Abstract
The underwater curing agents have demonstrated promising potential in various applications, especially in underwater repair engineering, yet have proven considerable challenging. Here, we report a cardanol-based curing agent for epoxy resin that achieves robust adhesion to steel and concrete substrates both in air and underwater. Cardanol, paraformaldehyde, and m-phenyldimethylamine are selected as the polymeric monomers to synthesize curing agent by Mannich reaction in the absence of chemical cross-linker agents. The coating is completely cured within 46 ± 1 min in air and 54 ± 2 min under water with an adhesion of 0 or 1 and a hardness of 5H. The impact strength, shear strength, and tensile strength of coating on underwater concrete were 9.58 ± 0.41 kJ·m−2, 13.1 ± 0.3, and 10.5 ± 0.2 MPa, respectively, demonstrating exceptional flexibility and mechanical strength as well as favorable hydrophobicity. This work paves the way for the rehabilitation of underwater drainage network for urban infrastructure and water conservancy projects.
1 Introduction
Drainage pipeline is an essential element of urban infrastructure and water conservancy platform, undertaking a number of functions such as drainage and sewage disposal. Drainage pipeline networks in cities along the Yangtze River and even in the whole China, especially the traditional concrete pipes, generally suffer from the problems of aging and leakage, which seriously affects drainage efficiency and pollution prevention and treatment. Therefore, the rehabilitation of the underwater pipeline network is imminent. However, the limited space and complex environment of the drainage network puts forward stringent requirements for the coating technology, especially the curing of coating in the moisture- and water-related environment.
Epoxy resins are widely used in anti-corrosion coatings because of superior mechanical performance, chemical resistance, insulation, and good adhesive properties (1,2,3,4). However, epoxy resins applied for underwater drainage pipeline rehabilitation require the involvement of an underwater curing agent. Low molecular weight epoxy needs to be converted into three-dimensional crosslinked thermoset networks using suitable curing agents to achieve better performance (5,6). Currently, the majority of the curing agents available for underwater curing are modified amine curing agents, of which the most commonly used are phenalkamines prepared by Mannich modification (7). Phenalkamines, as a promising curing agent, combine the unique properties of aliphatic amines and polyamides and are suitable for humid conditions (5). Biosourced materials are widespread for being eco-friendly and having unique properties. Jayaprakash et al. (8) prepared nanocomposites by water evaporation using cellulose nanofibers (CNFs) as fillers and natural rubber (NR) latex as the matrix, the study found that pineapple fruit residue-based CNFs enhance mechanical and thermal properties of NR composites. Batubara et al. (9) fabricated hybrid nanocomposite films composed of chitosan, cinnamaldehyde, Nigella sativa or blackseed oil, and silver nanoparticles biosynthesized in Azadirachta indica or neem leaves’ extract, and the films were biodegradable in soil and showed good thermal stability. Pradeepa et al. (10) developed three sisal fiber-reinforced sodium alginate composites, and the untreated sample with the maximum sodium alginate gum concentration had significantly enhanced mechanical properties and low moisture absorption rate. Jie et al. (11) fabricated an innovative environmentally friendly nanofiber membrane adsorbent by electrospinning chitosan using urushiol as the cross-linking agent, thereby achieving rapid and high-capacity adsorption of Cr(vi) in water. Hu et al. (12) synthesized a high-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing, and the hydrogel exhibits a natural formulation, high mechanical strength, and electrical conductivity. In addition, biosourced materials exhibit exceptional performance in emerging applications, particularly within the realm of sensors and energy devices. Liu et al. (13) developed a biodegradable aerogel that possesses sensing and fire-warning capabilities, utilizing resource-abundant graphite and green carboxymethyl cellulose. These resultant aerogels exhibit exceptional electrical conductivity and mechanical strength. The authors, Trano et al. (14), designed a lignin-based membrane through the crosslinking of a pre-oxidized Kraft lignin matrix with an ethoxylated difunctional oligomer, and the lignin-based electrolyte attains significant electrochemical performances. Manarin et al. (15) developed biobased gel polymer electrolyte membranes via the esterification reaction of a cardanol-based epoxy resin, and these membranes exhibited excellent electrochemical stability toward potassium metal and suitable ionic conductivity. Amici et al. (16) proposed a composite gel polymer electrolyte consisting of a highly cross-linked polymer matrix, containing a dextrin-based nanosponge and activated with a liquid electrolyte. The nanosponge allows good ionic conductivity at room temperature. Because of these sustainability advantages, biosourced materials quickly replace synthetic composites in composites. Cardanol is a natural and environmentally friendly material extracted from cashew shell oil (17,18,19,20) and is an essential raw material for the production of phenalkamines (21). Moreover, the presence of a long C15 aliphatic chain and aromatic ring in cardanols provides exceptional flexibility, hardness, and hydrophobicity, rendering them appropriate candidates for curing agent feedstocks (22,23,24).
Furthermore, a lot of literatures (5,7,21,23,25,26,27) reported that underwater cardanol-based curing agents are developed for epoxy resin, but these studies in the laboratory are mainly concentrated on the steel substrate and very few studies referred to the concrete substrates to evaluate the performance of the coatings, especially the moisture and underwater concrete substrates.
Here we prepare a cardanol-based curing agent suitable for underwater curing of drainage pipeline networks by Mannich reaction using cardanol, paraformaldehyde (PA), and m-phenyldimethylamine (MXDA) as monomers. The structure of the curing agent is confirmed by Fourier transform infrared spectra (FTIR) and 1H nuclear magnetic resonance spectra (1H NMR). The effects of monomer molar concentration and reaction condition on the viscosity and the amine value of the curing agent are investigated. Furthermore, the hardness, adhesion, curing time, and mechanical properties of coatings prepared by mixing cardanol-based curing agents with commercial epoxy resin E44 in different ratios to steel and concrete substrates both in air and underwater are also studied. In addition, commercial MXDA is used for comparative study. More importantly, modified MXDA achieves higher shear strength (13.1 ± 0.3 MPa), higher tensile strength (10.5 ± 0.2 MPa), and more rigid and harder film compared with commercial MXDA.
