv S, Gao J. Studies on the molecular interactions between plant-derived protein zein and small molecules. ACS Food Sci Technol. 2021;1(6):1077. 10.1021/acsfoodscitech.1c00068.Search in Google Scholar
(33) Song R, Xie J, Yu X, Ge J, Liu M, Guo L. Preparation of molecularly imprinted polymer microspheres for selective solid-phase extraction of capecitabine in urine samples. Polymers. 2022;14(19):3968. 10.3390/polym14193968.Search in Google Scholar PubMed PubMed Central
(34) Paine AJ. Dispersion polymerization of styrene in polar solvents. IV. Solvency control of particle size from hydroxypropyl cellulose stabilized polymerizations. J Polym Sci A: Polym Chem. 1990;28(9):2485. 10.1002/pola.1990.080280921.Search in Google Scholar
(35) Matveev YI, Askadskii AA. The effect of the degree of polymerization and lyophobic interaction on the condition of solubility of polymers. Polym Sci Ser A. 2010;52(12):1245. 10.1134/S0965545X10120011.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