2 Materials and methods
2.1 Materials
Cardanol, PA, and epoxy resin (E44) are purchased from Shanghai Liming Chemical Co., Ltd. MXDA and perchloric acid are purchased from Sinopharm Chemical Reagent Co., Ltd. All the chemicals are industrial grade and used without further purification.
2.2 Preparation of the curing agent
The curing agent is prepared by the one-pot method (Figure 1). First, 3.02 g cardanol and 1.77 g MXDA are placed into a 500 mL four-necked round-bottom flask at room temperature (22–25°C). The 500 mL four-necked round-bottom flask is suspended in a temperature-controlled water bath on a programmable hotplate with an external temperature probe and equipped with a magnetic stirrer and reflux condenser. The mixture is stirred for 20–30 min at a rotor speed of 600 rpm until all the chemicals are dissolved. Subsequently, the mixture is slowly heated to 80°C at a rate of 1°C·min−1 under stirring at 600 rpm, and then, an equal amount of 0.33 g PA is mixed into the mixture in five portions at intervals of 2 min. The solution is continued to be slowly heated to 100°C at 1°C·min−1 and maintained condensation reflux for 4 h under stirring at 600 rpm. When the reaction is completed, the curing agent is obtained by decompression distillation and dehydration.
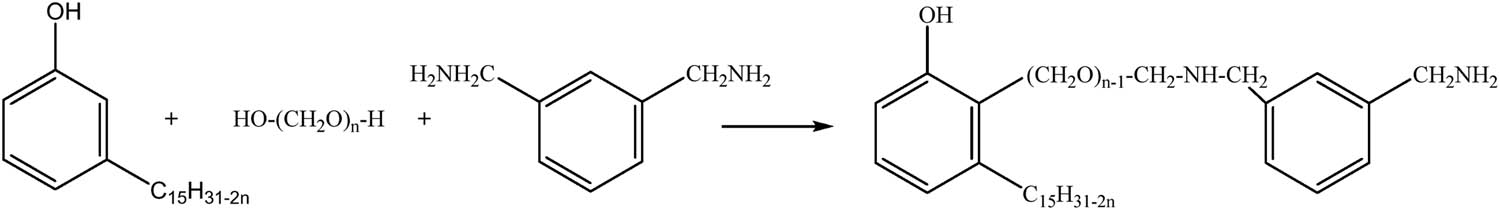
Reaction scheme for the preparation of curing agent.
2.3 The fabrication of coatings
Curing agents are mixed with epoxy resins with 1:1.2, 1:1.3, 1:1.4, and 1:1.5 ratios. Similarly, an unmodified curing agent (commercial MXDA) serves as a control. The mixture is coated on mild steel substrates and cured for 16–24 h at the ambient environment to get a completely dried coating. The dry coating thickness is controlled at 120 ± 20 μm. Besides, the mixture is coated on a concrete substrate soaked in water for 24 h and then cured for 24 ± 2 h underwater. The dry coating thickness is controlled at 150 ± 20 μm.
2.4 Characterizations
To test the structure and properties of the prepared samples, various characterizations are conducted.
2.4.1 1H NMR analysis
1H NMR spectra of as-prepared samples are carried out by spectrometer (Bruker Avance neo 400) with deuterated dimethyl sulfoxide (DMSO-d6) or deuterated chloroform (CDCl3) as solvents.
2.4.2 FTIR analysis
Fourier transform infrared spectra of as-prepared samples are acquired using a Nicolet 6700 IR spectrophotometer (Bruker, Bremen, Germany) in the wavelength range of 500–4,000 cm−1.
2.4.3 Amine value measurements (28)
The amine value is measured by the nonaqueous titration method with perchloric acid and calculated using the following equation:
where c is the concentration of the perchloric acid standard solution, V is the consumption of the standard solution, and m is the mass of a sample.
2.4.4 Viscosity measurements
The viscosity of the prepared samples is measured according to GB/T 2994-1995 and acquired using an NDJ-5S digital rotary viscometer at 25°C.
2.4.5 Hardness measurements
The hardness of coatings is measured according to GB/T 6739-2022 and acquired using a QHQ-type pencil hardness tester.
2.4.6 Adhesion strength measurements
The adhesion strength of coatings is evaluated by the scribe tester according to GB/T 9286-2021.
2.4.7 Surface drying time measurements
The surface drying time of coatings is evaluated according to GB/T 1728-2020. The surface drying is tested three times and averaged.
2.4.8 Water absorption measurements
To measure water absorption of coatings, the coating with known weight is immersed in water for 24 h at room temperature. The water absorption is calculated according to the following equation based on the weight difference between the coating before and after immersion (23).
where M t is the water absorption, W t is the weight of the sample after immersion in water, and W 0 is the weight of the sample before immersion in water.
2.4.9 Water contact angle measurements
The hydrophobicity of the coatings is determined by the water contact angle and measured by a contact angle analyzer (JCY-3, Suzhou Qi Le Electronic Technology Co., Ltd., Suzhou, China). The contact angle is tested five times and averaged.
2.4.10 Mechanic properties measurements
The tensile strength is tested according to GB/T 50081-2003 and the tensile rate of the universal tester (Jinjian, China) is 5 mm·min−1. The shear adhesive strength is tested according to GB/T 7124-2008 and the tensile rate is 5 mm·min−1. The impact strength is investigated according to the method of the no-notched Charpy impact plastics sample. These data are tested five times and averaged.
3 Results and discussion
3.1 Structure of the curing agent
The structure of the curing agent is determined by 1H NMR spectrum (CDCl3 is used as the solvent). As shown in Figure 2, the peaks at about 6.64–7.05 and 7.28–7.59 ppm are the protons of benzene rings. The peak assignments of –OH are observed at 9.68 ppm, and the peaks at 0.88–5.43 ppm belong to C15 long aliphatic chains of cardanol. The peaks at about 5.43 ppm belong to the protons of ethylene on C15 long aliphatic chains. Compared to the spectrum of the MXDA and cardanol, the 1H NMR spectrum of the curing agent has similar peaks that correspond to the main chain of polymers. In addition, the new emerging peaks at about 3.76–4.80 ppm can be observed in 1H NMR spectrum of the curing agent and the attenuation of –NH2 peaks, which demonstrate that the curing agent was successfully synthesized through reaction.

1H NMR spectra: (a) MXDA; (b) cardanol; and (c) as-prepared curing agent.
Moreover, the structure of the curing agent is further confirmed through FTIR spectra. FTIR spectra of MXDA, cardanol, and as-prepared curing agent are shown in Figure 3. MXDA exhibits two characteristic peaks at near 3,400 cm−1, which are ascribed to the N–H stretching vibrations. The sharp peak at 1,300 cm−1 is observed due to the stretching vibrations of the C–N. The distinct absorption peaks appear at 1,458, 1,489, and 1,623 cm−1, corresponding to the C═C stretching vibration of the aromatic ring. Absorption peaks around 2,860, 3,360, and 3,006 cm−1 are assigned to the –CH2– stretching vibration, O–H stretching vibration, and C═H stretching vibration, respectively.
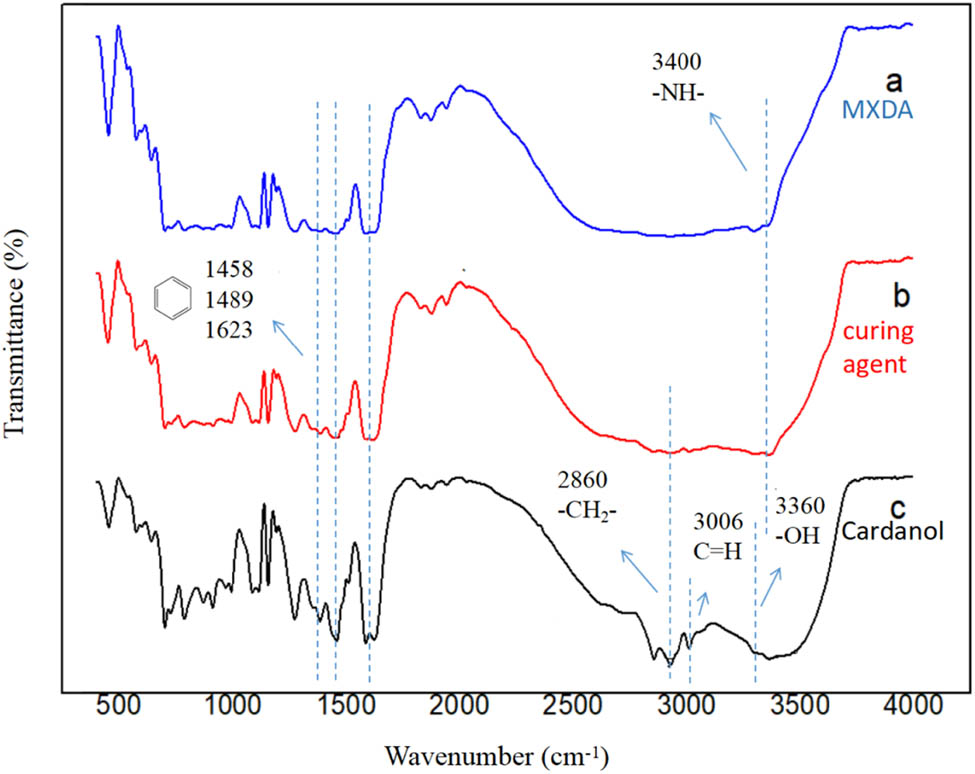
FTIR spectra: (a) MXDA; (b) cardanol; and (c) as-prepared curing agent.
The FTIR spectrum of as-prepared curing agent shows the absorption peak between 3,200 and 3,500 cm−1 due to the overlap of the stretching vibration peak between the N–H generated by the reaction and the phenolic hydroxyl. The FTIR spectrum of as-prepared curing agent not only contains the long C15 aliphatic chains of cardanol but also exhibits the N–H absorption peaks of MXDA. Moreover, there is no peak observed in the region between 1,735 and 1,750 cm−1, suggesting that the curing agent does not contain C═O functional group; therefore, confirming the PA is completely reacted. The absorption peak at 880–730 cm−1 represents the substitution of ortho, indicating that the active H on the ortho position of phenol is replaced by the intermediates of formaldehyde and amine. The above results indicate that the curing agent was successfully synthesized.
3.2 Effect of different molar concentrations of monomer
The properties of thermosets depend on the kind of polyamine and molecular weight of the resultant phenalkamine. However, the curing characteristics depend on amine value (29). The effect of the different concentrations of monomer on viscosity and the amine value of the curing agent is evaluated. The ratios of MXDA to PA and cardanol are (1, 1.1, 1.2, 1.3, 1.4):1:1, respectively. The ratios of PA to MXDA and cardanol are (0.9, 1.0, 1.1, 1.2, 1.3):1:1, respectively. The ratio of curing agent to epoxy resin is 1:1.3. The mixtures coated on steel substrate are evaluated for hardness, adhesion, and drying time.
The viscosity and the amine value of the curing agents with various concentrations of MXDA are shown in Figure 4. The viscosity of curing agent decreases from 9,374 to 4,901 mPa·s and the amine value of curing agent increases from 214 to 401 mgKOH·g−1, respectively, as the MXDA concentration increases from 1.0 to 1.4. As a result of the ring-opening polymerization reaction between the epoxy groups and the amine, the amine value gradually decreases, indicating that the reaction is nearly complete.

Effect of MXDA concentration on the viscosity and the amine value of curing agent.
All coatings exhibit excellent adhesion (0 or 1) to the steel substrate because the phenolic hydroxyl groups in the curing agent and the structure of the epoxy resin can form chemical bonds with the hydroxyl on the surface of the substrate (Table 1). It is observed that the hardness of coatings with low concentration of MXDA is stronger than the coatings with high concentration of MXDA, which could be attributed to the fact that the straight chain molecules in the excess MXDA are free into the network structure of the coating after the reaction is completed. Furthermore, drying time decreases with increasing MXDA concentration. The more active hydrogen in the molecule involved in the reaction, the shorter of curing time, indicating that the curing rate of coatings essentially depends on the amine value of the curing agent. The results show that the curing agent with an MXDA concentration of 1.3 exhibits optimum curing performance.
Effect of the curing agent prepared by different MXDA concentrations on the coating performance
| Characterization | 1:1:1 | 1.1:1:1 | 1.2:1:1 | 1.3:1:1 | 1.4:1:1 |
|---|---|---|---|---|---|
| Pencil hardness/H | 6 | 6–5 | 5 | 5 | 4 |
| Adhesion/series | 0 | 0 | 0–1 | 0–1 | 1 |
| Surface drying time (min) | 62 ± 1 | 55 ± 2 | 52 ± 2 | 50 ± 2 | 49 ± 1 |
The viscosity and amine value of the curing agent prepared by different PA concentrations are shown in Figure 5. As the concentration of PA increases, the color of the curing agent changes from a light brown to a dark brown with white colloidal matter (Table 2). The white colloidal pieces could be unreacted PA or phenolic intermediates. It could be inferred that excessive PA is detrimental to the reaction process because the increase of viscosity of the system weakens adequate mixing. The amine value of the curing agent decreases as the concentration of PA increases. Therefore, the reaction of MXDA, PA, and cardanol is accelerated as the PA concentration increases, leading to a rapid increase in the degree of polymerization and the average molecular weight of the product.

Effect of the PA concentration on the viscosity and amine value of curing agent.
Effect of PA concentration on the physicochemical properties of curing agent
| n(PA):n(MXDA):n(cardanol) | Colour and viscosity |
|---|---|
| 0.9:1:1 | Light brown liquid, transparent and viscous |
| 1:1:1 | Brown liquid, transparent and viscous |
| 1.1:1:1 | Dark brown liquid, transparent and viscous |
| 1.2:1:1 | Dark brown liquid, transparent and viscous, and with some white colloidal pieces |
| 1.3:1:1 | Dark brown liquid, transparent and viscous, and with some white colloidal pieces |
The coatings cured with different PA concentrations show excellent adhesion (a rating of 0 or 1). The coatings cured with different concentrations of PA exhibited rigid and harder films, while the hardness decreased as the concentration of PA increased. Moreover, the surface drying time decreases and then increases with increasing PA concentration. The results indicate that the curing agent prepared with a PA concentration of 1.0 provides the best curing performance (Table 3).
Effect of the as-prepared curing agent with different PA concentrations on the coating performance
| Characterization | 0.9:1:1 | 1.0:1:1 | 1.1:1:1 | 1.2:1:1 | 1.3:1:1 |
|---|---|---|---|---|---|
| Pencil hardness/H | 6 | 6 | 5 | 4 | 4 |
| Adhesion/series | 1 | 1 | 1 | 0–1 | 0 |
| Surface drying time (min) | 52 ± 2 | 48 ± 1 | 51 ± 1 | 55 ± 3 | 62 ± 2 |
3.3 Effect of reaction condition
The effect of reaction temperature and reaction time is investigated by testing viscosity and amine value at 80, 90, 100, 110, and 120°C and at 3.0, 3.5, 4, 4.5, and 5 h, respectively.
As shown in Table 4, the curing agent exhibits a white colloidal matter accompanied by a small amount of granular intermediate of phenolic aldehyde. When the reaction temperature is increased to 100°C, the precipitates disappear, confirming that the reaction is totally completed. Therefore, the reaction of the monomers to generate products is accelerated as the temperature increases, and the amount of active hydrogen on the amine is continuously consumed, leading to that the amine value decreases and the viscosity rises, respectively (Figure 6).
Effect of reaction temperature on physicochemical properties of curing agent
| Temperature T/°C | Reaction processes | Colour and viscosity |
|---|---|---|
| 80 | Some white gelatinous substances were attached to the wall of the bottle | Light brown liquid, transparent, and viscous |
| 90 | A small amount of granular material was deposited at the bottom of the bottle | Dark brown liquid, transparent, and viscous |
| 100 | No sedimentation | Dark brown liquid, transparent, and viscous |
| 110 | No sedimentation | Dark brown liquid, transparent, and viscous |
| 120 | No sedimentation | Dark brown liquid, transparent, and viscous |
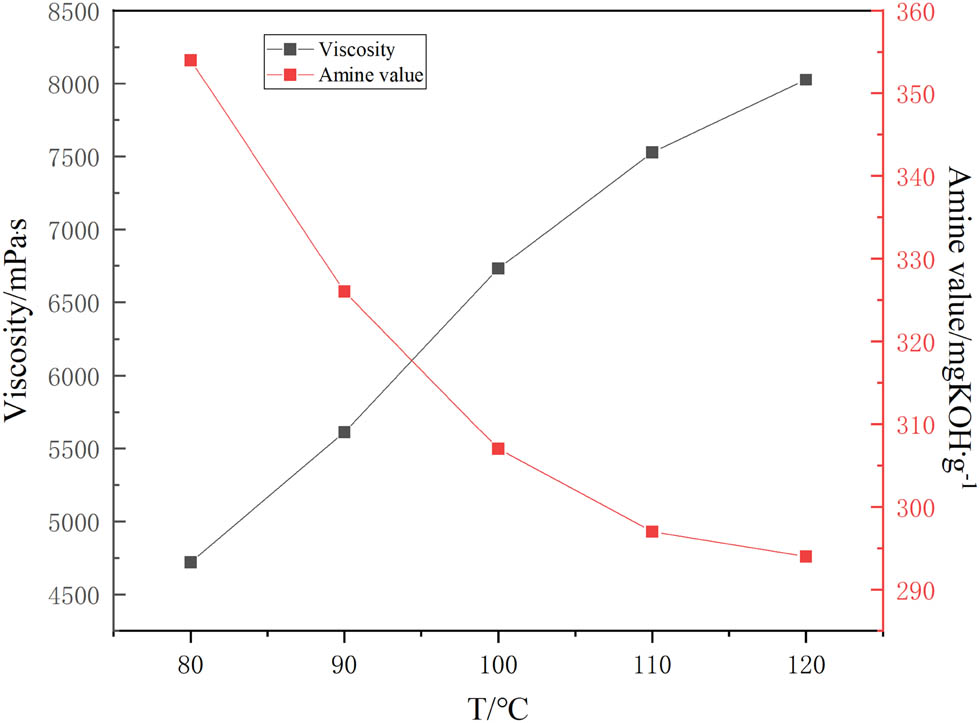
Effect of reaction temperature on viscosity and amine value of curing agent.
All coatings display extremely favorable adhesion to the steel substrate, achieving a rating of 0 or 1 (Table 5). Coatings cured with curing agents with higher preparation temperatures provide better adhesion and hardness than those cured with curing agents with lower preparation temperatures. The set-to-touch time is gradually prolonged with increasing reaction temperature. It can be attributed to the fact that the increase in reaction temperature led to the sufficient ring-opening addition reaction and reduction of reactive hydrogen on primary amines. The results show that the curing agent prepared with a reaction temperature of 100°C exhibits optimal curing properties.
Effect of the as-prepared curing agent with different reaction temperature on the coating performance
| Characterization | 80°C | 90°C | 100°C | 110°C | 120°C |
|---|---|---|---|---|---|
| Pencil hardness/H | 4 | 5 | 5 | 5–6 | 6 |
| Adhesion/series | 1 | 1–0 | 0 | 0 | 0 |
| Surface drying time (min) | 46 ± 3 | 48 ± 2 | 49 ± 2 | 53 ± 1 | 55 ± 3 |
As can be seen from Figure 7, the amine value decreases as the reaction time increases. However, the viscosity changes in the opposite trend to the amine value. In addition, for the amine value, the viscosity, and the coating performance, the reaction time shows a similar trend as the reaction temperature. The results show that the curing agent prepared with a reaction time of 4.5 h exhibits optimal curing properties (Tables 6 and 7).

Effect of reaction time on viscosity and amine value of curing agent.
Effect of reaction time on physicochemical properties of curing agent
| Reaction time (h) | Reaction processes | Colour and viscosity |
|---|---|---|
| 3 | Some white gelatinous substances were attached to the wall of the bottle | Light brown liquid, transparent, and viscous |
| 3.5 | A small amount of granular material was deposited at the bottom of the bottle | Light brown liquid, transparent, and viscous |
| 4 | No sedimentation | Dark brown liquid, transparent, and viscous |
| 4.5 | No sedimentation | Dark brown liquid, transparent, and viscous |
| 5 | No sedimentation | Dark brown liquid, transparent, and viscous |
Effect of the as-prepared curing agent with different reaction time on the coating performance
| Characterization | 3.0 h | 3.5 h | 4.0 h | 4.5 h | 5.0 h |
|---|---|---|---|---|---|
| Pencil hardness/H | 4 | 4–5 | 5 | 5 | 5–6 |
| Adhesion/series | 1 | 1 | 1–0 | 0 | 0 |
| Surface drying time (min) | 58 ± 2 | 52 ± 1 | 51 ± 2 | 48 ± 1 | 46 ± 2 |
3.4 The performance of the coatings
The performance of the coatings is further investigated in both air and underwater environments. The curing agent synthesized by the optimal synthetic process is employed for these studies (the ratio of MXDA to the PA and cardanol is 1.3:1:1, a reaction temperature set at 100°C, and a reaction duration of 4.5 h).
Table 8 demonstrates surface drying time, water contact angle, and water absorption of the coatings coated mild steel substrates. It was observed that, when the ratio of epoxy resin in the coating is low, the water absorption of the coating is less.
Effect of curing agent and epoxy resin ratio on coating performance
| Characterization | 1:1.2 | 1:1.3 | 1:1.4 | 1:1.5 |
|---|---|---|---|---|
| Surface drying time (min) | 58 ± 2 | 46 ± 1 | 53 ± 2 | 62 ± 2 |
| Water absorption (%) | 0.75 | 0.87 | 1.21 | 1.51 |
| Water contact angle (°) | 89.23 ± 1.01 | 87.67 ± 0.23 | 85.34 ± 0.43 | 82.19 ± 0.27 |
The water contact angle decreases with increasing epoxy resin concentration in the coating, indicating that the presence of C15 long aliphatic chains and phenyl rings increases the hydrophobicity of the coating and limits the diffusion of water through coatings. Moreover, when the ratio of the curing agent to the epoxy resin is 1:1.3, the coating exhibited the shortest surface drying time. Overall, the curing agent to epoxy resin ratio of 1:1.3 is chosen to prepare the coatings and to evaluate the underwater curing performance of the coatings.
Table 9 shows the underwater curing performance of the curing agent (the modified MXDA) and unmodified MXDA. It can be seen that the surface drying time of the coating cured with modified MXDA (54 min) is shorter than that of the coating cured with MXDA (73 min). The hardness of the coating cured with modified MXDA on concrete substrates is 5H, while the hardness of the coating cured with unmodified MXDA is only 2H. Mechanically, the shear strength of coating cured with modified MXDA reached 13.1 ± 0.3 MPa compared to the MXDA (9.4 ± 0.2 MPa), which is much higher than previously reported curing agents for underwater applications.
Comparison of coating performance using modified and unmodified curing agents
| Characterization | Surface drying time (min) | Adhesion/series | Pencil hardness/H | Impact resistance (kJ·m−2) | Shear strength (MPa) | Tensile strength (MPa) |
|---|---|---|---|---|---|---|
| MXDA | 73 ± 1 | 3 | 3 | 7.41 ± 0.23 | 9.4 ± 0.2 | 4.9 ± 0.1 |
| Modified MXDA | 54 ± 2 | 1 | 5 | 9.58 ± 0.41 | 13.1 ± 0.3 | 10.5 ± 0.2 |
The tensile strength of the coating with modified MXDA is 10.5 ± 0.2 MPa, which is 2.14 times higher than that of pristine MXDA (4.9 ± 0.1 MPa). The dramatic enhancement in mechanical strength is attributed to the reinforcing network formed by the strong interactions between the homogeneous and ordered structure of the curing agent and the compactness of the coating, while C15 long aliphatic chains in the structure provide flexibility to the coating. Therefore, results suggest that coatings cured with modified MXDA exhibit better adhesion and mechanical strength than the coatings cured with MXDA.
4 Conclusion
In conclusion, a cardanol-based curing agent is successfully synthesized via the Mannich reaction using cardanol, PA, and MXDA. The effects of monomer ratio and reaction conditions on the properties of the curing agent have been investigated. The optimal performance of the coatings is achieved when the ratio of curing agent to epoxy resin is 1:1.3. Moreover, the coatings cured with modified MXDA exhibit superior shear strength (13.1 ± 0.3 MPa) and tensile strength (10.5 ± 0.2 MPa) compared to those cured with MXDA. Considering the advantages of ideal mechanical characteristics and exceptional adhesion both in air and under water, the cardanol-based curing agent has potential applications in the emergency rehabilitation of damaged drainage pipelines.
Acknowledgements
The authors thank China Three Gorges Corp for its research funding.
-
Funding information: This work was supported by the Research Project of China Three Gorges Corperation (No. NBWL202300013).
-
Author contributions: Yu Li: conceptualization, methodology, data curation, formal analysis, validation, writing – original draft. Guoqing Wang: data curation, formal analysis, methodology, validation, writing – review & editing. Yali Guo: supervision, writing – review & editing. Ning Fang: investigation, supervision, resources, writing – review & editing. Jingxiang Li: validation, resources, writing – review & editing. Zheng Li: validation. Junhan Li: data curation. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: The raw/processed data are available from the corresponding author on a reasonable request.
References
(1) Zhang X, Wang Z, Xie J. Synthesis and curing properties of fluorinated curing agent for epoxy resin based on natural soybean isoflavones. React Funct Polym. 2023;184:105511. 10.1016/j.reactfunctpolym.2023.105511.Search in Google Scholar
(2) Zhao H, Xu S, Guo A, Li J, Liu D. The curing kinetics analysis of four epoxy resins using a diamine terminated polyether as curing agent. Thermochim Acta. 2021;9:178987. 10.1016/j.tca.2021.178987.Search in Google Scholar
(3) Jin FL, Li X, Park S. Synthesis and application of epoxy resins: A review. J Ind Eng Chem. 2015;29:1–11. 10.1016/j.jiec.2015.03.026.Search in Google Scholar
(4) Liu S, Chevali VS, Xu Z, Hui D, Wang H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos Part B Eng. 2017,136:197–214. 10.1016/j.compositesb.2017.08.020.Search in Google Scholar
(5) Zhang JH, Xu SA. Curing kinetics of epoxy cured by cardanol-based phenalkamines synthesized from different polyethylenepolyamines by Mannich reaction. Iran Polym J. 2017;26(7):499–509. 10.1007/s13726-017-0538-9.Search in Google Scholar
(6) Stanzione III J, Scala LJ. Sustainable polymers and polymer science: dedicated to the life and work of Richard P. Wool. J Appl Polym Sci. 2016;133(45):44212. 10.1002/app.44212.Search in Google Scholar
(7) Liu Y, Wang J, Xu S. Synthesis and curing kinetics of cardanol-based curing agents for epoxy resin by in situ depolymerization of paraformaldehyde. J Polym Sci Part A: Polym Chem. 2014;52(4):472–80. 10.1002/pola.27018.Search in Google Scholar
(8) Jayaprakash S, Chellappan S, Prasannan S, Padil V. Pineapple fruit residue-based nanofibre composites: Preparation and characterizations. e-Polymers. 2023;23(1):20230094. 10.1515/EPOLY-2023-0094.Search in Google Scholar
(9) Batubara AS, Obaid NA, Alharbi HM, Altalhi TM, Alasmari MA, Alghamdi AZ, et al. Chitosan nanocomposite film incorporating Nigella sativa oil, Azadirachta indica leaves’ extract, and silver nanoparticles. e-Polymers. 2023;23(1):20228083. 10.1515/EPOLY-2022-8083.Search in Google Scholar
(10) Pradeepa BR, Kiruthika AV. Preparation and characterization of sisal fibre reinforced sodium alginate gum composites for non-structural engineering applications. e-Polymers. 2023;23(1):787–312. 10.1515/epoly-2023-0027.Search in Google Scholar
(11) Jie X, Shiu B, Wu H, Zhang Y, Ye Y, Lin C, et al. Adsorption and recovery of Cr(vi) from wastewater by Chitosan–Urushiol composite nanofiber membrane. e-Polymers. 2023;23(1):20230157. 10.1515/EPOLY-2023-0157. Search in Google Scholar
(12) Hu Y, Luo J, Luo S, Fei T, Song M, Qin H. High-strength polyvinyl alcohol-based hydrogel by vermiculite and lignocellulosic nanofibrils for electronic sensing. e-Polymers. 2023;23(1):20230081. 10.1515/EPOLY-2023-0081.Search in Google Scholar
(13) Liu Y, Cheng F, Li K, Yao J, Li X, Xia Y. Lightweight, flame retardant Janus carboxymethyl cellulose aerogel with fire-warning properties for smart sensor. Carbohydr Polym. 2024;328:121730. 10.1016/j.carbpol.2023.121730.Search in Google Scholar PubMed
(14) Trano S, Corsini F, Pascuzzi G, Giove E, Fagiolari L, Amici J, et al. Lignin as polymer electrolyte precursor for stable and sustainable potassium batteries. ChemSusChem. 2022;15(12):e202200294. 10.1002/cssc.202200294.Search in Google Scholar PubMed PubMed Central
(15) Manarin E, Corsini F, Trano S, Fagiolari L, Amici J, Francia C, et al. Cardanol-derived epoxy resins as biobased gel polymer electrolytes for potassium-ion conduction. ACS Appl Polym Mater. 2022;4(5):3855–65. 10.1021/acsapm.2c00335.Search in Google Scholar PubMed PubMed Central
(16) Amici J, Torchio C, Versaci D, Dessantis D, Marchisio A, Caldera F, et al. Nanosponge-based composite gel polymer electrolyte for safer Li-O2 batteries. Polymers. 2021;13(10):1625. 10.3390/polym13101625.Search in Google Scholar PubMed PubMed Central
(17) Afroz J, Shumaila M, Fahmina Z, Ahmed RS, Manawwer A, Nahid HQMRN. Ambient-cured cardanol-derived polyurea coatings for anti-corrosive and anti-bacterial applications. Prog Org Coat. 2023;182:107638. 10.1016/J.PORGCOAT.2023.107638.Search in Google Scholar
(18) Phani Kumar P, Paramashivappa R, Vithayathil PJ, Subba Rao PV, Srinivasa Rao A. Process for isolation of cardanol from technical cashew (Anacardium occidentale L.) nut shell liquid. J Agric food Chem. 2002;50(16):4705–8. 10.1021/jf020224w.Search in Google Scholar PubMed
(19) Paramashivappa R, Kumar PP, Vithayathil PJ, Rao AS. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J Agric food Chem. 2001;49(5):2548–51. 10.1021/jf001222j.Search in Google Scholar PubMed
(20) Lubi MC, Thachil ET. Cashew nut shell liquid (CNSL) – a versatile monomer for polymer synthesis. Des Monomers Polym: An Int J Monomer Macromol Synth. 2000;3(2):123–53. 10.1163/156855500300142834.Search in Google Scholar
(21) Atta AM, Al-Hodan HA, Hameed RSA, Ezzat AO. Preparation of green cardanol-based epoxy and hardener as primer coatings for petroleum and gas steel in marine environment. Prog Org Coat. 2017;111:283–93. 10.1016/j.porgcoat.2017.06.002.Search in Google Scholar
(22) More AS, Pasale SK, Wadgaonkar PP. Synthesis and characterization of polyamides containing pendant pentadecyl chains. Eur Polym J. 2010;46(3):557–67. 10.1016/j.eurpolymj.2009.11.014.Search in Google Scholar
(23) Wazarkar K, Kathalewar M, Sabnis A. Anticorrosive and insulating properties of cardanol based anhydride curing agent for epoxy coatings. React Funct Polym. 2018;122:148–57. 10.1016/j.reactfunctpolym.2017.11.015.Search in Google Scholar
(24) Baik JH, Kim DG, Shim J, Lee JH, Choi YS, Lee JC. Solid polymer electrolytes containing poly(ethylene glycol) and renewable cardanol moieties for all-solid-state rechargeable lithium batteries. Polymer. 2016;99:704–12. 10.1016/j.polymer.2016.07.058.Search in Google Scholar
(25) Panda R, Tjong J, Nayak SK, Sain M. Effect of alkyl phenol from cashew nutshell liquid and sisal fiber reinforcement on dry sliding wear behavior of epoxy resin. J Nat Fibers. 2017;14(5):747–58. 10.1080/15440478.2017.1279100.Search in Google Scholar
(26) Huang K, Zhang Y, Li M, Lian J, Yang X, Xia J. Preparation of a light color cardanol-based curing agent and epoxy resin composite: Cure-induced phase separation and its effect on properties. Prog Org Coat. 2012;74(1):240–7. 10.1016/j.porgcoat.2011.12.015.Search in Google Scholar
(27) Pathak SK, Rao BS. Structural effect of phenalkamines on adhesive viscoelastic and thermal properties of epoxy networks. J Appl Polym Sci. 2006;102(5):4741–8. 10.1002/app.25005.Search in Google Scholar
(28) Zhang LF, Zhou XL. Determination of amine value of epoxy curing agent. Coat Technol Abstr. 2007;2004(01):47–8. 10.3969/j.issn.1672-2418.2004.01.012.Search in Google Scholar
(29) Sabnis A, Kathalewar M. Effect of molecular weight of phenalkamines on the curing, mechanical, thermal and anticorrosive properties of epoxy based coatings. Prog Org Coat An Int Rev J. 2015;84:79–88. 10.1016/j.porgcoat.2015.02.014.Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings
Articles in the same Issue
- Research Articles
- Flame-retardant thermoelectric responsive coating based on poly(3,4-ethylenedioxythiphene) modified metal–organic frameworks
- Highly stretchable, durable, and reversibly thermochromic wrapped yarns induced by Joule heating: With an emphasis on parametric study of elastane drafts
- Molecular dynamics simulation and experimental study on the mechanical properties of PET nanocomposites filled with CaCO3, SiO2, and POE-g-GMA
- Multifunctional hydrogel based on silk fibroin/thermosensitive polymers supporting implant biomaterials in osteomyelitis
- Marine antifouling coating based on fluorescent-modified poly(ethylene-co-tetrafluoroethylene) resin
- Preparation and application of profiled luminescent polyester fiber with reversible photochromism materials
- Determination of pesticide residue in soil samples by molecularly imprinted solid-phase extraction method
- The die swell eliminating mechanism of hot air assisted 3D printing of GF/PP and its influence on the product performance
- Rheological behavior of particle-filled polymer suspensions and its influence on surface structure of the coated electrodes
- The effects of property variation on the dripping behaviour of polymers during UL94 test simulated by particle finite element method
- Experimental evaluation on compression-after-impact behavior of perforated sandwich panel comprised of foam core and glass fiber reinforced epoxy hybrid facesheets
- Synthesis, characterization and evaluation of a pH-responsive molecular imprinted polymer for Matrine as an intelligent drug delivery system
- Twist-related parametric optimization of Joule heating-triggered highly stretchable thermochromic wrapped yarns using technique for order preference by similarity to ideal solution
- Comparative analysis of flow factors and crystallinity in conventional extrusion and gas-assisted extrusion
- Simulation approach to study kinetic heterogeneity of gadolinium catalytic system in the 1,4-cis-polyisoprene production
- Properties of kenaf fiber-reinforced polyamide 6 composites
- Cellulose acetate filter rods tuned by surface engineering modification for typical smoke components adsorption
- A blue fluorescent waterborne polyurethane-based Zn(ii) complex with antibacterial activity
- Experimental investigation on damage mechanism of GFRP laminates embedded with/without steel wire mesh under low-velocity-impact and post-impact tensile loading
- Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance
- Assessing the recycling potential of thermosetting polymer waste in high-density polyethylene composites for safety helmet applications
- Mesoscale mechanics investigation of multi-component solid propellant systems
- Preparation of HTV silicone rubber with hydrophobic–uvioresistant composite coating and the aging research
- Experimental investigation on tensile behavior of CFRP bolted joints subjected to hydrothermal aging
- Structure and transition behavior of crosslinked poly(2-(2-methoxyethoxy) ethylmethacrylate-co-(ethyleneglycol) methacrylate) gel film on cellulosic-based flat substrate
- Mechanical properties and thermal stability of high-temperature (cooking temperature)-resistant PP/HDPE/POE composites
- Preparation of itaconic acid-modified epoxy resins and comparative study on the properties of it and epoxy acrylates
- Synthesis and properties of novel degradable polyglycolide-based polyurethanes
- Fatigue life prediction method of carbon fiber-reinforced composites
- Thermal, morphological, and structural characterization of starch-based bio-polymers for melt spinnability
- Robust biaxially stretchable polylactic acid films based on the highly oriented chain network and “nano-walls” containing zinc phenylphosphonate and calcium sulfate whisker: Superior mechanical, barrier, and optical properties
- ARGET ATRP of styrene with low catalyst usage in bio-based solvent γ-valerolactone
- New PMMA-InP/ZnS nanohybrid coatings for improving the performance of c-Si photovoltaic cells
- Impacts of the calcinated clay on structure and gamma-ray shielding capacity of epoxy-based composites
- Preparation of cardanol-based curing agent for underwater drainage pipeline repairs
- Preparation of lightweight PBS foams with high ductility and impact toughness by foam injection molding
- Gamma-ray shielding investigation of nano- and microstructures of SnO on polyester resin composites: Experimental and theoretical study
- Experimental study on impact and flexural behaviors of CFRP/aluminum-honeycomb sandwich panel
- Normal-hexane treatment on PET-based waste fiber depolymerization process
- Effect of tannic acid chelating treatment on thermo-oxidative aging property of natural rubber
- Design, synthesis, and characterization of novel copolymer gel particles for water-plugging applications
- Influence of 1,1′-Azobis(cyclohexanezonitrile) on the thermo-oxidative aging performance of diolefin elastomers
- Characteristics of cellulose nanofibril films prepared by liquid- and gas-phase esterification processes
- Investigation on the biaxial stretching deformation mechanism of PA6 film based on finite element method
- Simultaneous effects of temperature and backbone length on static and dynamic properties of high-density polyethylene-1-butene copolymer melt: Equilibrium molecular dynamics approach
- Research on microscopic structure–activity relationship of AP particle–matrix interface in HTPB propellant
- Three-layered films enable efficient passive radiation cooling of buildings
- Electrospun nanofibers membranes of La(OH)3/PAN as a versatile adsorbent for fluoride remediation: Performance and mechanisms
- Preparation and characterization of biodegradable polyester fibers enhanced with antibacterial and antiviral organic composites
- Preparation of hydrophobic silicone rubber composite insulators and the research of anti-aging performance
- Surface modification of sepiolite and its application in one-component silicone potting adhesive
- Study on hydrophobicity and aging characteristics of epoxy resin modified with nano-MgO
- Optimization of baffle’s height in an asymmetric twin-screw extruder using the response surface model
- Effect of surface treatment of nickel-coated graphite on conductive rubber
- Experimental investigation on low-velocity impact and compression after impact behaviors of GFRP laminates with steel mesh reinforced
- Development and characterization of acetylated and acetylated surface-modified tapioca starches as a carrier material for linalool
- Investigation of the compaction density of electromagnetic moulding of poly(ether-ketone-ketone) polymer powder
- Experimental investigation on low-velocity-impact and post-impact-tension behaviors of GFRP T-joints after hydrothermal aging
- The repeated low-velocity impact response and damage accumulation of shape memory alloy hybrid composite laminates
- Exploring a new method for high-performance TPSiV preparation through innovative Si–H/Pt curing system in VSR/TPU blends
- Large-scale production of highly responsive, stretchable, and conductive wrapped yarns for wearable strain sensors
- Preparation of natural raw rubber and silica/NR composites with low generation heat through aqueous silane flocculation
- Molecular dynamics simulation of the interaction between polybutylene terephthalate and A3 during thermal-oxidative aging
- Crashworthiness of GFRP/aluminum hybrid square tubes under quasi-static compression and single/repeated impact
- Review Articles
- Recent advancements in multinuclear early transition metal catalysts for olefin polymerization through cooperative effects
- Impact of ionic liquids on the thermal properties of polymer composites
- Recent progress in properties and application of antibacterial food packaging materials based on polyvinyl alcohol
- Additive manufacturing (3D printing) technologies for fiber-reinforced polymer composite materials: A review on fabrication methods and process parameters
- Rapid Communication
- Design, synthesis, characterization, and adsorption capacities of novel superabsorbent polymers derived from poly (potato starch xanthate-graft-acrylamide)
- Special Issue: Biodegradable and bio-based polymers: Green approaches (Guest Editors: Kumaran Subramanian, A. Wilson Santhosh Kumar, and Venkatajothi Ramarao)
- Development of smart core–shell nanoparticles-based sensors for diagnostics of salivary alpha-amylase in biomedical and forensics
- Thermoplastic-polymer matrix composite of banana/betel nut husk fiber reinforcement: Physico-mechanical properties evaluation
- Special Issue: Electrospun Functional Materials
- Electrospun polyacrylonitrile/regenerated cellulose/citral nanofibers as active food packagings

